Abstract
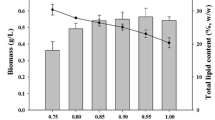
Microalgal-biofilm culture has the advantages of less water requirement and higher lipid productivity. For efficient lipid production in an aerial phase condition, it is ideal to simultaneously induce microalgal biofilm growth and lipid accumulation. Biomass production and lipid accumulation in the aerial microalga Coccomyxa subellipsoidea KGU-D001 were investigated in the logarithmic growth phase. Initially, the alga was cultured in three different conditions: in liquid phase using Bold’s basal medium (BBM), and in the aerial phase in the presence or absence of BBM. In aerial phase conditions with nutrients (i.e., BBM), biomass production was prioritized over cell proliferation until approximately the 7th day of culture. In the liquid phase conditions, the ratio of total organic carbon to total nitrogen (C/N) in cells was almost constant during the culture period. However, in both aerial phase conditions, the C/N ratio increased during the culture period, more so in the absence of nutrients. The total fatty acid content also increased throughout the culture period. Then, algal cells were cultured for a 17-day culture; there would be a growth period of 10 days followed by a lipid accumulation period of 7 days. In such cultures in the presence of BBM, the biomass and lipid productivities (388 and 94.2 mg/m2/day) were both significantly higher in the aerial phase than in the liquid phase during the lipid production period. C. subellipsoidea can produce lipids even during growth if the CO2 supply is efficient and the C/N ratio is high.




Similar content being viewed by others
Data Availability
All data reported is included in the article or in the Supplementary Material.
Code Availability
Not applicable
References
Behera B, Selvam M, Paramasivan B (2022) Research trends and market opportunities of microalgal biorefinery technologies from circular bioeconomy perspectives. Bioresour Technol 351:127038. https://doi.org/10.1016/j.biortech.2022.127038
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenerg Res 1:20–43. https://doi.org/10.1007/s12155-008-9008-8
Ananthi V, Brindhadevi K, Pugazhendhi A, Arun A (2021) Impact of abiotic factors on biodiesel production by microalgae. Fuel 284:118962. https://doi.org/10.1016/j.fuel.2020.118962
Chhandama MVL, Satyan KB, Changmai B, Vanlalveni C, Rokhum SL (2021) Microalgae as a feedstock for the production of biodiesel: a review. Bioresour Technol Rep 15:100771. https://doi.org/10.1016/j.biteb.2021.100771
Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N (2008) Biofuels from microalgae. Biotechnol Progr 24:815–820. https://doi.org/10.1021/bp070371k
Moreira BRA, Viana CRA, Cruz VH, Lopes PRM, Viana RS, Ramos RAV (2022) Meta-analytic review on third-generation biodiesel. Bioenerg Res 15:27–45. https://doi.org/10.1007/s12155-020-10232-6
Katiyar R, Gurjar BR, Biswas S, Pruthi V, Kumar N, Kumar P (2017) Microalgae: an emerging source of energy based bio-products and a solution for environmental issues. Renew Sustain Energy Rev 72:1083–1093. https://doi.org/10.1016/j.rser.2016.10.028
Faried M, Abdelsalam E, Yousef RS, Attia YA, Ali AS (2017) Biodiesel production from microalgae: processes, technologies and recent advancements. Renew Sustain Energy Rev 79:893–913. https://doi.org/10.1016/j.rser.2017.05.199
Rahpeyma SS, Raheb J (2019) Microalgae biodiesel as a valuable alternative to fossil fuels. Bioenerg Res 12:958–965. https://doi.org/10.1007/s12155-019-10033-6
Brar A, Kumar M, Soni T, Vivekanand V, Pareek N (2021) Insights into the genetic and metabolic engineering approaches to enhance the competence of microalgae as biofuel resource: a review. Bioresour Technol 339:125597. https://doi.org/10.1016/j.biortech.2021.125597
Hoffmann L (1989) Algae of terrestrial habitats. Bot Rev 55:77–105. https://doi.org/10.1007/BF02858529
Nowicka-Krawczyk P, Komar M, Gutarowska B (2022) Towards understanding the link between the deterioration of building materials and the nature of aerophytic green algae. Sci Total Environ 802:149856. https://doi.org/10.1016/j.scitotenv.2021.149856
Abe K, Ishiwatari T, Wakamatsu M, Aburai N (2014) Fatty acid content and profile of the aerial microalga Coccomyxa sp. isolated from dry environments. Appl Biochem Biotechnol 174:1724–1735. https://doi.org/10.1007/s10811-013-0106-4
Aburai N, Nishida A, Abe K (2020) Effects of light-emitting diodes (LEDs) on lipid production of the aerial microalga Coccomyxa sp. KGU-D001 under liquid- and aerial-phase conditions. J Biotechnol 323:274–282. https://doi.org/10.1016/j.jbiotec.2020.09.005
Aburai N, Nishida A, Abe K (2021) Aerial microalgae Coccomyxa simplex isolated from a low-temperature, low-light environment, and its biofilm growth and lipid accumulation. Algal Res 60:102522. https://doi.org/10.1016/j.algal.2021.102522
Meher LC, Vidya Sagar D, Naik SN (2006) Technical aspects of biodiesel production by transesterification-a review. Renew Sust Energ Rev 10:248–268. https://doi.org/10.1016/j.rser.2004.09.002
Ohkubo K, Aburai N, Miyashita H, Tsuzuki M, Abe K (2017) CO2 fixation and lipid accumulation in biofilms of the aerial microalga Coccomyxa sp. KGU-D001 (Trebouxiophyceae). J Appl Phycol 29:1745–1753. https://doi.org/10.1007/s10811-017-1123-5
Schnurr PJ, Allen DG (2015) Factors affecting algae biofilm growth and lipid production: a review. Renew Sust Energ Rev 52:418–429. https://doi.org/10.1016/j.rser.2015.07.090
Behera B, Acharya A, Gargey IA, Aly N, Paramasivan B (2019) Bioprocess engineering principles of microalgal cultivation for sustainable biofuel production. Bioresour Technol Rep 5:297–316. https://doi.org/10.1016/j.biteb.2018.08.001
Wang C, Wang Z, Luo F, Li Y (2017) The augmented lipid productivity in an emerging oleaginous model alga Coccomyxa subellipsoidea by nitrogen manipulation strategy. World J Microbiol Biotechnol 33:160. https://doi.org/10.1007/s11274-017-2324-4
Danesh AF, Ebrahimi S, Salehi A, Parsa A (2017) Impact of nutrient starvation on intracellular biochemicals and calorific value of mixed microalgae. Biochem Eng J 125:56–64. https://doi.org/10.1016/j.bej.2017.05.017
Kwok ACM, Wong JTY (2005) Lipid biosynthesis and its coordination with cell cycle progression. Plant Cell Physiol 46:1973–1986. https://doi.org/10.1093/pcp/pci213
Peccia J, Haznedaroglu B, Gutierrez J, Zimmerman JB (2013) Nitrogen supply is an important driver of sustainable microalgae biofuel production. Trends Biotechnol 31:134–138. https://doi.org/10.1016/j.tibtech.2013.01.010
Mirizadeh S, Nosrati M, Shojaosadati SA (2020) Synergistic effect of nutrient and salt stress on lipid productivity of Chlorella vulgaris through two-stage cultivation. Bioenerg Res 13:507–517. https://doi.org/10.1007/s12155-019-10077-8
Aburai N, Maruyama S, Shimizu K, Abe K (2019) Production of bioactive oligopeptide hydrolyzed by protease derived from aerial microalga Vischeria helvetica. J Biotechnol 294:67–72. https://doi.org/10.1016/j.jbiotec.2019.01.021
Saha SK, Brewer CF (1994) Determination of the concentrations of oligosaccharides, complex type carbohydrates, and glycoproteins using the phenol-sulfuric acid method. Carbohydr Res 254:157–167. https://doi.org/10.1016/0008-6215(94)84249-3
Aburai N, Kitajima E, Morita R, Fujii K (2023) Nitrogen-assisted lipid production by biofilms of aerial microalga Coccomyxa subellipsoidea KGU-D001 in the aerial phase. Arch Microbiol in press. https://doi.org/10.1007/s00203-022-03389-5
Maltsev Y, Maltseva I, Maltseva S, Kociolek J, Kulikovskiy M (2019) Fatty acid content and profile of the novel strain of Coccomyxa elongata (Trebouxiophyceae, Chlorophyta) cultivated at reduced nitrogen and phosphorus concentrations. J Phycol 55:1154–1165. https://doi.org/10.1111/jpy.12903
Msanne J, Xu D, Konda AR, Casas-Mollano JA, Awada T, Cahoon EB, Cerutti H (2012) Metabolic and gene expression changes triggered by nitrogen deprivation in the photoautotrophically grown microalgae Chlamydomonas reinhardtii and Coccomyxa sp. C-169. Phytochemistry 75:50–59. https://doi.org/10.1016/j.phytochem.2011.12.007
Abe K, Bito T, Sato A, Aburai N (2014) Effects of light intensity and magnesium supplementation in pretreatment cycle on ammonium removal from wastewater of photobioreactor using a biofilter composed of the aerial microalga Trentepohlia aurea. J Appl Phycol 26:341–347. https://doi.org/10.1007/s12010-014-1181-y
Tripathi S, Choudhary S, Poluri KM (2021) Insights into lipid accumulation features of Coccomyxa sp. IITRSTKM4 under nutrient limitation regimes. Environ Technol Innov 24:101786. https://doi.org/10.1016/j.eti.2021.101786
Miyauchi H, Okada K, Fujiwara S, Tsuzuki M (2020) Characterization of CO2 fixation on algal biofilms with an infrared gas analyzer and importance of a space-rich structure on the surface. Algal Res 46:101814. https://doi.org/10.1016/j.algal.2020.101814
Ruiz-Domínguez MC, Vaquero I, Obregón V, de laMorena B, Vílchez C, Vega JM (2015) Lipid accumulation and antioxidant activity in the eukaryotic acidophilic microalga Coccomyxa sp. (strain onubensis) under nutrient starvation. J Appl Phycol 27:1099–1108. https://doi.org/10.1007/s10811-014-0403-6
Yin Z, Zhu L, Li S, Hu T, Chu R, Mo F, Hu D, Liu C, Li B (2020) A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: environmental pollution control and future directions. Bioresour Technol 301:122804. https://doi.org/10.1016/j.biortech.2020.122804
Acton E (1909) Coccomyxa subellipsoidea, a new member of the Palmellaceae. Ann Bot 23:573–578. https://doi.org/10.1093/oxfordjournals.aob.a089239
Cheng Y-S, Labavitch JM, VanderGheynst JS (2014) Elevated CO2 concentration impacts cell wall polysaccharide composition of green microalgae of the genus Chlorella. Lett Appl Microbiol 60:1–7. https://doi.org/10.1111/lam.12320
González-Hourcade M, del Campo EM, Casano LM (2021) The under-explored extracellular proteome of aero-terrestrial microalgae provides clues on different mechanisms of desiccation tolerance in non-model organisms. Microb Ecol 81:437–453. https://doi.org/10.1007/s00248-020-01604-8
Vingiani GM, Gasulla F, Barón-Sola Á, Sobrino-Plata J, Henández LE (2021) Physiological and molecular alterations of phycobionts of genus Trebouxia and Coccomyxa exposed to cadmium. Microb Ecol 82:334–343. https://doi.org/10.1007/s00248-021-01685-z
Minhas AK, Hodgson P, Barrow CJ, Adholeya A (2016) A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front Microbiol 7:546. https://doi.org/10.3389/fmicb.2016.00546
Behera B, Selvam M, Dey B, Paramasivan B (2020) Algal biodiesel production with engineered biochar as a heterogeneous solid acid catalyst. Bioresour Technol 310:123392. https://doi.org/10.1016/j.biortech.2020.123392
Bermejo E, Ruiz-Domínguez MC, Cuaresma M, Vaquero I, Ramos-Merchante A, Vega JM, Vílchez C, Garbayo I (2018) Production of lutein, and polyunsaturated fatty acids by the acidophilic eukaryotic microalga Coccomyxa onubensis under abiotic stress by salt or ultraviolet light. J Biosci Bioeng 125:669–675. https://doi.org/10.1016/j.jbiosc.2017.12.025
Acknowledgements
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This work was supported by the Strategic Research Foundation Grant-aided Project for Private Universities from Ministry of Education, Culture, Sport, Science, and Technology, Japan (S1411005) and the Research Institute for Science and Technology of Kogakuin University for a special Grant-in-Aid to earn KAKENHI by the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
Nobuhiro Aburai: conceptualization, resources, writing—review and editing, visualization, supervision, project administration, funding acquisition. Naritaka Kawashima: conceptualization, methodology, validation, formal analysis, investigation, data curation, writing—original draft. Rei Morita: methodology, validation, formal analysis. Hiroki Miyauchi: methodology, validation, formal analysis. Katsuhiko Okada: formal analysis, investigation, resources. Norihiro Sato: formal analysis, investigation, resources. Shoko Fujiwara: formal analysis, investigation, resources. Katsuhiko Fujii: supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aburai, N., Kawashima, N., Morita, R. et al. Biomass and Lipid Production in the Aerial Microalga Coccomyxa subellipsoidea KGU-D001 in the Liquid and Aerial Phases. Bioenerg. Res. 16, 2479–2488 (2023). https://doi.org/10.1007/s12155-023-10569-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10569-8