Abstract
The environmental sustainability of the microalgae Nannochloropsis oceanica cultivation for total fatty acid (TFA) production was analyzed using life cycle assessment (LCA). Pilot data provided by the plant operator from cultivation in Italy using Green Wall Panel (GWP®) photobioreactors were upscaled to a 20-ha production process, and an LCA was conducted and assessed for the Italian regions of Tuscany and Sicily. Two additional scenarios were modelled to analyze the influence of more sustainable framework conditions, respectively nutrient recycling and renewable energy supply. The results show that environmental impacts per functional unit are around 15% less at the site with optimal growth conditions. Between 60 and 80% of the impacts are due to the energy demand during plant operation, infrastructure, and nutrient demand. Nutrient recycling and the gain of an energy credit from the separated biocrude with the hydrothermal liquefaction (HTL) process reduce the environmental impacts in all six International Reference Life Cycle Data System (ILCD) impact categories by an average of 11% compared to a scenario without nutrient recycling. The additional consideration of a renewable energy supply allows for an average reduction of 36% and together with the nutrient recycling of an average of 45% for the global warming potential (GWP) and most of the other impact categories.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae are considered an innovative and alternative source of lipids, proteins, and high valuable compounds for both food and feed supplements for human and animal nutrition. Additionally, microalgae are valued as a natural source of high-value compounds for the nutraceutical, cosmetic, and pharmaceutical industries. The promise of third-generation biofuels to produce energy while balancing the increasing demand for feed and food has pushed for the industrialization of bioenergy from microalgae across the world [1,2,3]. Microalgae of the species Nannochloropsis are well-known and already exploited for aquaculture due to its high content in polyunsaturated fatty acids (PUFAs), carotenoids, polyphenols, and vitamins [4,5,6,7,8,9,10]. Nannochloropsis is considered a possible source of third-generation biofuels as it attains higher oil yields than currently available agricultural crops and can be cultivated in seawater and on non-arable land and thus does not trigger land use competition with food production [10,11,12,13]. Nannochloropsis can be cultivated in an environmentally friendly way without the use of pesticides and other chemicals to prevent biological contaminations [14,15,16]. Although different food and cosmetic applications of microalgae are already established in the market, biodiesel from microalgae is not yet commercialized [17,18,19].
Despite these advantages of microalgae, including the potential to use wastewater and capture CO2 from exhaust gas emissions as a nutrient source (e.g., [20,21,22,23]), sustainably cultivating and applying the microalgae is challenging [24,25,26]. In this context, a transparent, detailed, and comprehensive accounting of the environmental effects is required. The method of choice in this work is life cycle assessment (LCA) as it is a recognized and standardized method to identify the environmental weaknesses of algal production from cradle to gate by critically assessing the sustainability of systems. Despite the high appreciation of LCA among scientists, engineers, and politicians, the number of LCAs on products produced with Nannochloropsis is very limited (e.g., [27, 28]). Furthermore, information regarding the environmental impact of large-scale algae production is scarce and therefore prompts the need for scenario assessment before implementation [29]. It is indispensable to carry out LCAs already at an early stage of development to ensure that the desired goal of environmental compatibility will be achieved with the technologies and processes under development. Respectively, the impact of renewable energy supply and the application of hydrothermal liquefaction (HTL) for energy recovery and nutrient recycling is crucial to assess since environmental impacts are expected [30, 31].
A number of studies on the LCA of biofuel or feed production from microalgae have been published in the last decade. Although many of these studies deal with microalgae cultivation in raceway ponds, several consider production in closed systems (photobioreactors), mostly tubular and flat panel reactors [32,33,34,35,36,37,38,39]. Different cultivation systems may influence the results of the assessment because of the different microalgae biomass yield achievable (and thus nutrient consumption, water use, etc.), of the different energy requirements for their operation and for the impact due to the production of their building materials. Focusing on those studies adopting the same typology of reactor used in this work, flat panels, it has to be considered that they deal with the production of different algal species and this may have an impact on the assessment that should be considered when comparing the results obtained. Dufour et al. [40] found that flat plate reactors are the most remarkable for the production of Nannochloropsis gaditana due to lower power supply requirements and lower contamination rates of the culture when compared to raceway ponds. Brentner et al. [32] found that the impact for biodiesel production from Scenedesmus dimorphus (25% triglycerides content) in Arizona (USA), expressed as greenhouse gas (GHG) emissions, is lower when flat panels are used instead of raceway ponds, although the major differences are due to application of different downstream processes (from harvesting to lipid extraction), while the cultivation process shows similar contributions. Starting from the best case reported by Brentner et al. [32], Monari et al. [34] analyzed biodiesel production from Nannochloropsis with algal cultivation in Denmark, including scenarios with use of waste CO2 and nutrients. These scenarios, coupled with an increase of biomass lipid content from 29 to 60%, led to much improved results in terms of GHG emissions and use of non-renewable energy. However, the energy balance for microalgae biodiesel was still negative and, to improve this balance, energy requirements during the cultivation phase represent the most important input to reduce, whereas in terms of lipid extraction, as already found by Brentner et al. [32], supercritical CO2 appears as the less impacting method. Two recent LCA analyses deal with products different from biodiesel. Onorato and Rösch [38] analyzed astaxanthin production in different culture systems, including Green Wall Panels, although integrated with LEDs. In this case, artificial illumination becomes the higher contributor to all the impacts considered. Maiolo et al. [37] analyzed a Green Wall Panel configuration very similar to that used in this work and in a similar location (Italy), with the purpose to obtain algal biomass (Tetraselmis suecica and Tisochrysis lutea) as partial fishmeal substitute. The major contributors for global warming potential (GWP) are fertilizers and pure CO2 production, while energy consumption is the major score for all the other impacts considered (acidification, eutrophication, water use, and cumulative energy demand). High values were found for all impacts analyzed compared to other protein sources (insects and poultry by-product).
From the reported literature, it clearly emerges that it is important to know how the results of the LCA will change under different scenarios. For instance, the German target for a 100% renewable electricity supply by 2050 [41] would implement a different energy scenario than at present, ultimately creating different environmental LCA results than with the present energy system. Beyond that, the concept of a circular bioeconomy, as presented by the European Commission as a Circular Economy Strategy and ‘Closing the Loop’ Action Plan [42], is likely to be implemented in order to increase resource efficiencies and reduce wastes and the environmental burdens associated with them. In total fatty acid (TFA) production from microalgae, the concept of a circular bioeconomy would include the recycling of nutrients in the production to reduce external inputs and reduce waste [43].
At present, LCAs of total fatty acid (TFA) or biodiesel production from microalgae, which consider these future sustainability scenarios, do not yet exist in the scientific literature. Similarly, LCA of the Green Wall Panel (GWP®) for the production of TFA from Nannochloropsis oceanica does not exist. Furthermore, while some literature on LCAs of nutrient recycling for algal biodiesel exist (e.g., [30, 44]), the inclusion of upscaled production and renewable electricity to consider future socio-political targets is limited within scientific literature. Conducting such an LCA is vital to understand the chances and challenges of the GWP®, as well as of how hot spots of the process can be reduced or even eliminated.
The aims of this study are:
-
(i)
To provide valuable environmental system insights in the ecological chances and challenges of LCAs on TFA production with Nannochloropsis oceanica in upscaled systems
-
(ii)
To compare different sites of production to assess the impact of natural site conditions
-
(iii)
To quantify the environmental impacts associated with different scenarios for TFA production with Nannochloropsis oceanica.
Methods and Materials
Against this background, an LCA was conducted for the ecological evaluation of total fatty acid (TFA) production for both feed and biodiesel production with Nannochloropsis oceanica. Building on real experimental data from pilot scale production in Italy, we upscaled the pilot-scale algae cultivation system at two different sites still in Italy and developed two scenarios to consider changes to renewable energy supply and nutrient recycling. Recent work has shown that nutrient recycling and electricity production largely influence the energy and nutrient demands of the algal production process [21, 30, 43, 45,46,47,48,49,50]. By upscaling a pilot-scale algae cultivation system into three scenarios, we were focusing on those changes in production processes that will have the most significant impacts on the LCA results for TFA production.
This study considers the naturally occurring marine alga Nannochloropsis oceanica for the production of TFA. The environmental impact of the production of 1 kg of TFA is quantified using three scenarios. The first scenario, considered to be the Baseline Scenario, is the upscaled production of TFA production from pilot scale (28.1 m2) to industrial scale with a plant size of 20 ha. For the upscaling approach, we used actual, original data from a GWP® pilot plant provided by the University of Florence (UNIFI) and techno-economic data for a 1-ha GWP® plant published by Tredici et al. [51]. The second scenario is the Resource Efficiency Scenario, in which the Baseline Scenario assumptions are optimized using nutrient recycling and energy credit via HTL. The third scenario is the Energy Transition Scenario, in which the Resource Efficiency Scenario is further optimized to consider the production of 1 kg TFA using a renewable electricity mix. Each scenario builds off the previous scenario, and all scenarios utilize the GWP® photobioreactor (PBR) in a two-stage cultivation process. The following paragraphs describe the methods of the LCA of Nannochloropsis oceanica within a GWP® PBR, starting with the Life Cycle Inventory (LCI), the straight-forward accounting of the input and output flows within the system [52].
Goal, Scope, and Functional Unit
The goal of the LCA is to define, assess, and compare the environmental impacts of TFA production with Nannochloropsis oceanica considering scenarios of upscaled production in novel GWP®s at different sites in Italy (Tuscany and Sicily) to identify the ecological hot spots of the production processes. The LCA for upscaled production under different scenarios will give valuable insights concerning the setup and implementation of large-scale TFA production and the need to change socio-technical framework conditions to further improve the benefits from microalgae.
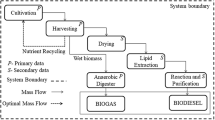
The system boundaries of the 20-ha upscaled LCA are shown in Fig. 1 and are similar to those reported by Khoo et al. [53]. The upstream system includes algae cultivation and harvest with centrifuges. The downstream system includes the process of cell disruption via homogenization, the extraction of TFA, which includes the triacylglycerols (TAGs) applicable for, e.g., biodiesel production, and solvent vaporization to obtain the final TFA product. The conversion of the TFA into feed or an algal biodiesel product is considered to be outside the system boundaries of the present work. Additionally, the first scenario to be assessed, also referred to as the Baseline Scenario, does not include the HTL process. No co-product allocation is considered. The LCA software used to conduct this study was OpenLCA version 1.7 with unit processes selected from the LCA database Ecoinvent 3.4 [54] in accordance to ISO 14,040/44. The functional unit of the LCA is defined as 1 kg TFA.
Life Cycle Impact Assessment Method
To quantify the environmental life cycle impacts, the ILCD Midpoint 2011 impact assessment method, developed and promoted by the JRC European Commission [55], was applied. In the context of evaluating microalgae for TFA production, six categories that are the most important to measure in regard to algae biofuel and feed production were chosen (see Table 1). These include the following: climate change, particulate matter formation, freshwater eutrophication, mineral resource depletion, water resource depletion, and land use. The categories are grouped into three classifications based on reliability. Therefore, as water resource depletion and land use are relevant to algal use [32, 56], these categories were chosen from classification III, despite the ILCD warning that these categories should be applied with caution.
Life Cycle Inventory and Data Collection
For data acquisition, a systematic and comprehensive questionnaire was developed to collect all relevant and detailed information and data on the process design, technology, and equipment used to model and calculate the LCA. This questionnaire was completed for the cultivation part by biologists at the University of Florence (UNIFI) and at Fotosintetica & Microbiologica S.r.l. (F&M) in Sesto Florentino, Italy, a spin-off company of the University of Florence. Personal exchange, virtual meetings, and email correspondence complemented the collection of data and assumptions needed for a comprehensive LCI. The final LCI was created using the collected data and supplemented with literature data where needed.
Two-Stage Algae Cultivation for Optimal Lipid Extraction
Since lipid accumulation in microalgae for TFA production does not take place under natural growing conditions, lipid accumulation in microalgae must be increased by different stress factors such as temperature, light intensity, or nitrogen or phosphorus starvation [65, 66]. Under such stress conditions, some microalgae synthesize lipids as energy and carbon reserves and accumulate them as TAGs in the cytoplasm [67].
However, applying any of these stress factors during cultivation to boost lipid accumulation decreases biomass productivity [68]. In order to maintain a high biomass productivity, a two-stage cultivation process is recommended [66]. Specifically, algal growth can be optimized in the first stage with nutrient repletion. In the second stage, conversion of biomass into lipids can be enhanced as well as TAG production by applying a stress factor such as nutrient starvation. This strategy was reported to be efficient for Nannochloropsis sp., reaching a fatty acid methyl ester (FAME) content of approximately 30% dry weight after the addition of sodium acetate [66] and a total lipid content of 48% dry weight when adjusting salinity [69]. However, the most frequently used and effective strategy to induce lipid accumulation is nutrient starvation, by which total lipid contents higher than 50% biomass can be reached [14, 65].
Applying this strategy in a two-stage cultivation approach requires a modification of the medium composition. To achieve this medium change, several strategies are possible, depending on the volume of the culture. Under laboratory conditions, for batch cultures the simplest way is to centrifuge the culture grown in nutrient-replete medium and to resuspend it in N-free medium. However, this approach is applicable only at small volumes [16, 70]. At larger volumes, the easiest way to perform the two-stage process is to grow the algae until it consumes a large part of the nitrogen in the medium and then dilute it in the same reactor or use it to inoculate a larger reactor or raceway pond, thus achieving both very low initial nitrogen concentrations in the culture medium and a high light availability to the cells, which is fundamental to boost lipid accumulation [14, 16, 43]. In this way, centrifugation is limited to the final phase when lipid-rich biomass is harvested and may be preceded by a pre-concentration step (e.g., ultrafiltration) to reduce the impact of the energy-intensive centrifugation process.
The cultivation of Nannochloropsis oceanica for TFA production was conducted in a GWP® pilot scale reactor operated by UNIFI personnel at F&M facilities in Sesto Fiorentino, Italy. The GWP® reactor, commercialized by F&M, is a flat disposable reactor containing a flexible culture chamber made of photosynthetic active radiation (PAR)-transparent low-density polyethylene (LDPE) film within a metal framework [71]. The pilot scale reactor used was a novel GWP®-II, in which the width of the culture chamber can be varied to suit the needs of the cultivated algae. The pilot reactor was made of four 9-m-long rows connected by manifolds. The reactor could be operated at full volume or at half volume by filling only two rows. Full volume (V) was 1.4 m3, directly illuminated surface (Si) was 23.9 m2, with a Si/V ratio of about 17 m−1. Mixing was achieved by bubbling air, provided by a blower, through a perforated tube placed at the bottom of the culture chamber and by circulating the culture by means of a membrane pump. The culture was cooled by circulation through a titanium plate heat exchanger in which tap water refrigerated by a chiller was circulated. Cooling water was disposed of. The pH was regulated by injecting CO2 in the airflow. CO2 also provided carbon for algal growth. Culture parameters were regulated through a programmable logic controller (PLC). Consumption parameters (electric energy, cooling water, CO2) were recorded. The reactor and ancillary equipment configuration allowed simulation of the biomass production process in larger, industrial GWP® reactors.
Tredici et al. [51] have conducted a techno-economic analysis of a 1-ha GWP® plant located in Tuscany, which was used as a reference for this study. The plant consists of eight GWP®-II modules, each occupying a land surface area of 1250 m2. Air is pumped at 0.12 bar through a perforated polyethylene pipe into the culture chambers by means of a 7.5-kW three-lobe blower, and CO2 is injected at the blower level at need according to pre-set pH values and functions as both carbon supply and pH regulator for the culture. The culture is circulated through a titanium-plate heat exchanger, which is cooled by seawater, in order to regulate the temperature of the culture. Temperature, pH, air bubbling rate, CO2, and O2 levels are measured and controlled via the PLC system that is contained within a polyethylene box and mounted on a stainless-steel plate near the GWP® PBR.
Nutrient stock solution is produced from technical-grade fertilizers within high-density polyethylene (HDPE) tanks on the same site as the GWP® PBR. The stock nutrient solution is composed of sodium nitrate (only during nutrient replete growth), sodium dihydrogen phosphate, iron chloride chelated by disodium EDTA and micronutrients (Mn, Zn, Co, Mo, Cu) concentrated 100 times with respect to the final medium. The (N:)P:Fe ratio is (80:)8:1, whereas micronutrients are provided at a ratio Mn:Zn:Co:Mo:Cu of 20:2:1:1:1, with a Fe:Mn ratio of 13:1. The stock is pumped via a 0.75-kW centrifugal pump and a polyethylene pipeline to a growth medium tank, where the stock and filtered seawater combine to become a growth medium. This growth medium is transferred to the GWP® panels via a 5.5-kW centrifugal pump and polyethylene pipeline.
After the nitrogen-deplete phase, the culture is pumped through a polyethylene pipeline into a centrifugal separator with the use of open impeller pumps. The Westfalia centrifugal separators (model SSD8) produce an algal paste of approximately 20% dry weight. The exhausted culture medium is disposed of directly in the sea, as it is assumed that nutrients (mainly phosphorous, as nitrogen was not added to the medium in this phase) were completely utilized during algae growth and the remaining organic load within the culture medium is below the national and regional allowances for ocean discharge. Italian Regulation (D.L. 152/2006) [72] sets a chemical oxygen demand (COD) limit of 125 mg/L for discharge; exhausted culture medium of Nannochloropsis salina was shown to contain about 35 mg of soluble organic carbon per milliliter [73], corresponding to < 115 mg/L of COD, using the conversion equation for effluents by Dubber and Gray [74]. The discharged effluent is pumped via open-impeller pumps and a polyethylene pipeline. Assuming that the plant is located close to the sea, seawater is pumped from the ocean via a polyethylene pipeline, through a 60-µm filter, used to cool the heat exchanger, and finally back into the sea using submersible pumps. An open impeller centrifugal pump per module is used to transfer the culture through the heat exchanger. Circulation pumps are used to pump the CO2-enriched air through the chambers, the seawater through the heat exchanger, and the culture between the panels of the PBR modules.
Upscaling Upstream Data from Pilot to Industrial Scale
Data was provided by UNIFI for production of Nannochloropsis oceanica in a pilot-scale GWP® reactor. The cultivation trial, conducted in April 2018, used half of a GWP® reactor with an occupied land area of 14 m2 for the biomass accumulation phase and full volume reactor (28.1 m2 of occupied land area) for the nitrogen starvation/lipid accumulation phase. The progress of lipid accumulation during the starvation phase was followed by daily determination of total lipid content in the biomass. The pH of the culture was set to a range of 7.5–7.8 and the temperature was set to a maximum value of 27 °C. The biomass concentration when leaving the PBR was 1.6 g/L culture. This pilot-scale cultivation data was upscaled to fit to a 20-ha industrial GWP® plant. The plant is assumed to be made of several 1250-m2 GWP®-II modules structured as in Tredici et al. [51] (see Online Resource 1Footnote 1). The total volume of the plant is 6300 m3 with an Si of 128,000 m2 (see Table 2) meaning that the Si/V ratio is about 20, close to that of the pilot reactor.
To give greater perspective to the results, the 20-ha industrial plant was assigned to two different geographical locations with different solar radiation and growth temperatures throughout the year: Tuscany (region of the pilot plant) and Sicily, for which 240 and 330 productive days per year are assumed, respectively, to avoid the colder winter months with lower radiation, during which TFA accumulation would last too long compared to the other periods of the year (about 4–6 days in summer and 7–9 days in spring/autumn in pilot reactors). Sicily was chosen as the second geographical location as it has been identified as an ideal location for biomass fuel facilities based on the site parameters such as irradiance and temperatures [75]. The TFA concentration within the algal biomass was assumed to be 50% at industrial scale, assuming an optimization of TFA content from the data measured in the biomass obtained in the pilot reactor (45% of dry biomass). It was estimated that a 20-ha industrial site would be able to produce 10.5 t of TFA/ha/year in Tuscany and 15 t of TFA/ha/year in Sicily.
The equipment and electricity demand for the cultivation and harvesting from the 1-ha plant were linearly upscaled (similar to [76, 77]) to fit a 20-ha plant, and a 15% reduction in the linear upscaling of electricity was assumed thanks to adoption of equipment with improved efficiency (see Fig. 3). The Italian electricity mix from Ecoinvent 3.4 was used for the 20-ha Tuscany and Sicily baseline scenarios. Nutrients, cooling, and cleaning water were calculated per kg TFA from the pilot reactor data and upscaled linearly based on the productivity value for a 20-ha scenario (see Table 3).
As the pilot reactor trial in April 2018 did not include downstream processes (cell homogenization, TFA extraction via solvent and centrifugation, HTL and solvent vaporization), these values for input flows (such as electricity, infrastructure, water) per production volume were taken from literature and calculated based on the volume of TFA produced on 20 ha.
Upstream Inventory Analysis: Data and Assumptions
Data assumptions were made for the upstream inventory to produce an LCA with the most optimistic outcome. Nutrient uptake efficiency is considered in a simplified way to be 100% [51] knowing that nutrient uptake is not all due to algae metabolism but also to bacteria that are associated to algal cells in the cultures, as no axenic culture is currently performable at large scale. When the culture is harvested, the consortium of algae and bacteria cannot be separated in the two components, and thus, to be extremely precise, we should refer to algae/bacteria biomass. It is important to point out that bacterial load is usually low in a healthy algal culture; thus, biomass is substantially algal biomass. Moreover, the algae biomass in our case is obtained under nitrogen starvation, so no nitrogen is present in the culture medium. In contrast, phosphate, which represents the other major nutrient, is provided according to productivity to avoid excess feeding and is often taken up rather quickly by algae by virtue of luxury consumption mechanisms. With these considerations, a 100% consumption of the nutrients in the culture medium is an acceptable simplification.
The supply of CO2 in higher concentrations than available in the air is crucial to increase algal growth and achieve high productivities [78]. Since the environmental benefits would be counteracted if fossil-based CO2 from a coal-fired plant or industrial non-renewable produced CO2 would be applied, we assume in all scenarios that biogenic CO2 is provided, e.g., by a biogas plant nearby. As the CO2 is only temporarily removed from the atmosphere, the CO2 consumption was considered to be neutral and not used as an input flow in the LCI. However, as CO2 must be provided to the algal culture to ensure pH regulation and provide carbon source, the infrastructure and electricity needed for the pumping of CO2 from the production site to the blower were considered [51]. The infrastructure and electricity needed for pumping the air (at need enriched with CO2) to mix the culture and avoid sedimentation and gradient formation [51] was also considered.
Because the seawater used to cool the culture is pumped back into the ocean, seawater was considered to be a neutral value, but the infrastructure and electricity for pumping the seawater is considered. Similarly, as 99% of the seawater in the culture is removed from centrifugation and pumped back into the sea, the seawater required for the culture is considered to be neutral. For the same reasons as for the pilot reactor and 1-ha GWP reference plant from Tredici et al. [51], the exhausted culture medium is considered to be disposed of directly in the sea.
Downstream Inventory Analysis: Data and Assumptions
In order to remove the TFA from the concentrated Nannochloropsis oceanica biomass, the first critical step is to completely disrupt the cell wall [79]. Techniques for cell wall rupture have been widely explored in algal research, but many are too energy- or cost-intensive. Cell disruption via homogenization was chosen for this study, as it has proven to be energy-efficient with Nannochloropsis sp. with up to 25% dry weight [80]. For the 20-ha plant, the GEA Ariete NS2006 Homogenizer and High Pressure Pump [81] was chosen, as this model is optimal for industrial-scale wet algal biomass homogenization due to its maximum flow rate of 80 L/h and lower power consumption (1200 bar) when compared to other models [80, 81]. This homogenizer requires lubricating and cooling water, as well as lubricating oil for proper functioning [81]. Materials considered for the downstream machinery were assumed to be stainless steel at 70% of the overall weight.
Angles et al. [82] found that extraction of lipids from wet microalgae (which have already been disrupted) was more efficient than lipid extraction from dried algal biomass. This downstream phase of lipid extraction involves the use of a solvent and centrifugation of the solvent-wet biomass mixture. Methyl-tert-butyl ether (MTBE) is used as a solvent due to the low solubility in water and low heat requirement for recycling via vaporization [82]. The concentration of MTBE per g of dry algae is 3.73 ml [82]. The amount of solvent required for the 20-ha plant was calculated based on the final yield of dry algal biomass. The solvent-biomass mixture is separated using a three-phase separator centrifuge (Flottweg, based on [83]). The three-phase separator requires 6660 kWh/a in order to separate the solvent-biomass mixture into solvent and TFA, residual slurry, and residual water. This residual slurry is processed via HTL to recover nutrients and energy from the biocrude oil within the biomass. The HTL converts the protein and carbohydrates into energy (e.g., oil), and the incorporated nutrients such as nitrogen are released and have the potential to be re-used in the cultivation process, which has implications for the LCA [30, 84]. However, this nutrient and energy-recovery process is only considered in the Resource Efficiency and Energy Transition Scenarios; in the Baseline scenario, the residual slurry does not pass through the HTL and is instead assumed to be used for further refinery of valuable components (e.g., protein), for which only the energy and materials for transfer to the next processing phase are included in the analysis. The solvent and TFA mixture is vaporized to remove the TFA and to allow the separated MTBE solvent to be recycled in the following cycles. The emissions related to solvent use for lipid extraction can be reduced if the recovery of the solvent is considered [85]. Methyl tert-butyl ether requires the least energy (J) per mg of extracted lipids when compared to 11 other viable solvents [82]. At lab scale, 235 J/mg extracted lipids are required to vaporize the solvent from the lipids; however, at large scale, the energy demand for solvent recycling can be reduced to 8–10 J/mg due to an assumed increase in algae-to-solvent ratio. For this LCA, 8 J/mg extracted lipids are assumed for recycling of the methyl tert-butyl ether from the TFA. This equates to 666 MWh/year at the 20-ha plant in Sicily and 466 MWh/year at the 20-ha plant in Tuscany. Due to this recycling consideration, cultivation at the Sicily site would require 101 t MTBE solvent/year and 93 t MTBE solvent /year in Tuscany.
The residual water separated from the solvent-biomass mixture is pumped back into the ocean after centrifugation via open-impeller pumps.
Inventory Analysis: Scenarios
In addition to the 20-ha industrial-scale plant, which is referred to as the Baseline Scenario, two scenarios were explored in this study in order to determine the influence of more efficient processes.
Resource Efficiency Scenario
There is evidence that after the extraction of algal fatty acids, the residual biomass should be valorized in order to improve the overall efficiency of the process, e.g., by utilizing the energetic output and recycling of nutrients (see Fig. 2). For this study, the hydrothermal liquefaction (HTL) process has been considered the most suitable technology to convert the residual algae biomass (see Fig. 3 [23]) since HTL in general is appropriate for the conversion of wet feedstock [30, 83]. A so-called bio-crude oil is produced from the residual biomass including carbohydrates and proteins via the HTL process, which can also be upgraded and used, e.g., as fuel.
Hydrothermal liquefaction (HTL) process flows (N, nitrogen; P, phosphorous) (*[83])
The majority of HTL research has been performed using small batch reactors, typically a few hundred milliliters in volume. The Resource Efficiency Scenario considers an HTL process operating at the end of each harvest cycle. At the Tuscany plant, 74,375 L of biomass flows through the HTL process per cycle, which was designed according to [83] and [86] and complemented by experimental data provided by [87]. Similarly, at the 20-ha Sicily plant, 77,272 L of biomass flows through the HTL process per cycle. These volumes of biomass were processed and converted by a high temperature (350 °C) and pressure (210 bar) reaction into four streams [88]: 56 Vol.% bio-crude, 39 Vol.% of aqueous phase, 5 Vol.% of product gas, and 1 Vol.% of solids. The nitrogen and phosphorus bound in the solid and aqueous phases are internally recycled and used as a credit in the model, reducing the external nutrient demand during cultivation. This is a simplified approach to model the nutrient recycling step as it is known that the HTL solids would require an additional conversion step (such as acid digestion) to make it bio-available before re-use [89]. However, any further processing of nutrients to enhance bioavailability was considered outside the system boundaries. A generic organic co-solvent (1,1 dimethylcyclopentan was chosen as a reference) is also included in order to support the separation of the bio-oil from the other products. The solvent flowrate was set to 10% of the total flow entering the HTL process [88]. To further optimize the results of this scenario, a credit was applied to recycle the solvent at 99% for each HTL harvest cycle after the first. However, as this credit reduces the solvent amount and therefore the impact of the HTL solvent without considering the energy and infrastructure needed to recycle the solvent, this optimization should be taken only at face value. A list of the equipment used in the HTL phase is given (see Table 4). All equipment inputs were scaled according to the volume of flows through the system. After the HTL process, the product flow is separated using a ceramic filter as a first step and a 3-phase separator subsequently.
The bio-crude oil produced (0.56 kg per 1 kg TFA produced), with a high heating value (HHV) of 35 MJ/kg [83, 90], is implemented in the model as an energy credit. In the LCA model, it is assumed that this bio-crude oil is being used to produce electricity with a conversion efficiency of 40%. The produced gas is not recycled or used as its amount is negligible (5 Vol.%).
Energy Transition Scenario
The scenario that represents a potential energy transition is the exact same as the Resource Efficiency Scenario (see Fig. 2), except that electricity flows are changed to present a renewable energy mix (see Table 5). In other words, the Italian electricity mix used for the baseline and resource efficiency scenarios is replaced by the Norwegian electricity mix, of which 98% is produced by hydropower [91]. Although an electricity mix based more on solar power would be more realistic for the Italian sites, such an electricity mix for Europe does not yet exist in Ecoinvent 3.4 and therefore the Norwegian renewable electricity mix was chosen as a proxy.
Results
In this section, the LCA results are first shown for each scenario: the Baseline Scenario, the Resource Efficiency Scenario, and the Energy Transition Scenario. The focus is on the six ILCD categories mainly relevant for the environmental evaluation of algal TFA production. The results are aggregated by the contributions of each process group: electricity, infrastructure, nutrients, other operational material, and tap water (see Table 6). Then, these results are compared with each other and soybean oil as reference.
Baseline Scenario Results
The results of the Baseline Scenario demonstrate the large influence of infrastructure and electricity in most categories (Fig. 4). The infrastructure required for the plants (such as pumps, PVC pipes, GWP® chambers per year) in both locations is identical, but as the productivity is higher in Sicily than in Tuscany, the impact of infrastructure per 1 kg of TFA produced in Tuscany is greater because more infrastructure is required per kg of TFA. However, more electricity is required for the Sicilian plant because the plant operates 90 days more than the plant in Tuscany. Electricity is required for all pumps, the PLC system, blowers, centrifugal harvesting, seawater recycling, cell homogenization, the three-phase centrifugal separator, and the solvent vaporization for solvent recycling.
Resource Efficiency Scenario Results
The LCA results of the Resource Efficiency Scenario (Fig. 5) show that the HTL process is beneficial if the yield is high enough to counter the additional infrastructure and electricity required for the HTL process. Using the HTL aqueous phase to fertilize the algae during cultivation can jeopardize energy and emissions reductions since nutrient recycling is associated with energy consumption and emissions [30]. The relatively small reduction in the emissions of CO2 equivalents between the Baseline and the Resource Efficiency Scenario shows that there is a small reduction due to a little energy gain by applying the HTL process for nutrient recycling.
The HTL process allows for a significant reduction of nutrient input (63% of N and 90% of P are recycled), thus decreasing the impacts in all categories compared to the Baseline Scenario at both sites. The inclusion of the biocrude energy credit (2.18 kWh/kg TFA) also allows for a reduction in the electricity demand and resulting impact. In the case of the Tuscany site, the electricity contribution to the impact category of climate change decreased by 0.8 kg CO2-eq. from the Baseline Scenario. In contrast, the infrastructure required for the HTL process increases the impacts slightly (e.g., an increase of 0.09 kg CO2-eq. from the infrastructure processes in the climate change impact category for the Tuscany site). Infrastructure and electricity are still the largest contributors for most categories.
Energy Transition Scenario Results
The LCA results of the Energy Transition Scenario (Fig. 6) demonstrate the large influence that a renewable electricity mix can have on the environmental impacts of an energy-intensive production system. The only change adapted for this scenario from the Resource Efficiency Scenario is the change from the Italian electricity mix to the Norwegian electricity mix, which was considered an example of renewable European energy as 98% of the electricity is produced via hydropower [91]. The results show a significant reduction of impacts related to electricity in all categories except for mineral, fossil, and renewable resource depletion, as this category is largely impacted by nutrient input and not electricity.
Scenario Result Comparison to Soybean Reference
In order to gain perspective on the impacts of a 20-ha TFA plant based in Tuscany or Sicily, the LCA results were compared with refined soybean oil as a reference system (Fig. 7). Soybean was chosen as reference due to its high market share and its crucial role in the feed and fuel market; e.g., soybean oil is the second-most used resource for biodiesel production in Italy [92]. Of greatest significance, this graph shows the difference between impact category results from algal TFA production and soybean oil production. In all categories except for land use (kg C deficit), the soybean oil reference system creates a smaller environmental impact than the TFA production from Nannochloropsis oceanica: in the most extreme case, the soybean oil reference impact of water depletion is approximately 5000% smaller than the Resource Efficiency Scenario results. Electricity production is considered to deplete water resources according to the ILCD Midpoint 2011 LCA methodology and for TFA production from Nannochloropsis oceanica is much larger per kg than for the refined soybean oil reference system (about 22 kWh/kg TFA and 0.00437 kWh/kg refined soybean oil [93]). Additionally, the Ecoinvent 3.4 refined soybean process handles the waste byproduct as a negative value for wastewater, which reduces the water resource depletion impact category values, thus further increasing the difference between the algal TFA scenarios and the soybean reference system. Land use by algae as a source of TFA is an important impact category when considering algal TFA for feed and biofuel production, because they are preferred to feed and biofuels produced from food crops due to the lack of competition for arable land. The type of land use considered for the 20-ha scenarios is industrial land, which was not originally used for arable crops. In contrast, the soybean oil reference system considers that the soybeans were grown in Brazil on arable cropland, which was originally secondary and primary forest and therefore required clear-cutting to produce the soybean [93]. Due to these considerations, the reference system requires more land per 1 kg algal TFA product.
Discussion
Discussion of the Results
The environmental impacts of the upscaled TFA production with Nannochloropsis oceanica were assessed with the LCA methodology for a 20-ha-sized plant in two locations in Italy (Sicily and Tuscany) and for three scenarios. The system boundaries of these scenarios included the upstream and downstream processes involved from algae cultivation to the final TFA extraction from the algal biomass; the pilot reactor data collected from the University of Florence determined the upstream processes, whereas the downstream processes were defined with data from literature. The single processes chosen were selected and optimized to create a comprehensive algal TFA production system and to design a system layout applicable for large-scale production at industrial scale. According to that rationale, a technical process to recycle the nutrients in the Resource Efficiency Scenario was considered to reduce the external demand for nutrients. For the purposes of this study, it was assumed that the nutrient flows from the HTL process could directly replace a percentage of the technical-grade fertilizers in the nutrient stock, as this is technically feasible from any hydrothermal methods [94]. However, there is evidence that this could lead to biological challenges in the upstream process such as limited algal growth due to possible low bioavailability of nutrients [94,95,96] or the accumulation of inhibitory matter [97]. A pre-treatment of the HTL liquid phase could be recommended, if not necessary before its reuse for the cultivation of Nannochloropsis oceanica [98]. Therefore, the assumptions made in this scenario, as well as the LCA results achieved, have to be considered preliminary and rather optimistic. However, in order to include a pretreatment process, more research at lab and pilot scale is required to find out which process steps are required for a safe and sustainable reuse of the nutrient-rich phase from HTL [99, 100]. As an alternative to this technical approach to improve resource efficiency by a circular reuse of nutrients, nutrient-rich digestate from anaerobic digestion plants could be used at low costs and with low environmental impacts to cover the nutrient demand of microalgae cultivation in the upstream process [101,102,103,104]. Although the digestate also needs to be pretreated, this only requires ceramic filters to remove solids and for mechanical sterilization to prevent bacteria to enter the PBR that could negatively affect algal growth. This would improve the resource efficiency of the overall agricultural system, as biogas digestate is a waste source of nutrients and there is an urgent need to valorize these nutrients. This process would give an additional credit to algal TFA production as the current use of biogas digestate as field fertilizer leads to significant negative environmental impacts [105]; in particular, an increased concentration of nitrate in the groundwater and the deterioration of the limited amount of phosphate fertilizer resources can be a result of applying biogas digestate as a fertilizer.
Another technical alternative which could improve overall efficiency of the process would be to use a different method of TFA extraction, such as supercritical CO2 extraction, which has been studied for the potential to selectively extract lipids without the use of solvents [32, 106,107,108].
In this LCA study, CO2 is not considered an input or output flow, although the energy and infrastructure required for CO2 injection in the culture is included. As bioenergy systems are considered to be carbon–neutral due to the carbon present within the material originating from the atmosphere, the carbon is removed temporarily from the atmosphere. Therefore, the biological or thermochemical conversion of algal biomass that releases CO2 into the atmosphere does not create additional greenhouse gas emissions [109]. In the scenarios, the biogenic source for the CO2 needed to achieve high algal productivities was modelled in an ideal way considering that the biogas plant is located nearby the algal TFA production site. Possible additional infrastructure or energy to provide the biogenic CO2 for algae cultivation was not included in the LCA calculations since agriculture is obliged to reduce their greenhouse gas emissions anyway to become more sustainable.
Comparison Between Algal TFA and Soybean Oil
To gain perspective on the environmental impact of the scenarios examined in this study, the results were compared with a soybean oil reference system. In the case of biodiesel produced in Italy, 40% is from rapeseed, which is imported from France, Germany, the Netherlands, and Belgium [92]. However, rapeseed processes do not yet exist in Ecoinvent 3.4 and therefore could not be used as a reference for this study. The second-most produced biodiesel in Italy (30%) is from imported soybeans. Both TFA from algal biomass and soybean oil must be transesterified, a process which is not currently included in this study’s system boundaries, in order to yield biodiesel and glycerin byproducts [110]. The Ecoinvent process “soybean oil refinery operation- ROW” [93] matches the system boundaries and functional unit of the algal TFA LCA scenarios and is therefore used here as a reference system.
The comparison between the reference system and the systems of focus in this study demonstrates a smaller impact in most environmental impact categories for soybean oil when compared to the two geographical locations and three scenarios of algal TFA. However, the impact categories of land use and water depletion do not fit this pattern. The Ecoinvent 3.4 database considers primary and secondary forest “clear-cutting,” as well as the land required for green manure cultivation, within the soybean oil flow. Clear-cutting is not an issue for the areas of Tuscany and Sicily, where the 20-ha plants would be located, and the land use considered for this study is from industrial area, meaning the removal of any forest at one point in time (if any) on these areas is not considered. Naturally, when the system boundaries include land use change in this way, 1 kg of soybean oil requires far more land than 1 kg of algal TFA. This difference in system boundaries, which are not to the benefit of the reference system in this case, should be considered when comparing the results.
It is important to note that the soybean oil does consider the transportation of soybeans, produced in Argentina and Brazil, to the soybean mill; however, this consideration is very basic as the geography of the process in Ecoinvent 3.4 is generalized as “Europe.” In other words, the transportation of soybeans from arrival in Europe to a theoretical soy mill located somewhere else in Europe is considered, while the transport of the soybeans from South America to Europe is not. Considering the oversea transport of soybeans via ship would increase the environmental impacts of soybean oil production, particularly in the impact categories of climate change, particulate matter, and minerals, fossils, renewables depletion due to the large fuel requirement for shipping.
In addition to reduced land use competition, another positive characteristic of algal TFA production is the ability of the microalgae to be cultivated with saltwater instead of tap water. Nannochloropsis oceanica, a naturally occurring seawater alga, requires saltwater during cultivation and only small amounts of tap water for cleaning and cell homogenization. Therefore, in the case of water depletion, a higher impact of soybean oil than algal TFA was expected, as soybean production requires heavy inputs of tap water for irrigation: Gerbens-Leenes et al. [111] found that 1 kg of soybean biodiesel requires 7521 kg of blue water. However, the reference process has a lower impact on water depletion than the algal TFA in all scenarios for two reasons: the ILCD midpoint 2011 LCA methodology considers water turbine use for electricity generation to deplete water resources, creating high water depletion values for the algal TFA scenarios; and the waste byproduct handling in the Ecoinvent 3.4 refined soybean process considers a negative value for wastewater, which reduces the water resource depletion impact category values. This byproduct handling is not considered in the TFA LCA, which therefore creates an inaccurate reference for this impact category.
Environmental Benefits by Energy-Efficient Harvesting and Replacing Soybean Protein
This study has calculated three scenarios for Nannochloropsis oceanica cultivation for TFA production by using collected data and optimistic assumptions. However, Nannochloropsis as a TFA producer precursor still generally lags behind other biogenic oil-producing options producible in Italy or other countries, as was shown by the example of soybean oil. Further improvements can be made to the algal TFA LCA and the methodology for more reliable results. For instance, as per the pilot data collected at the University of Florence, harvesting of the algal biomass is conducted by centrifuge. By replacing the centrifuge usually applied for the harvesting process and to dewater the biomass by alternative harvesting technologies, e.g., the wet-end of a paper machine, potential environmental benefits could be gained [112]. The composition of total lipids, carbohydrates, and protein is similar for biomass harvested via alkaline flocculation or centrifugation. Flocculation, however, is more energy- and cost-efficient than centrifugation for harvesting of algae [113]. Although this method was not used during pilot cultivation of Nannochloropsis and was therefore not included in the upscaled scenarios, centrifugation can be replaced with alkaline flocculation to harvest Nannochloropsis oceanica in the cultivation stage to increase the environmental friendliness of TFA production.
Additionally, by incorporating a system expansion method within the LCA to consider the use of the residual slurry, the results can be improved. After extracting the lipids from Nannochloropsis biomass, the remaining defatted material could be used as an animal feed ingredient [114] or even a high-value human protein supplement [115] rather than recycling the nutrients in the protein-fraction with the HTL process. Crude protein content is around 45.2% of the defatted Nannochloropsis dried biomass [114]. These proteins could replace soybean protein and be an alternative for people with soy allergies.
Replacing soybean could save the high water and land demands for their cultivation, and even biodiversity could be protected as no rainforest would be cut down for soybean cultivation. In a study conducted by Lamminen et al. in 2019 [116] on the effect of substituting soybean meal with microalgae for cow feed, dried Nannochloropsis biomass was found to contain comparable amounts of crude protein to soybean meal at 150 and 154 g/kg DM, respectively. Moreover, the algal biomass replacement allowed the cows to consume more dietary fiber and fatty acids per day than with the soybean feed. Cows consumed 8.82 kg/day of fiber and 1744 g/day total fatty acids when consuming soybean meal feed in comparison to the 9.09 g/day of fiber and 1901 g/day total fatty acids consumed from the Nannochloropsis-based feedstock. Despite that Nannochloropsis sp. is one of the few species accepted as feed and the advantages of algal protein, the protein-rich residual biomass is not yet used for feed due to high capital and operating costs as well as the lack of technology and knowledge. This was also proved by Taelman et al. [117], who concluded that feeding livestock imported protein-rich soy meal is less energy-intensive than algal meal due to currently inefficient algal cultivation systems. The biorefinery concept for zero-waste and multiple-products should be further investigated to fully exploit the advantages of Nannochloropsis and to improve the environmental benefits.
Conclusions
Using life cycle assessment, this study quantified the environmental impacts from six impact categories for an upscaled 20-ha algal TFA production plant under three different scenarios (Baseline, Resource Efficiency, and Energy Transition Scenario) and in two different locations (Tuscany and Sicily). The results of this study contribute to a new body of knowledge regarding the methodological approach as well as the results based on data from real pilot reactors: as indicated by the scenario calculations, the achievement of socio-political targets can significantly improve LCA results.
The study shows that nutrient recycling as seen in the Resource Efficiency Scenario or a renewable electricity mix, as seen in the Energy Transition Scenario, can improve the environmental friendliness of TFA production from microalgae. The results indicated a significant improvement in all impact categories when considering the nutrient recycling and biocrude energy credit possibilities in the Resource Efficiency Scenario when compared to the Baseline Scenario. The improvements were slightly smaller in less optimal locations, such as in Central Italy, as the yield is lower and does not compete as well as the more optimal location with the additional energy and infrastructure required for the nutrient recycling and energy credit. Furthermore, the adaptation of a renewable energy mix in the Energy Transition Scenario presented further reductions for both plant locations when compared to the Baseline scenario. These results conclude that a high productivity system would benefit from the adaptation of nutrient recycling, bioenergy credits, and renewable energy.
Data availability
Not applicable.
Code availability
Not applicable.
Notes
Reprinted from Algal Research (M.R. Tredici, L. Rodolfi, N. Biondi, N. Bassi, G. Sampietro, Techno-economic analysis of microalgal biomass production in a 1-ha Green Wall Panel (GWP®) plant, 253–263, 2016) [51] with permission from Elsevier.
Abbreviations
- COD:
-
Chemical oxygen demand
- GHG:
-
Greenhouse gas
- GWP®:
-
Green Wall Panel
- HDPE:
-
High-density polyethylene
- HHV:
-
High heating value
- HTL:
-
Hydrothermal liquefaction
- LCA:
-
Life cycle assessment
- LCI:
-
Life Cycle Inventory
- LCIA:
-
Life Cycle Impact Assessment
- LDPE:
-
Low-density polyethylene
- PAR:
-
Photosynthetic active radiation
- PBR:
-
Photobioreactor
- PLC:
-
Programmable logic controller
- PUFA:
-
Polyunsaturated fatty acids
- TAG:
-
Triacylglycerols
- TFA:
-
Total fatty acids
References
Coelho MS, Barbosa FG, da RAZ de Souza M (2014) The scientometric research on macroalgal biomass as a source of biofuel feedstock. Algal Res. https://doi.org/10.1016/j.algal.2014.11.001
Moreno AD, Susmozas A, Oliva JM, Negro MJ (2020) Overview of bio-based industries. In: Biobased Products and Industries
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact 17:26
Zanella L, Vianello F (2020) Microalgae of the genus Nannochloropsis: chemical composition and functional implications for human nutrition. J Funct Foods 68:103919. https://doi.org/10.1016/j.jff.2020.103919
Sharma K, Schenk PM (2015) Rapid induction of omega-3 fatty acids (EPA) in Nannochloropsis sp. by UV-C radiation. Biotechnol Bioeng. https://doi.org/10.1002/bit.25544
Molino A, Iovine A, Casella P, et al (2018) Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph15112436
Lemahieu C, Bruneel C, Termote-Verhalle R, et al (2013) Impact of feed supplementation with different omega-3 rich microalgae species on enrichment of eggs of laying hens. Food Chem. https://doi.org/10.1016/j.foodchem.2013.06.078
Macías-Sánchez MD, Mantell C, Rodríguez M, et al (2005) Supercritical fluid extraction of carotenoids and chlorophyll a from Nannochloropsis gaditana. J Food Eng. https://doi.org/10.1016/j.jfoodeng.2004.03.021
Rebolloso-Fuentes MM, Navarro-Pérez A, García-Camacho F, et al (2001) Biomass nutrient profiles of the microalga Nannochloropsis. J Agric Food Chem. https://doi.org/10.1021/jf0010376
Khozin-Goldberg I, Solovchenko A, Pal-Nath D et al (2013) Omega-3 and omega-6 LC-PUFA from photosynthetic microalgae: studies on Parietochloris incisa and Nannochloropsis sp. In: Catala A (ed) Polyunsaturated fatty acids: sources, antioxidant properties and health benefits. Nova Science Publishers
Gurumoorthy P, Saravanan A (2019) Biofuel production from marine microalgae Nannochloropsis salina using paper mill effluents. Int J Mech Eng Technol 10:1471–1477
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the U.S. Department of Energy’s Aquatic Species Program- biodiesel from algae. Golden
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science (80- ) 329:796–799. https://doi.org/10.1126/science.1189003
Rodolfi L, Zittelli GC, Bassi N et al (2009) Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112. https://doi.org/10.1002/bit.22033
Pal D, Khozin-Goldberg I, Didi-Cohen S, et al (2013) Growth, lipid production and metabolic adjustments in the euryhaline eustigmatophyte Nannochloropsis oceanica CCALA 804 in response to osmotic downshift. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-013-5092-6
Benvenuti G, Bosma R, Klok AJ et al (2015) Microalgal triacylglycerides production in outdoor batch-operated tubular PBRs. Biotechnol Biofuels 8:1–9. https://doi.org/10.1186/s13068-015-0283-2
Rösch C, Roßmann M, Weickert S (2019) Microalgae for integrated food and fuel production. GCB Bioenergy 11:326–334. https://doi.org/10.1111/gcbb.12579
Ruiz J, Olivieri G, De Vree J et al (2016) Towards industrial products from microalgae. Energy Environ Sci 9:3036–3043. https://doi.org/10.1039/c6ee01493c
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96. https://doi.org/10.1263/jbb.101.87
Fortier MOP, Roberts GW, Stagg-Williams SM, Sturm BSM (2014) Life cycle assessment of bio-jet fuel from hydrothermal liquefaction of microalgae. Appl Energy 122:73–82. https://doi.org/10.1016/j.apenergy.2014.01.077
Handler RM, Shonnard DR, Kalnes TN, Lupton FS (2014) Life cycle assessment of algal biofuels: Influence of feedstock cultivation systems and conversion platforms. Algal Res 4:105–115. https://doi.org/10.1016/j.algal.2013.12.001
Craggs RJ, Heubeck S, Lundquist TJ, Benemann JR (2011) Algal biofuels from wastewater treatment high rate algal ponds. Water Sci Technol. https://doi.org/10.2166/wst.2011.100
Quiroz Arita CE, Peebles C, Bradley TH (2015) Scalability of combining microalgae-based biofuels with wastewater facilities: a review. Algal Res
Leite GB, Abdelaziz AEM, Hallenbeck PC (2013) Algal biofuels: challenges and opportunities. Bioresour Technol 145:134–141. https://doi.org/10.1016/j.biortech.2013.02.007
Usher PK, Ross AB, Camargo-Valero MA et al (2014) An overview of the potential environmental impacts of large-scale microalgae cultivation. Biofuels 5:331–349. https://doi.org/10.1080/17597269.2014.913925
Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenerg 53:29–38. https://doi.org/10.1016/j.biombioe.2012.12.019
Dufour J, Moreno J, Rodríguez R (2011) Life cycle assessment of biodiesel production from microalgae oil: effect of algae species and cultivation system. In: Finkbeiner M (ed) Towards life cycle sustainability management. Dordrecht: Springer Netherlands, pp 437–442
Soh L, Montazeri M, Haznedaroglu BZ et al (2014) Evaluating microalgal integrated biorefinery schemes: empirical controlled growth studies and life cycle assessment. Bioresour Technol 151:19–27. https://doi.org/10.1016/j.biortech.2013.10.012
van Boxtel AJB, Perez-Lopez P, Breitmayer E, Slegers PM (2015) The potential of optimized process design to advance LCA performance of algae production systems. Appl Energy 154:1122–1127. https://doi.org/10.1016/j.apenergy.2015.01.036
Frank ED, Elgowainy A, Han J, Wang Z (2013) Life cycle comparison of hydrothermal liquefaction and lipid extraction pathways to renewable diesel from algae. Mitig Adapt Strateg Glob Chang 18:137–158. https://doi.org/10.1007/s11027-012-9395-1
Singh A, Olsen SI (2013) Comparison of algal biodiesel production pathways using life cycle assessment tool. In: Singh A, Pant D, Olsen S (eds) Life cycle assessment of renewable energy sources. Springer, pp 145–168
Brentner LB, Eckelman MJ, Zimmerman JB (2011) Combinatorial life cycle assessment to inform process design of industrial production of algal biodiesel. Environ Sci Technol 45:7060–7067. https://doi.org/10.1021/es2006995
Silva AG, Carter R, Merss FLM et al (2015) Life cycle assessment of biomass production in microalgae compact photobioreactors. GCB Bioenergy 7:184–194. https://doi.org/10.1111/gcbb.12120
Monari C, Righi S, Olsen SI (2016) Greenhouse gas emissions and energy balance of biodiesel production from microalgae cultivated in photobioreactors in Denmark: a life-cycle modeling. J Clean Prod 112:4084–4092
Pérez-López P, de Vree JH, Feijoo G et al (2017) Comparative life cycle assessment of real pilot reactors for microalgae cultivation in different seasons. Appl Energy 205:1151–1164. https://doi.org/10.1016/j.apenergy.2017.08.102
Mata TM, Cameira M, Marques F et al (2018) Carbon footprint of microalgae production in photobioreactor. Energy Procedia 153:432–437. https://doi.org/10.1016/j.egypro.2018.10.039
Maiolo S, Parisi G, Biondi N et al (2020) Fishmeal partial substitution within aquafeed formulations: life cycle assessment of four alternative protein sources. Int J Life Cycle Assess 25:1455–1471. https://doi.org/10.1007/s11367-020-01759-z
Onorato C, Rösch C (2020) Comparative life cycle assessment of astaxanthin production with Haematococcus pluvialis in different photobioreactor technologies. Algal Res 50:Article no: 102005. https://doi.org/10.1016/j.algal.2020.102005
Schade S, Meier T (2020) Distinct microalgae species for food—part 1: a methodological (top-down) approach for the life cycle assessment of microalgae cultivation in tubular photobioreactors. J Appl Phycol. https://doi.org/10.1007/s10811-020-02177-2
Dufour J, Moreno J, Rodríguez R (2011) Life cycle assessment of biodiesel production from microalgae oil: effect of algae species and cultivation system. In: Towards life cycle sustainability management
Klaus T, Vollmer C, Werner K, et al (2010) Energy Target 2050: 100% renewable electricity supply. Dessau-Roßlau
Carus M, Dammer L (2018) The “Circular Bioeconomy”-concepts, opportunities and limitations. Hürth
Mayers JJ, Ekman A, Albers E, Flynn KJ (2017) Nutrients from anaerobic digestion e ffl uents for cultivation of the microalga Nannochloropsis sp. — impact on growth, biochemical composition and the potential for cost and environmental impact savings. Algal Res 26:275–286. https://doi.org/10.1016/j.algal.2017.08.007
Yuan J, Kendall A, Zhang Y (2015) Mass balance and life cycle assessment of biodiesel from microalgae incorporated with nutrient recycling options and technology uncertainties. GCB Bioenergy 7:1245–1259. https://doi.org/10.1111/gcbb.12229
Frank ED, Han J, Palou-Rivera I et al (2011) Life-cycle analysis of algal lipid fuels with the GREET model. Argonne
Lardon L, Hélias A, Sialve B et al (2009) Life-cycle assessment of biodiesel production from microalgae. Environ Sci Technol 43:6475–6481. https://doi.org/10.1021/es900705j
Stephenson AL, Kazamia E, Dennis JS et al (2010) Life-cycle assessment of potential algal biodiesel production in the united kingdom: a comparison of raceways and air-lift tubular bioreactors. Energy Fuels 24:4062–4077. https://doi.org/10.1021/ef1003123
Campbell PK, Beer T, Batten D (2008) Greenhouse gas sequestration by algae – energy and greenhouse gas life cycle studies. CSIRO Aust 102:50–56. https://doi.org/10.1016/j.biortech.2010.06.048
Clarens AF, Resurreccion EP, White MA, Colosi LM (2010) Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ Sci Technol 44:1813–1819. https://doi.org/10.1021/es902838n
Rösch C, Skarka J, Wegerer N (2012) Materials flow modeling of nutrient recycling in biodiesel production from microalgae. Bioresour Technol 107:191–199. https://doi.org/10.1016/j.biortech.2011.12.016
Tredici MR, Rodolfi L, Biondi N et al (2016) Techno-economic analysis of microalgal biomass production in a 1-ha Green Wall Panel (GWP®) plant. Algal Res 19:253–263. https://doi.org/10.1016/j.algal.2016.09.005
Athena Sustainable Materials Institute (2020) LCA, LCI, LCIA, LCC: what’s the difference? In: Athena Sustain Mater Inst Resour. http://www.athenasmi.org/resources/about-lca/whats-the-difference/. Accessed 11 Mar 2020
Khoo HH, Sharratt PN, Das P et al (2011) Life cycle energy and CO2 analysis of microalgae-to-biodiesel: Preliminary results and comparisons. Bioresour Technol 102:5800–5807. https://doi.org/10.1016/j.biortech.2011.02.055
ecoinvent Center (2017) Activity overview for ecoinvent 3.4, excel data sheet. In: ecoinvent.org. http://www.ecoinvent.org/support/documents-and-files/documents-and-files.html. Accessed 10 Oct 2019
European Commission, Joint Research Center, Institute for Environment and Sustainability (2012) Characterisation factors of the ILCD recommended life cycle impact assessment methods, 1st edn. Publications Office of the European Union, Luxembourg
Collet P, Hélias A, Lardon L et al (2015) Recommendations for life cycle assessment of algal fuels. Appl Energy 154:1089–1102. https://doi.org/10.1016/j.apenergy.2015.03.056
IPCC Core Writing Team (2007) Climate Change 2007 Synthesis Report. Geneva
Spadaro JV, Rabl A (2004) Pathway analysis for population-total health impacts of toxic metal emissions. Risk Anal 24:1121–1141. https://doi.org/10.1111/j.0272-4332.2004.00514.x
Greco SL, Wilson AM, Spengler JD, Levy JI (2007) Spatial patterns of mobile source particulate matter emissions-to-exposure relationships across the United States. Atmos Environ 41:1011–1025
van Oers L, de Koning A, Guinee JB, Huppes G (2002) Abiotic resource depletion in LCA: improving characterization factors for abiotic resource depletion as recommended in the new Dutch LCA handbook. Road and Hydraulic Engineering Institute, Ministry of Transport and Water, Directorate-General for Public Works and Water Management of the Netherlands, Amsterdam
Guinee JB, Gorree M, Heijungs R et al (2002) Handbook on life cycle assessment: operational guide to the ISO standards. Dordrecht: Kluwer Academic Publishers
Struijs J, Beusen A, van Jaarsveld H, Huijbregts M (2009) Struijs J, Beusen A, van Jaarsveld H, Huijbregts M (2009) Aquatic eutrophication. In: ReCiPe 2008: A life cycle impact assessment method which comprises harmonised category indicators at the midpoint and the endpoint level, 1st edn. Ministerie van Volkshuisvesting, Ruimtelijke Ordening en Milieubeheer, Den Haag
Mila i Canals L, Romanya J, Cowell SJ (2007) Method for assessing impacts on life support functions (LSF) related to the use of ‘fertile land’ in Life Cycle Assessment (LCA). J Clean Prod 15:1426–1440
Frischknecht R, Steiner R, Jungbluth N (2009) The ecological scarcity method – eco-factors 2006. A method for impact assessment in LCA. Federal Office for the Environment (FOEN), Bern
Pal D, Khozin-Goldberg I, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90:1429–1441. https://doi.org/10.1007/s00253-011-3170-1
Doan YTT, Obbard JP (2015) Two-stage cultivation of a Nannochloropsis mutant for biodiesel feedstock. J Appl Phycol 27:2203–2208. https://doi.org/10.1007/s10811-014-0490-4
Jia J, Han D, Gerken HG et al (2015) Molecular mechanisms for photosynthetic carbon partitioning into storage neutral lipids in Nannochloropsis oceanica under nitrogen-depletion conditions. Algal Res 7:66–77. https://doi.org/10.1016/j.algal.2014.11.005
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186. https://doi.org/10.1016/j.plipres.2006.01.001
Su C, Chien L, Gomes J (2011) Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. 903–908. https://doi.org/10.1007/s10811-010-9609-4
Pal D, Khozin-Goldberg I, Didi-Cohen S et al (2013) Growth, lipid production and metabolic adjustments in the euryhaline eustigmatophyte Nannochloropsis oceanica CCALA 804 in response to osmotic downshift. Appl Microbiol Biotechnol 97:8291–8306. https://doi.org/10.1007/s00253-013-5092-6
Tredici MR, Bassi N, Prussi M et al (2015) Energy balance of algal biomass production in a 1-ha “Green Wall Panel” plant: how to produce algal biomass in a closed reactor achieving a high Net Energy Ratio. Appl Energy 154:1103–1111. https://doi.org/10.1016/j.apenergy.2015.01.086
President of the Italian Republic (2006) Legislative Decree No. 152 approving the Code on the Environment, pp 1–207
Baroni É, Cao B, Webley PA, et al (2020) Nitrogen availability and the nature of extracellular organic matter of microalgae. Ind Eng Chem Res. https://doi.org/10.1021/acs.iecr.9b05426
Dubber D, Gray NF (2010) Replacement of chemical oxygen demand (COD) with total organic carbon (TOC) for monitoring wastewater treatment performance to minimize disposal of toxic analytical waste. J Environ Sci Health A Toxic/Hazardous Subst Environ Eng. https://doi.org/10.1080/10934529.2010.506116
Brusca S, Famoso F, Lanzafame R et al (2017) A site selection model to identify optimal locations for microalgae biofuel production facilities in sicily (Italy). Int J Appl Eng Res 12:16058–16067
Passell H, Dhaliwal H, Reno M et al (2013) Algae biodiesel life cycle assessment using current commercial data. J Environ Manage 129:103–111. https://doi.org/10.1016/j.jenvman.2013.06.055
Taelman SE, Sfez S (2015) Environmental life cycle assessment (LCA) of algae production in North West Europe (NWE). Public Output Rep En Algae Proj Swansea 35
Tredici MR (2019) Personal Communication 03.12.2019. 1–2
Olmstead ILD, Kentish SE, Scales PJ, Martin GJO (2013) Low solvent, low temperature method for extracting biodiesel lipids from concentrated microalgal biomass. Bioresour Technol 148:615–619
Yap BHJ, Dumsday GJ, Scales PJ, Martin GJO (2015) Energy evaluation of algal cell disruption by high pressure homogenisation. Bioresour Technol 184:280–285. https://doi.org/10.1016/j.biortech.2014.11.049
GEA Mechanical Equipment Italia S.p.A. (2008) GEA Ariete Homogenizer 5400 Technical Datasheet. 2
Angles E, Jaouen P, Pruvost J, Marchal L (2017) Wet lipid extraction from microalga Nannochloropsis sp.: disruption, physiological effects and solvent screening. Algal Res 21:27–34
Jones S, Zhu Y, Anderson D et al (2014) Process design and economics for the conversion of algal biomass to hydrocarbons: whole algae hydrothermal liquefaction and upgrading
Patel B, Guo M, Chong C, et al (2016) Hydrothermal upgrading of algae paste: inorganics and recycling potential in the aqueous phase. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2016.06.041
Dutta S, Neto F, Coelho MC (2016) Microalgae biofuels: a comparative study on techno-economic analysis & life-cycle assessment. Algal Res 20:44–52. https://doi.org/10.1016/j.algal.2016.09.018
Zhang B, Wu J, Deng Z et al (2017) A comparison of energy consumption in hydrothermal liquefaction and pyrolysis of microalgae. Trends Renew Energy 3:76–85. https://doi.org/10.17737/tre.2017.3.1.0013
Wagner JL, Lee-Lane D, Monaghan M et al (2019) Recovery of excreted n-butanol from genetically engineered cyanobacteria cultures: process modelling to quantify energy and economic costs of different separation technologies. Algal Res 37:92–102. https://doi.org/10.1016/j.algal.2018.11.008
Boatman T (2019) HTL reactor design and process data: personal communication (14.12.2019). 8
European Commission (2019) New study investigates properties of bio kerosene fuel blends. https://ec.europa.eu/energy/en/news/new-study-investigates-properties-bio-kerosene-fuel-blends. Accessed 10 Oct 2019
Faeth JL, Valdez PJ, Savage PE (2013) Fast hydrothermal liquefaction of nannochloropsis sp. to produce biocrude. Energy Fuels 27:1391–1398. https://doi.org/10.1021/ef301925d
Ecoinvent (2014) Ecoinvent 3.4 dataset documentation: Market for electricity, medium voltage- NO
Bettini O, Sloop C (2015) Biofuels overview 2015. Rome
ecoinvent Center (2017) Ecoinvent 3.4 dataset documentation: soybean oil refinery operation- Rest of World (2000-2017)
Bagnoud-velásquez M, Schmid-staiger U, Peng G et al (2015) First developments towards closing the nutrient cycle in a biofuel production process. ALGAL 8:76–82. https://doi.org/10.1016/j.algal.2014.12.012
Valdez PJ, Nelson MC, Wang HY et al (2012) Hydrothermal liquefaction of Nannochloropsis sp.: Systematic study of process variables and analysis of the product fractions. Biomass Bioenerg 46:317–331. https://doi.org/10.1016/j.biombioe.2012.08.009
Biller P, Ross AB, Skill SC et al (2012) Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res 1:70–76. https://doi.org/10.1016/j.algal.2012.02.002
Tian C, Li B, Liu Z et al (2014) Hydrothermal liquefaction for algal biore fi nery : a critical review. Renew Sustain Energy Rev 38:933–950. https://doi.org/10.1016/j.rser.2014.07.030
Hu Y, Feng S, Yuan Z et al (2017) Investigation of aqueous phase recycling for improving bio-crude oil yield in hydrothermal liquefaction of algae. Bioresour Technol 239:151–159. https://doi.org/10.1016/j.biortech.2017.05.033
Guo Y, Yeh T, Song W et al (2015) A review of bio-oil production from hydrothermal liquefaction of algae. Renew Sustain Energy Rev 48:776–790. https://doi.org/10.1016/j.rser.2015.04.049
Chiaramonti D, Prussi M, Buffi M et al (2017) Review and experimental study on pyrolysis and hydrothermal liquefaction of microalgae for biofuel production. Appl Energy 185:963–972. https://doi.org/10.1016/j.apenergy.2015.12.001
Stiles WAV, Styles D, Chapman SP et al (2018) Using microalgae in the circular economy to valorise anaerobic digestate: challenges and opportunities. Bioresour Technol 267:732–742. https://doi.org/10.1016/j.biortech.2018.07.100
Bjornsson WJ, Nicol RW, Dickinson KE, McGinn PJ (2013) Anaerobic digestates are useful nutrient sources for microalgae cultivation: functional coupling of energy and biomass production. J Appl Phycol. https://doi.org/10.1007/s10811-012-9968-0
Fouilland E, Vasseur C, Leboulanger C, et al (2014) Coupling algal biomass production and anaerobic digestion: production assessment of some native temperate and tropical microalgae. Biomass Bioenerg. https://doi.org/10.1016/j.biombioe.2014.08.027
Zuliani L, Frison N, Jelic A, et al (2016) Microalgae cultivation on anaerobic digestate of municipalwastewater, sewage sludge and agro-waste. Int J Mol Sci. https://doi.org/10.3390/ijms17101692
Timonen K, Sinkko T, Luostarinen S et al (2019) LCA of anaerobic digestion: emission allocation for energy and digestate. J Clean Prod 235:1567–1579. https://doi.org/10.1016/j.jclepro.2019.06.085
Mendes RL, Fernandes HL, Coelho J et al (1995) Supercritical CO2 extraction of carotenoids and other lipids from Chlorella vulgaris. Food Chem 53:99–103. https://doi.org/10.1016/0308-8146(95)95794-7
Ecoinvent 3.3 (2016) Banana Production- Global (2009–2012). 2015–2016. https://doi.org/10.1002/14356007.a25
Taher H, Giwa A, Abusabiekeh H, Al-Zuhair S (2020) Biodiesel production from Nannochloropsis gaditana using supercritical CO2 for lipid extraction and immobilized lipase transesterification: economic and environmental impact assessments. Fuel Process Technol 198:106249. https://doi.org/10.1016/j.fuproc.2019.106249
Thornley P, Adams P (2018) Policy lessons: the role of policy regimes in maximising GHG savings in bioenergy systems. In: Greenhouse gas balances of bioenergy systems. Academic Press, pp 245–260
Farm-Energy (2019) Soybeans for biodiesel production – farm energy. https://farm-energy.extension.org/soybeans-for-biodiesel-production/#Production_and_Agronomic_Information. Accessed 27 Aug 2019
Gerbens-Leenes W, Hoekstra AY, Van Der Meer TH (2009) The water footprint of bioenergy. Proc Natl Acad Sci U S A 106:10219–10223. https://doi.org/10.1073/pnas.0812619106
Musa M (2020) Dewatering of microalgae from dilute suspensions for biofuels production: a single stage approach. Queensland University of Technology
Maji G, Choudhury S, Hamid S et al (2018) Microalgae harvesting via flocculation: impact of PH, algae species and biomass concentration. Methods Microbiol Mol Biol 1:1–5
Valente LMP, Custódio M, Batista S et al (2019) Defatted microalgae (Nannochloropsis sp.) from biorefinery as a potential feed protein source to replace fishmeal in European sea bass diets. Fish Physiol Biochem 45:1067–1081. https://doi.org/10.1007/s10695-019-00621-w
Chua ET, Schenk PM (2017) A biorefinery for Nannochloropsis: induction, harvesting, and extraction of EPA-rich oil and high-value protein. Bioresour Technol 244:1416–1424. https://doi.org/10.1016/j.biortech.2017.05.124
Lamminen M, Halmemies-Beauchet-Filleau A, Kokkonen T et al (2019) Different microalgae species as a substitutive protein feed for soya bean meal in grass silage based dairy cow diets. Anim Feed Sci Technol. https://doi.org/10.1016/j.anifeedsci.2018.11.005
Taelman SE, De Meester S, Van Dijk W et al (2015) Environmental sustainability analysis of a protein-rich livestock feed ingredient in the Netherlands: microalgae production versus soybean import. Resour Conserv Recycl 101:61–72. https://doi.org/10.1016/j.resconrec.2015.05.013
Acknowledgements
The authors would like to acknowledge with appreciation the PHOTOFUEL project partners for providing data on the Photofuel process chain.
Funding
Open Access funding enabled and organized by Projekt DEAL. The present work was supported by the European Union’s Horizon 2020 research and innovation program under grant agreement 640720.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design.
Kirsten Gaber: conceptualization; formal analysis; investigation; resources; software; validation; visualization; writing–original draft; writing–review and editing.
Christine Rösch: methodology; supervision; funding acquisition; project administration; resources; writing–review and editing.
Natascia Biondi: data curation; supervision; resources; writing–review and editing.
Corresponding author
Ethics declarations
Ethics approval and Consent to participate
This work does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All authors whose names appear on this work have approved this version of the work, contributed substantially to the work, taken part in the drafting and revising of the work, and have consented to the publication of this work.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12155_2021_10279_MOESM1_ESM.png
Online Resource 1 Scheme of the 1 ha GWP®-II plant and process flow sheet from Tredici et al. [51] (PNG 99 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gaber, K., Rösch, C. & Biondi, N. Life Cycle Assessment of Total Fatty Acid (TFA) Production from Microalgae Nannochloropsis oceanica at Different Sites and Under Different Sustainability Scenarios. Bioenerg. Res. 15, 1595–1615 (2022). https://doi.org/10.1007/s12155-021-10279-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10279-z