Abstract
Objective
To create the 3D convolutional neural network (CNN)-based system that can use whole-body [18F]FDG PET for recurrence/post-therapy surveillance in ovarian cancer (OC).
Methods
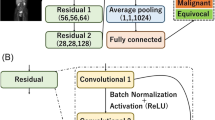
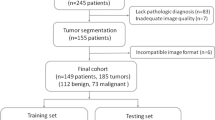
In this study, 1224 image sets from OC patients who underwent whole-body [18F]FDG PET/CT at Kowsar Hospital between April 2019 and May 2022 were investigated. For recurrence/post-therapy surveillance, diagnostic classification as cancerous, and non-cancerous and staging as stage III, and stage IV were determined by pathological diagnosis and specialists’ interpretation. New deep neural network algorithms, the OCDAc-Net, and the OCDAs-Net were developed for diagnostic classification and staging of OC patients using [18F]FDG PET/CT images. Examinations were divided into independent training (75%), validation (10%), and testing (15%) subsets.
Results
This study included 37 women (mean age 56.3 years; age range 36–83 years). Data augmentation techniques were applied to the images in two phases. There were 1224 image sets for diagnostic classification and staging. For the test set, 170 image sets were considered for diagnostic classification and staging. The OCDAc-Net areas under the receiver operating characteristic curve (AUCs) and overall accuracy for diagnostic classification were 0.990 and 0.92, respectively. The OCDAs-Net achieved areas under the receiver operating characteristic curve (AUCs) of 0.995 and overall accuracy of 0.94 for staging.
Conclusions
The proposed 3D CNN-based models provide potential tools for recurrence/post-therapy surveillance in OC. The OCDAc-Net and the OCDAs-Net model provide a new prognostic analysis method that can utilize PET images without pathological findings for diagnostic classification and staging.





Similar content being viewed by others
References
Dondi F, Albano D, Bertagna F, Giubbini R. [18F] FDG PET/CT and CA-125 in the evaluation of ovarian cancer relapse or persistence: is there any correlation? Nucl Med Rev. 2022;25(2):78–84.
Ovarian Cancer—Cancer Stat Facts [Internet]. [cited 2023 Jan 2]. https://seer.cancer.gov/statfacts/html/ovary.html.
Perrone AM, Dondi G, Lima GM, Castellucci P, Tesei M, Coluccelli S, et al. Potential prognostic role of 18F-FDG PET/CT in invasive epithelial ovarian cancer relapse. A preliminary study. Cancers (Basel). 2019;11(5):713.
Wang J, Liu L, Pang H, Liu L, Jing X, Li Y. Preoperative PET/CT score can predict incomplete resection after debulking surgery for advanced serous ovarian cancer better than CT score, MTV, tumor markers and hematological markers. Acta Obstet Gynecol Scand. 2022;101(11):1315–27.
Salvi A, Hardy LR, Heath KN, Watry S, Pergande MR, Cologna SM, et al. PAX8 modulates the tumor microenvironment of high grade serous ovarian cancer through changes in the secretome. Neoplasia. 2023;36:100866.
Rusu G, Achimaș-Cadariu P, Piciu A, Căinap SS, Căinap C, Piciu D. A comparative study between 18F-FDG PET/CT and conventional imaging in the evaluation of progressive disease and recurrence in ovarian carcinoma. In: Healthcare. Multidisciplinary Digital Publishing Institute; 2021. p. 666.
Virarkar M, Ganeshan D, Gulati AT, Palmquist S, Iyer R, Bhosale P. Diagnostic performance of PET/CT and PET/MR in the management of ovarian carcinoma—a literature review. Abdom Radiol. 2021;46(6):2323–49.
Mallum A, Mkhize T, Akudugu JM, Ngwa W, Vorster M. The role of positron emission tomography and computed tomographic (PET/CT) imaging for radiation therapy planning: a literature review. Diagnostics. 2022;13(1):53.
Pillay V, Hadebe B, Vorster M. Molecular imaging and theranostics in ovarian cancer: the role of nuclear medicine. Brisbane: Exon Publications; 2022. p. 69–85.
Tsuyoshi H, Yoshida Y. Diagnostic imaging using positron emission tomography for gynecological malignancy. J Obstet Gynaecol Res. 2017;43(11):1687–99.
Kim SJ, Lee SW. Diagnostic accuracy of 18F-FDG PET/CT for detection of peritoneal carcinomatosis; a systematic review and meta-analysis. Br J Radiol. 2018;91(1081):20170519.
Vahidfar N, Farzanefar S, Ahmadzadehfar H, Molloy EN, Eppard E. A review of nuclear medicine approaches in the diagnosis and the treatment of gynecological malignancies. Cancers (Basel). 2022;14(7):1779.
Hirata K, Sugimori H, Fujima N, Toyonaga T, Kudo K. Artificial intelligence for nuclear medicine in oncology. Ann Nucl Med. 2022;36(2):123–32. https://doi.org/10.1007/s12149-021-01693-6.
Saba L, Biswas M, Kuppili V, Godia EC, Suri HS, Edla DR, et al. The present and future of deep learning in radiology. Eur J Radiol. 2019;114:14–24.
Satoh Y, Imokawa T, Fujioka T, Mori M, Yamaga E, Takahashi K, et al. Deep learning for image classification in dedicated breast positron emission tomography (dbPET). Ann Nucl Med. 2022;36(4):401–10. https://doi.org/10.1007/s12149-022-01719-7.
Sharma G, Prabha C. A systematic review for detecting cancer using machine learning techniques. In: AIP conference proceedings. aIP Publishing LLC; 2022. p. 40007.
Akazawa M, Hashimoto K. Artificial intelligence in gynecologic cancers: current status and future challenges—a systematic review. Artif Intell Med. 2021;120: 102164.
Saba T. Recent advancement in cancer detection using machine learning: systematic survey of decades, comparisons and challenges. J Infect Public Health. 2020;13(9):1274–89.
Wang S, Liu Z, Rong Y, Zhou B, Bai Y, Wei W, et al. Deep learning provides a new computed tomography-based prognostic biomarker for recurrence prediction in high-grade serous ovarian cancer. Radiother Oncol. 2019;132:171–7.
Bogani G, Rossetti D, Ditto A, Martinelli F, Chiappa V, Mosca L, et al. Artificial intelligence weights the importance of factors predicting complete cytoreduction at secondary cytoreductive surgery for recurrent ovarian cancer. J Gynecol Oncol. 2018. https://doi.org/10.3802/jgo.2018.29.e66.
Avanzo M, Porzio M, Lorenzon L, Milan L, Sghedoni R, Russo G, et al. Artificial intelligence applications in medical imaging: a review of the medical physics research in Italy. Phys Medica. 2021;83:221–41.
Trivizakis E, Manikis GC, Nikiforaki K, Drevelegas K, Constantinides M, Drevelegas A, et al. Extending 2-D convolutional neural networks to 3-D for advancing deep learning cancer classification with application to MRI liver tumor differentiation. IEEE J Biomed Health inform. 2018;23(3):923–30.
Li X, Gao H, Zhu J, Huang Y, Zhu Y, Huang W, et al. 3D deep learning model for the pretreatment evaluation of treatment response in esophageal carcinoma: a prospective study (ChiCTR2000039279). Int J Radiat Oncol Biol Phys. 2021;111(4):926–35.
Schwartz D, Sawyer TW, Thurston N, Barton J, Ditzler G. Ovarian cancer detection using optical coherence tomography and convolutional neural networks. Neural Comput Appl. 2022;34(11):8977–87.
Potočnik B, Šavc M. Deeply-supervised 3D convolutional neural networks for automated ovary and follicle detection from ultrasound volumes. Appl Sci. 2022;12(3):1246.
Berek JS, Kehoe ST, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynecol Obstet. 2018;143:59–78.
Schaefferkoetter J, Yan J, Ortega C, Sertic A, Lechtman E, Eshet Y, et al. Convolutional neural networks for improving image quality with noisy PET data. EJNMMI Res. 2020;10(1):1–11.
Khan AI, Shah JL, Bhat MM. Coronet: a deep neural network for detection and diagnosis of COVID-19 from chest X-ray images. Comput Methods Programs Biomed. 2020;196:105581.
Giannini A, Di Dio C, Di Donato V, D’oria O, Salerno MG, Capalbo G, et al. PARP inhibitors in newly diagnosed and recurrent ovarian cancer. Am J Clin Oncol. 2023;46:414–9.
Matulonis UA, Sood AK, Fallowfield L, Howitt BE, Sehouli J, Karlan BY. Ovarian cancer. Nat Rev Dis Prim. 2016;2(1):1–22.
Shen WC, Chen SW, Wu KC, Hsieh TC, Liang JA, Hung YC, et al. Prediction of local relapse and distant metastasis in patients with definitive chemoradiotherapy-treated cervical cancer by deep learning from [18 F]-fluorodeoxyglucose positron emission tomography/computed tomography. Eur Radiol. 2019;29(12):6741–9.
Kawauchi K, Furuya S, Hirata K, Katoh C, Manabe O, Kobayashi K, et al. A convolutional neural network-based system to classify patients using FDG PET/CT examinations. BMC Cancer. 2020;20(1):1–10.
He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. In: Proceedings of the IEEE conference on computer vision and pattern recognition. 2016. p. 770–778.
Iandola FN, Han S, Moskewicz MW, Ashraf K, Dally WJ, Keutzer K. SqueezeNet: AlexNet-level accuracy with 50x fewer parameters and < 0.5 MB model size. arXiv Prepr arXiv160207360. 2016.
Zagoruyko S, Komodakis N. Wide residual networks. arXiv Prepr arXiv160507146. 2016.
Matsubara K, Ibaraki M, Nemoto M, Watabe H, Kimura Y. A review on AI in PET imaging. Ann Nucl Med. 2022;36(2):133–43. https://doi.org/10.1007/s12149-021-01710-8.
Xu Y, Hosny A, Zeleznik R, Parmar C, Coroller T, Franco I, et al. Deep learning predicts lung cancer treatment response from serial medical imaging longitudinal deep learning to track treatment response. Clin Cancer Res. 2019;25(11):3266–75.
Acknowledgements
This work was supported by the International Atomic Energy Agency (IAEA) under the coordinated research project (CRP) E13050—the International Multicenter Trial on FDG PET/CT in Ovarian Cancer (POCA). The authors would like to acknowledge the instrumental and technical support of the Nuclear Medicine Department, Kowsar Hospital.
Funding
This work was supported by Shiraz University (Grant numbers 1GBC1M148325). In addition, the work was funded by the International Atomic Energy Agency (IAEA) under the coordinated research project (CRP) E13050—the International Multicenter Trial on FDG PET/CT in Ovarian Cancer (POCA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sadeghi, M.H., Sina, S., Alavi, M. et al. The OCDA-Net: a 3D convolutional neural network-based system for classification and staging of ovarian cancer patients using [18F]FDG PET/CT examinations. Ann Nucl Med 37, 645–654 (2023). https://doi.org/10.1007/s12149-023-01867-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-023-01867-4