Abstract
Objectives
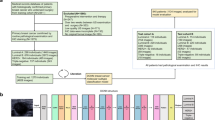
There currently lacks a noninvasive and accurate method to distinguish benign and malignant ovarian lesion prior to treatment. This study developed a deep learning algorithm that distinguishes benign from malignant ovarian lesion by applying a convolutional neural network on routine MR imaging.
Methods
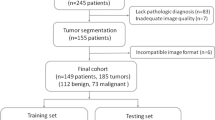
Five hundred forty-five lesions (379 benign and 166 malignant) from 451 patients from a single institution were divided into training, validation, and testing set in a 7:2:1 ratio. Model performance was compared with four junior and three senior radiologists on the test set.
Results
Compared with junior radiologists averaged, the final ensemble model combining MR imaging and clinical variables had a higher test accuracy (0.87 vs 0.64, p < 0.001) and specificity (0.92 vs 0.64, p < 0.001) with comparable sensitivity (0.75 vs 0.63, p = 0.407). Against the senior radiologists averaged, the final ensemble model also had a higher test accuracy (0.87 vs 0.74, p = 0.033) and specificity (0.92 vs 0.70, p < 0.001) with comparable sensitivity (0.75 vs 0.83, p = 0.557). Assisted by the model’s probabilities, the junior radiologists achieved a higher average test accuracy (0.77 vs 0.64, Δ = 0.13, p < 0.001) and specificity (0.81 vs 0.64, Δ = 0.17, p < 0.001) with unchanged sensitivity (0.69 vs 0.63, Δ = 0.06, p = 0.302). With the AI probabilities, the junior radiologists had higher specificity (0.81 vs 0.70, Δ = 0.11, p = 0.005) but similar accuracy (0.77 vs 0.74, Δ = 0.03, p = 0.409) and sensitivity (0.69 vs 0.83, Δ = -0.146, p = 0.097) when compared with the senior radiologists.
Conclusions
These results demonstrate that artificial intelligence based on deep learning can assist radiologists in assessing the nature of ovarian lesions and improve their performance.
Key Points
• Artificial Intelligence based on deep learning can assess the nature of ovarian lesions on routine MRI with higher accuracy and specificity than radiologists.
• Assisted by the deep learning model’s probabilities, junior radiologists achieved better performance that matched those of senior radiologists.




Similar content being viewed by others
Change history
26 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00330-021-07854-5
Abbreviations
- AI:
-
Artificial intelligence
- AUC:
-
Area under the curve
- MRI:
-
Magnetic resonance imaging
- PR:
-
Precision-recall curve
- ROC:
-
Receiver operating characteristic curve
- US:
-
Ultrasound
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30
Levine D, Brown DL, Andreotti RF et al (2010) Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of radiologists in ultrasound consensus conference statement. Ultrasound Q 26(3):121–131
Patel MD, Ascher SM, Paspulati RM et al (2013) Managing incidental findings on abdominal and pelvic CT and MRI, part 1: white paper of the ACR incidental findings committee II on adnexal findings. J Am Coll Radiol 10(9):675–681
Jacobs IJ, Parmar M, Skates SJ, Menon U (2016) Ovarian cancer screening: UKCTOCS trial – authors’ reply. Lancet 387(10038):2603–2604
van Nagell JR Jr., Hoff JT (2013) Transvaginal ultrasonography in ovarian cancer screening: current perspectives. Int J Womens Health 6:25–33
Sohaib SA, Reznek RH (2007) MR imaging in ovarian cancer. Cancer Imaging 7 Spec No A:S119–S129
Siegelman ES, Outwater EK (1999) Tissue characterization in the female pelvis by means of MR imaging. Radiology. 212(1):5–18
Pi S, Cao R, Qiang JW, Guo YH (2018) Utility of DWI with quantitative ADC values in ovarian tumors: a meta-analysis of diagnostic test performance. Acta Radiol 59(11):1386–1394
Kombächer P, Hamm B, Becker R, Hese S, Weitzel HK, Wolf KJ (1992) Tumors of the adnexa--a comparison of magnetic resonance tomography, endosonography and the histological findings. Rofo 156:303–308
Park SY, Oh YT, Jung DC (2016) Differentiation between borderline and benign ovarian tumors: combined analysis of MRI with tumor markers for large cystic masses (>/=5 cm). Acta Radiol 57(5):633–639
Moore BJ, Steiner CA, Davis PH, Stocks C, Barrett ML (2006) Trends in hysterectomies and oophorectomies in hospital inpatient and ambulatory settings, 2005–2013: Statistical Brief #214. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD)
Lass A (1999) The fertility potential of women with a single ovary. Hum Reprod Update 5(5):546–550
Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS (2005) Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol 106(2):219–226
Bi WL, Hosny A, Schabath MB et al (2019) Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin 69(2):127–157
Chang K, Beers AL, Bai HX et al (2019) Automatic assessment of glioma burden: a deep learning algorithm for fully automated volumetric and bidimensional measurement. Neuro Oncol 21(11):1412–1422
Papp L, Potsch N, Grahovac M et al (2018) Glioma survival prediction with combined analysis of in vivo (11)C-MET PET features, ex vivo features, and patient features by supervised machine learning. J Nucl Med 59(6):892–899
Zhang X, Yan LF, Hu YC et al (2017) Optimizing a machine learning based glioma grading system using multi-parametric MRI histogram and texture features. Oncotarget. 8(29):47816–47830
Chang K, Bai HX, Zhou H et al (2018) Residual convolutional neural network for the determination of IDH status in low- and high-grade gliomas from MR imaging. Clin Cancer Res 24(5):1073–1081
Meinhold-Heerlein I, Fotopoulou C, Harter P et al (2016) The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch Gynecol Obstet 293(4):695–700
Zafar HM, Chadalavada SC, Kahn CE Jr et al (2015) Code abdomen: an assessment coding scheme for abdominal imaging findings possibly representing cancer. J Am Coll Radiol 12(9):947–950
Zwanenburg A, Leger S, Vallières M, Löck S (2016) Image biomarker standardisation initiative. arXiv preprint arXiv:161207003
Agresti A, Coull BA (1998) Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 52(2):119–126
Efron B (1979) Bootstrap methods: another look at the jackknife. Ann Stat 7(1):1–26
Krippendorf K (1989) Content analysis, vol 1. Oxford University Press, New York
Vallieres M, Freeman CR, Skamene SR, El Naqa I (2015) A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol 60(14):5471–5496
Vallieres M, Kay-Rivest E, Perrin LJ et al (2017) Radiomics strategies for risk assessment of tumour failure in head-and-neck cancer. Sci Rep 7(1):10117
Olson RS, Urbanowicz RJ, Andrews PC, Lavender NA, Moore JH (2016) Automating biomedical data science through tree-based pipeline optimization. Paper presented at: European Conference on the Applications of Evolutionary Computation
Medeiros LR, Freitas LB, Rosa DD et al (2011) Accuracy of magnetic resonance imaging in ovarian tumor: a systematic quantitative review. Am J Obstet Gynecol 204(1):67 e61–67 e10
Medeiros LR, Rosa DD, da Rosa MI, Bozzetti MC (2009) Accuracy of ultrasonography with color Doppler in ovarian tumor: a systematic quantitative review. Int J Gynecol Cancer 19(7):1214–1220
Riccio TJ, Adams HG, Munzing DE, Mattrey RF (1990) Magnetic resonance imaging as an adjunct to sonography in the evaluation of the female pelvis. Magn Reson Imaging 8(6):699–704
Hricak H, Chen M, Coakley FV et al (2000) Complex adnexal masses: detection and characterization with MR imaging--multivariate analysis. Radiology. 214(1):39–46
Martinez-Mas J, Bueno-Crespo A, Khazendar S et al (2019) Evaluation of machine learning methods with Fourier transform features for classifying ovarian tumors based on ultrasound images. PLoS One 14(7):e0219388
Li Y, Jian J, Pickhardt PJ et al (2020) MRI-based machine learning for differentiating borderline from malignant epithelial ovarian tumors: a multicenter study. J Magn Reson Imaging 52:897–904. https://doi.org/10.1002/jmri.27084
Deng J, Dong W, Socher R et al (2009) ImageNet: A Large-Scale Hierarchical Image Database. Proceedings of IEEE Conference on Computer Vision and Pattern Recognition, Miami 2–9.
Xu Y, Hosny A, Zeleznik R et al (2019) Deep learning predicts lung cancer treatment response from serial medical imaging. Clin Cancer Res 25(11):3266–3275
Hamm CA, Wang CJ, Savic LJ et al (2019) Deep learning for liver tumor diagnosis part I: development of a convolutional neural network classifier for multi-phasic MRI. Eur Radiol 29(7):3338–3347
Zhang N, Yang G, Gao Z et al (2019) Deep learning for diagnosis of chronic myocardial infarction on nonenhanced cardiac cine MRI. Radiology. 291(3):606–617
Soni P, Vashisht S (2019) Image segmentation for detecting polycystic ovarian disease using deep neural networks. Int J Comput Civ Struct Eng 7(3)
Li W, Chu C, Cui Y, Zhang P, Zhu M (2012) Diffusion-weighted MRI: a useful technique to discriminate benign versus malignant ovarian surface epithelial tumors with solid and cystic components. Abdom Imaging 37(5):897–903
Zhao SH, Qiang JW, Zhang GF et al (2014) Diffusion-weighted MR imaging for differentiating borderline from malignant epithelial tumours of the ovary: pathological correlation. Eur Radiol 24(9):2292–2299
Abascal-Saiz A, Sotillo-Mallo L, de Santiago J, Zapardiel I (2014) Management of borderline ovarian tumours: a comprehensive review of the literature. Ecancermedicalscience. 8:403
Roth HR, Lu L, Seff A et al (2014) A new 2.5D representation for lymph node detection using random sets of deep convolutional neural network observations. In: Golland P, Hata N, Barillot C, Hornegger J, Howe R (eds) Medical Image Computing and Computer-Assisted Intervention – MICCAI 2014. MICCAI 2014. Lecture Notes in Computer Science, vol 8673
Tu X, Xie M, Gao J et al (2017) Automatic categorization and scoring of solid, part-solid and non-solid pulmonary nodules in CT images with convolutional neural network. Sci Rep 7(1):8533. https://doi.org/10.1038/s41598-017-08040-8
Acknowledgments
We would like to acknowledge and thank Huizhou Li (HZL) and Dan Li (DL) for performing the segmentation of MR images on the test set. We would like to thank Hui Liu (HL), Ting Huang (TH), Xin Su (XS), and Yijun Zhao (YJZ) for evaluating the test set as junior radiologists, and Dehong Peng (DHP), Shuanglin Zeng (SLZ), and Juan Chen (JC) for evaluating the test set as senior radiologists.
Funding
This work was supported by RSNA Research Scholar Grant to H. Bai, by a training grant from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health (5T32EB1680) to K. Chang and by the National Cancer Institute (NCI) of the National Institutes of Health (F30CA239407) to K. Chang. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. TL was partially supported by Institutional Development Award Number U54GM115677 from the National Institute of General Medical Sciences of the National Institutes of Health which funds Advance Clinical and Translational Research (Advance-CTR).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Harrison Bai.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• case-control study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Robin Wang is affiliated with two institutions.
Electronic supplementary material
ESM 1
(DOCX 97 kb)
Rights and permissions
About this article
Cite this article
Wang, R., Cai, Y., Lee, I.K. et al. Evaluation of a convolutional neural network for ovarian tumor differentiation based on magnetic resonance imaging. Eur Radiol 31, 4960–4971 (2021). https://doi.org/10.1007/s00330-020-07266-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07266-x