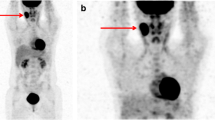
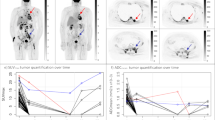
Abstract
Objective
Metabolic tumor burden (MTB) incorporates the advantages of the existing indices: the metabolic volume of the lesion calculated by size-dependent thresholding on positron emission tomography–computed tomography (PET–CT) along with its aggressiveness as determined by standardized uptake value (SUV). This study was conducted to investigate whether MTB can be used as an objective index for monitoring therapy response in pediatric lymphoma.
Methods
Forty-two pediatric patients (35 male and 7 female) with histologically proven lymphomas (26 Hodgkin’s and 16 non-Hodgkin’s) were evaluated. MTB was assessed in baseline, early interim (after 2 cycles) and post-therapy PET–CT studies using RT_Image software. Size-dependent thresholding based on a phantom study conducted at our institute was used for the calculation of metabolic tumor volume (MTV). MTB was given as the product of MTV and the SUVmean. Summation of MTB from all lesions gave the whole body MTB. Baseline, early interim and post-therapy SUVmax and whole body MTB of the partial and complete response group were compared.
Results
Of 42 patients, 37 had complete response and 5 had partial response at the end of therapy based on clinical, CECT and bone marrow biopsy findings. SUVmax showed an overall reduction of 87.4% while MTB showed a reduction of 96.4% between baseline and early interim PET–CT scan. Similarly, SUVmax showed an overall reduction of 95.2% while MTB showed a reduction of 99.6% between baseline and post-therapy scan. There was significant difference between MTB of partial response and complete response group at baseline and early interim PET–CT (p 0.031 and 0.012, respectively). No such significant difference was found for SUVmax.
Conclusion
Whole body MTB appears to be useful quantitative parameter for the assessment of treatment response using PET–CT in pediatric lymphoma patients.





Similar content being viewed by others
References
Sandland JT, Downing JR, Crist WM. Non Hodgkin’s lymphoma in childhood. N Eng J Med. 1996;334:1238–48.
Kumar R, Maillard I, Schuster SJ, Alavi A. The utility of FDG-PET imaging in management of patients with Hodgkin’s and Non Hodgkin’s lymphomas. Radiol Clin North Am. 2004;42:1083–100.
Murphy SB, Fairclough DL, Hutchinson RE, Berard CW. Non Hodgkin’s lymphomas of childhood: an analysis of histology, staging and response to treatment of 338 cases at a single institution. J Clin Oncol. 1989;7:186–93.
Mauch PM, Weinstein H, Botnick L, Belli J, Cassady JR. An evaluation of long term survival and treatment complications in children with Hodgkin’s disease. Cancer. 1988;51:925–32.
Schwartz CL. The management of Hodgkin’s disease in the young child. Curr Opin Pediatr. 2003;15:1–2.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Geus-Oei LF, van der Heijden HFM, Visser EP, Hermsen R, van Hoorn BA, Timmer-Bonte JN, et al. Chemotherapy response evaluation with 18F-FDG PET in patients with non-small cell lung cancer. J Nucl Med. 2007;48:1592–8.
de Wit M, Bumann D, Beyer W, Herbst K, Clausen M, Hossfeld DK. Whole body positron emission tomography for diagnosis of residual mass in patients with lymphoma. Ann Oncol. 1997;8:57–60.
Kumar R. Assessment of therapy response in malignant tumors with 18F-FLT. Eur J Nucl Med Mol Imaging. 2007;34:1334–8.
Ciernik IF, Dizendorf E, Baumert BG. Radiation treatment planning with an integrated positron emission and computer tomography (PET–CT): a feasibility study. Int J Radiat Oncol Biol Phys. 2003;57:853–63.
Amthauer H, Furth C, Denecke T, Hundsdoerfer P, Voelker T, Seeger K, et al. FDG-PET in 10 children with non-Hodgkin’s lymphoma: initial experience in staging and follow up. Klin Padiatr. 2005;217:327–33.
Riad R, Omar W, Kotb M, Hafez M, Sidhom I, Zamzam M, et al. Role of PET–CT in malignant pediatric lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:319–29.
Miller E, Metser U, Avrahami G, Dvir R, Valdman D, Sira LB, et al. Role of 18F-FDG PET–CT in staging and follow-up of lymphoma in pediatric and young adult patients. J Comput Assist Tomogr. 2006;30:689–94.
Benz MR, Evilevitch V, Allen-Auerbach MS, Eilber FC, Phelps ME, Czernin J, et al. Treatment monitoring by 18F-FDG PET–CT in patients with sarcomas: interobserver variability of quantitative parameters in treatment-induced changes in histopathologically responding and nonresponding tumors. J Nucl Med. 2008;49:1038–46.
Graves EE, Quon A, Loo BW. RT_Image: an open-source tool for investigating PET in radiation oncology. Technol cancer Res Treat. 2007;6:111–21.
Surbone A, Longo DL, DeVita VT Jr, Ihde DC, Duffey PL, Jaffe ES, et al. Residual abdominal masses in aggressive non-Hodgkin’s lymphoma after combination chemotherapy: significance and management. J Clin Oncol. 1988;6:1832–7.
Radford JA, Cowan RA, Flanagan M, Dunn G, Crowther D, Johnson RJ, et al. The significance of residual mediastinal abnormality on the chest radiograph following treatment for Hodgkin’s disease. J Clin Oncol. 1988;6:940–6.
Jochelson M, Mauch P, Balikian J, Rosenthal D, Canellos G, et al. The significance of the residual mediastinal mass in treated Hodgkin’s disease. J Clin Oncol. 1985;3:637–40.
Stewart FM, Williamson BR, Innes DJ, Hess CE. Residual tumor masses following treatment for advanced histiocytic lymphoma. Diagnostic and therapeutic implications. Cancer. 1985;55:620–3.
Hermann S, Wormann D, Pixberg M, Hunold A, Heindel W, Jürgens H, et al. Staging in childhood lymphoma: difference between FDG PET and CT. Nuklearmedizin. 2005;44:1–7.
Jerusalem G, Beguin Y, Fassotte MF, Najjar F, Paulus P, Rigo P, et al. Persistent tumor 18F-FDG uptake after a few cycles of polychemotherapy is predictive of treatment failure in non-Hodgkin’s lymphoma. Haematologica. 2000;85:613–8.
Jerusalem G, Hustinx R, Beguin Y, Fillet G. Evaluation of therapy for lymphoma. Semin Nucl Med. 2005;35:186–96.
Kumar R, Maillard I, Schuster SJ, Alavi A. Utility of fluorodeoxyglucose-PET imaging in the management of patients with Hodgkin’s and non-Hodgkin’s lymphomas. Radiol Clin North Am. 2004;42:1083–100.
Berkowitz A, Basu S, Srinivas S, Sankaran S, Schuster S, Alavi A. Determination of whole-body metabolic burden as a quantitative measure of disease activity in lymphoma: a novel approach with fluorodeoxyglucose-PET. Nucl Med Commun. 2008;29:521–6.
Lin C, Itti E, Haioun C, Petegnief Y, Luciani A, Dupuis J, et al. Early 18-F-FDG PET for prediction of prognosis in patients with patients with diffuse large cell lymphoma: SUV based assessment versus visual analysis. J Nucl Med. 2007;48:1626–32.
Alavi A, Newberg AB, Souder E, Berlin JA. Quantitative analysis of PET and MRI data in normal aging and Alzheimer’s disease: atrophy weighted total brain metabolism and absolute whole brain metabolism as reliable discriminators. J Nucl Med. 1993;34:1681–7.
Bural GG, Torigian DA, Chamroonrat W, Alkhawaldeh K, Houseni M, El-Haddad G, et al. Quantitative assessment of the atherosclerotic burden of the aorta by combined FDG-PET and CT image analysis: a new concept. Nucl Med Biol. 2006;33:1037–43.
Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2:159–71.
Lee P, Weerasuriya DK, Lavori PW, Quon A, Hara W, Maxim PG, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiation Oncology Biol Phys. 2007;2:328–33.
La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, et al. Metabolic tumor volume predicts for recurrence and death in head and neck cancer. Int J Radiation Oncology Biol Phys. 2009;5:1335–41.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, P., Gupta, A., Patel, C. et al. Pediatric lymphoma: metabolic tumor burden as a quantitative index for treatment response evaluation. Ann Nucl Med 26, 58–66 (2012). https://doi.org/10.1007/s12149-011-0539-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-011-0539-2