Abstract
TOCA1 is a downstream effector protein of the small GTPase, Cdc42. It is a multi-domain protein that includes a membrane binding F-BAR domain, a homology region 1 (HR1) domain, which binds selectively to active Cdc42 and an SH3 domain. TOCA1 is involved in the regulation of actin dynamics in processes such as endocytosis, filopodia formation, neurite elongation, cell motility and invasion. Structural insight into the interaction between TOCA1 and Cdc42 will contribute to our understanding of the role of TOCA1 in actin dynamics. The 1H, 15N and 13C NMR backbone and sidechain resonance assignment of the HR1 domain (12 kDa) presented here provides the foundation for structural studies of the domain and its interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Biological context
Transducer of Cdc42-dependent Actin assembly protein 1 (TOCA1) is a downstream effector of the small GTPase, Cdc42 (Ho et al. 2004). Cdc42 is a member of the Rho family of small G proteins, which are in turn, members of the Ras superfamily. The Rho family are particularly associated with regulation of actin dynamics, and bind to a number of effector proteins to achieve their functions. TOCA1 binding to Cdc42 is involved in the activation of another Cdc42 effector, N-WASP (the ubiquitously expressed homologue of the Wiskott–Aldrich Syndrome Protein, WASP) in Xenopus tropicalis extracts. The importance of TOCA1 in actin dynamics has been confirmed in a range of systems (Bu et al. 2009, 2010; Lee et al. 2010; Gallop et al. 2013). Furthermore, TOCA1 has been implicated in membrane invagination and endocytosis (Tsujita et al. 2006; Fricke et al. 2009; Giuliani et al. 2009; Bu et al. 2010; Bai and Grant 2015), filopodia formation (Bu et al. 2009), neurite elongation (Kakimoto et al. 2006) and cell motility and invasion (Hu et al. 2011; Chander et al. 2012).
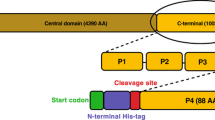
TOCA1 is a multidomain protein of the Pombe Cdc15 Homology (PCH) family. It has an N-terminal membrane binding domain (F-BAR), a central Cdc42-binding domain (HR1) and a C-terminal SH3 domain. The F-BAR domain binds to and deforms PI(4,5)P2-containing membranes (Itoh et al. 2005; Henne et al. 2007; Bu et al. 2009). The HR1 domain binds to Cdc42 (Ho et al. 2004), an interaction suggested to promote F-BAR-dependent membrane deformation (Bu et al. 2009). The SH3 domain has many known binding partners, including N-WASP (Ho et al. 2004) and the endocytosis protein, Dynamin (Itoh et al. 2005). It has been suggested that the TOCA1 HR1 domain may bind Cdc42 simultaneously with the N-WASP G protein-binding domain (GBD) (Ho et al. 2004; Bu et al. 2010). However, there is currently no direct evidence for such a complex. Structural insight into the TOCA1-Cdc42 interaction is of upmost importance in answering the question of whether these two effector proteins bind Cdc42 simultaneously.
In addition to its importance in understanding the pathway of actin dynamics, this resonance assignment will also be generally interesting with regards to G protein signalling. For example, HR1 domains show differential selectivity for specific G proteins. The HR1 domains from TOCA1 and from another TOCA family member, CIP4 bind to Cdc42 (Aspenström 1997; Ho et al. 2004) whereas the HR1 domains from the protein kinase C-related kinase (PRK) family show differential specificity for Rac1, RhoA, RhoB and RhoC (Modha et al. 2008; Hutchinson et al. 2013). Further structural analysis of G protein-HR1 domain interactions is therefore of significant interest.
The 1H, 15N and 13C NMR resonance assignment of the TOCA1 HR1 domain, presented here, will provide the foundation for structure determination and for studying its interaction with Cdc42. It will also be useful in determining whether TOCA1 and N-WASP bind Cdc42 simultaneously. The resonance assignment is, therefore, of importance for better understanding the pathways of Cdc42-dependent actin dynamics and in general for gaining understanding of G protein-effector interactions.
Materials and experiments
Protein cloning, expression, labelling and purification
The TOCA1 HR1 domain (residues 330-426 based on sequence alignments) was cloned from full-length Xenopus tropicalis TOCA1 cDNA, into the BamHI and EcoRI sites of pGEX-6P-1 (Addgene). The construct was expressed as a GST-fusion in E. coli BL21 cells (Invitrogen). Stationary cultures were diluted 1 in 10 and grown at 37 °C until an A600 ~ 0.8 was reached, then induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside for 20 h at 20 °C. To produce isotopically labelled protein for NMR studies, cells were grown in M9 media supplemented with 15NH4Cl and 13C-glucose (Sigma-Aldrich). The proteins were purified using glutathione-agarose beads (Sigma), and eluted from the beads by cleavage of the GST-tag by HRV 3C protease prior to gel filtration on a 16/60 S75 column (GE Healthcare).
NMR spectroscopy
All NMR experiments were run at 25 °C with 0.9 mM 13C/15N-labelled HR1 domain in 20 mM sodium phosphate pH 7.5, 150 mM NaCl, 5 mM MgCl2, 5 mM DTT, 10% D2O). 15N-HSQC, 15N-separated NOESY (100 ms mixing time), 15N-separated TOCSY (48 ms mixing time), HNCA (Nietlispach et al. 2002), HNCO, HN(CO)CA, HNCACB and HN(CO)CACB (reviewed in Ferentz and Wagner 2000) were recorded on a Bruker DRX500. A 13C-separated HCCH-TOCSY (FLOPSY-16 mixing, 18.6 ms mixing time) and 13C-separated NOESY (100 ms mixing time) were recorded on an Avance AV600. NMR data were processed using AZARA (W. Boucher).
Standard methodology (Cavanagh et al. 2006) was used to carry out the backbone assignment with reference to 2D 15N-HSQC and 13C-HSQC experiments using 15N-separated NOESY and TOCSY experiments, 3D HNCA, HNCO, HN(CO)CA, HNCACB and HN(CO)CACB in ANALYSIS (Vranken et al. 2005). The 3D HCCH-TOCSY and 13C-separated NOESY were used in addition to these experiments to assign the sidechain resonances.
Resonance assignment and data deposition
The TOCA1 HR1 domain construct gave well-dispersed spectra under the conditions described in "Materials and experiments" section (Fig. 1). The construct comprised residues 330-426 of TOCA1 with five additional residues at the N-terminus encoded by the expression vector (GPLGS). Assignment of 85 % of all 15N, 1H and 13C atoms in the construct was achieved. Excluding the five additional N-terminal residues, which are not of interest, 97.9 % of the backbone resonances have been assigned. This includes 100 % of the amide groups. The carbonyl carbons of Ser425, Glu426 and of the residues preceding the six Prolines, for which no HNCO transfer is possible, account for all of the unassigned backbone resonances. All of the observable sidechain resonances have been assigned, equating to 94.3 % of all sidechain NH and CH groups. The resonances of Lysine Nζ, Arginine Nη and Histidine Nδ and Nε were not observed for any of these residues. These resonances are rarely observable in NMR spectra. The NHε groups of Arginines 348, 358, 402, 404 and 424 were also not observed. These account for the remaining 5.7 % of unassigned resonances. The backbone 1H, 13C, and 15N chemical shifts were validated using TALOS-N (Shen and Bax 2013) before being deposited at the BioMagResBank (accession number 25945).
The difference in backbone chemical shifts (Cα, Cβ, and CαH) from the random coil positions were used to generate the Chemical Shift Index (CSI), which can be used to predict the secondary structure. The short-range NOEs and the CSI for each residue are shown in Fig. 2. The predicted secondary structure, predicted based on chemical shifts using TALOS-N (Shen and Bax 2013) is also shown. The data indicate that the TOCA1 HR1 domain comprises two α-helices separated by a region of no defined secondary structure. This is consistent with previously studied HR1 domains, which are known to adopt an anti-parallel coiled-coil fold (Maesaki et al. 1999; Modha et al. 2008; Kobashigawa et al. 2009). The completeness of this HR1 domain assignment will allow for structure determination using distance restraints derived from further NMR experiments as well as detailed investigation of its interaction with Cdc42. This information will be used to test the models of the role of the HR1 domain of TOCA1 in its regulation of actin dynamics.
Secondary structure summary of the TOCA1 HR1 domain. Black bars indicate NOEs for each of the atoms listed. The height of the bar represents the strength of the NOE. The atoms listed depict the following NOEs: dαN (dαδ) = CαH of residue i and the NH of residue i + 1, or CδH of i + 1 in the case of Prolines; dNN(dNδ,dδN) = NH of residue i and the NH of residue i + 1, or CδH in the case of Prolines; dβN (dβδ) = CβH of residue i and either the NH or CδH (Prolines) of residue i + 1. For the last four rows, dxy (i,i + z) is used, where x and y denote the atoms in residues i and i + z respectively. The Chemical Shift Index value (CSI) is calculated from all of the backbone atom chemical shifts. A value of +1 indicates a β-strand and a value of −1 indicates an α-helix. The secondary structure was predicted from the chemical shifts using TALOS-N (Shen and Bax 2013) and is depicted as a cartoon below the CSI. This figure was generated in CCPN ANALYSIS (Vranken et al. 2005)
References
Aspenström P (1997) A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr Biol 7:479–487
Bai Z, Grant BD (2015) A TOCA/CDC-42/PAR/WAVE functional module required for retrograde endocytic recycling. Proc Natl Acad Sci USA 112:E1443–E1452. doi:10.1073/pnas.1418651112
Bu W, Chou AM, Lim KB et al (2009) The Toca-1-N-WASP complex links filopodial formation to endocytosis. J Biol Chem 284:11622–11636. doi:10.1074/jbc.M805940200
Bu W, Lim KB, Yu YH et al (2010) Cdc42 interaction with N-WASP and Toca-1 regulates membrane tubulation, vesicle formation and vesicle motility: implications for endocytosis. PLoS ONE 5:e12153. doi:10.1371/journal.pone.0012153
Cavanagh J, Fairbrother W, Palmer A et al (2006) Protein NMR spectroscopy, 2nd edn. Academic Press, San Diego
Chander H, Truesdell P, Meens J, Craiga WB (2012) Transducer of Cdc42-dependent actin assembly promotes breast cancer invasion and metastasis. Oncogene. doi:10.1038/onc.2012.317
Ferentz AE, Wagner G (2000) NMR spectroscopy: a multifaceted approach to macromolecular structure. Quart Rev Biophys 33:29–65
Fricke R, Gohl C, Dharmalingam E et al (2009) Drosophila Cip4/Toca-1 integrates membrane trafficking and actin dynamics through WASP and SCAR/WAVE. Curr Biol CB 19:1429–1437. doi:10.1016/j.cub.2009.07.058
Gallop JL, Walrant A, Cantley LC, Kirschner MW (2013) Phosphoinositides and membrane curvature switch the mode of actin polymerization via selective recruitment of toca-1 and Snx9. Proc Natl Acad Sci USA 110:7193–7198. doi:10.1073/pnas.1305286110
Giuliani C, Troglio F, Bai Z et al (2009) Requirements for F-BAR proteins TOCA-1 and TOCA-2 in actin dynamics and membrane trafficking during Caenorhabditis elegans oocyte growth and embryonic epidermal morphogenesis. PLoS Genet 5:e1000675. doi:10.1371/journal.pgen.1000675
Henne WM, Kent HM, Ford MGJ et al (2007) Structure and analysis of FCHo2 F-BAR domain: a dimerizing and membrane recruitment module that effects membrane curvature. Structure 15:839–852. doi:10.1016/j.str.2007.05.002
Ho HH, Rohatgi R, Lebensohn AM et al (2004) Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell 118:203–216
Hu J, Mukhopadhyay A, Craig AWB (2011) Transducer of Cdc42-dependent actin assembly promotes epidermal growth factor-induced cell motility and invasiveness. J Biol Chem 286:2261–2272. doi:10.1074/jbc.M110.157974
Hutchinson CL, Lowe PN, Mclaughlin SH et al (2013) Differential binding of RhoA, RhoB, and RhoC to protein kinase C-related kinase (PRK) isoforms PRK1, PRK2, and PRK3: PRKs have the highest affinity for RhoB. Biochemistry 52:7999–8011
Itoh T, Erdmann KS, Roux A et al (2005) Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell 9:791–804. doi:10.1016/j.devcel.2005.11.005
Kakimoto T, Katoh H, Negishi M (2006) Regulation of neuronal morphology by Toca-1, an F-BAR/EFC protein that induces plasma membrane invagination. J Biol Chem 281:29042–29053. doi:10.1074/jbc.M604025200
Kobashigawa Y, Kumeta H, Kanoh D, Inagaki F (2009) The NMR structure of the TC10- and Cdc42-interacting domain of CIP4. J Biomol NMR 44:113–118. doi:10.1007/s10858-009-9317-z
Lee K, Gallop JL, Rambani K, Kirschner MW (2010) Self-assembly of filopodia-like structures on supported lipid bilayers. Science 329:1341–1345. doi:10.1126/science.1191710
Maesaki R, Ihara K, Shimizu T (1999) The structural basis of Rho effector recognition revealed by the crystal structure of human RhoA complexed with the effector domain of PKN/PRK1. Mol Cell 4:793–803
Modha R, Campbell LJ, Nietlispach D et al (2008) The Rac1 polybasic region is required for interaction with its effector PRK1. J Biol Chem 283:1492–1500. doi:10.1074/jbc.M706760200
Nietlispach D, Ito Y, Laue ED (2002) A novel approach for the sequential backbone assignment of larger proteins: selective intra-HNCA and DQ-HNCA. J Am Chem Soc 124:11199–11207
Shen Y, Bax A (2013) Protein backbone and sidechain torsion angles predicted from NMR chemical shifts using artificial neural networks. J Biomol NMR 56:227–241. doi:10.1007/s10858-013-9741-y
Tsujita K, Suetsugu S, Sasaki N et al (2006) Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol 172:269–279. doi:10.1083/jcb.200508091
Vranken WF, Boucher W, Stevens TJ et al (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59:687–696. doi:10.1002/prot.20449
Acknowledgments
JRW is supported by a Herchel Smith studentship. The TOCA1 cDNA was a kind gift from Dr. J. L. Gallop.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s12104-016-9679-6.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Watson, J.R., Nietlispach, D., Owen, D. et al. 1H, 13C and 15N resonance assignments of the Cdc42-binding domain of TOCA1. Biomol NMR Assign 10, 407–411 (2016). https://doi.org/10.1007/s12104-016-9677-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-016-9677-8