Abstract
Breast cancer (BC) is one of the most diagnosed cancers in women. Based on histological characteristics, they are classified as non-invasive, or in situ (tumors located within the milk ducts or milk lobules) and invasive. BC may develop from in situ carcinomas over time. Determining prognosis and predicting response to treatment are essential tools to manage this disease and reduce its incidence and mortality, as well as to promote personalized therapy for patients. However, over half of the cases are not associated with known risk factors. In addition, some patients develop resistance to treatment and relapse. Therefore, it is necessary to identify new biomarkers and treatment strategies that improve existing therapies. In this regard, the role of the microbiome is being researched as it could play a role in carcinogenesis and the efficacy of BC therapies. This review aims to describe specific microbiome patterns associated with BC. For this, a literature search was carried out in PubMed database using the MeSH terms “Breast Neoplasms” and “Gastrointestinal Microbiome”, including 29 publications. Most of the studies have focused on characterizing the gut or breast tissue microbiome of the patients. Likewise, studies in animal models and in vitro that investigated the impact of gut microbiota (GM) on BC treatments and the effects of the microbiome on tumor cells were included. Based on the results of the included articles, BC could be associated with an imbalance in the GM. This imbalance varied depending on molecular type, stage and grade of cancer, menopause, menarche, body mass index, and physical activity. However, a specific microbial profile could not be identified as a biomarker. On the other hand, some studies suggest that the GM may influence the efficacy of BC therapies. In addition, some microorganisms and bacterial metabolites could improve the effects of therapies or influence tumor development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Breast cancer (BC) is one of the most frequently diagnosed tumors in women worldwide. Its incidence has increased in recent decades. Although advances in diagnosis and treatment have improved survival rate, it remains one of the leading causes of cancer death in women [1].
BC is a multifactorial disease whose development involves genetic and environmental components that contribute to the complexity of its treatment and management. A wide variety of risk factors associated with BC have been identified: genetic, such as BRCA1/2 mutations, and environmental, such as alcohol intake, smoking, breast tissue density, sedentary lifestyle and obesity. However, the main risk factor is hormonal exposure throughout life, including physiological changes associated with puberty, menarche, pregnancy, menopause, hormonal contraceptives and hormone replacement therapy. In this context, the risk of BC is directly related to elevated levels of endogenous estrogens and differences in estrogen metabolism, especially in postmenopausal women [2, 3].
Several classifications have been developed to group BC. Based on histological characteristics, there are non-invasive (or in situ) and invasive BC. While non-invasive BC is referred to tumors contained in the milk ducts or lobules (such as ductal or lobular carcinoma in situ), invasive BC means that cancer has spread into the surrounding tissues or other body areas. In this case, BC can be ductal carcinoma no special type, or lobular carcinoma. Over time, in situ carcinomas may become invasive BC [4]. Invasive BC can be divided into different subtypes based on the expression of biomarkers, such as estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) and Ki67 antigen [2, 5, 6]. Perou and Sorlier [7] classify BC into four subtypes: luminal A and luminal B (expressing ER), HER2-enriched and basal-like. However, clinical practice uses a surrogate classification based on histological and molecular characteristics (Table 1). The progression of BC is divided into four stages based on the TNM system (classification of malignant tumors), which considers the size of the primary tumor (T), lymph node involvement (N) and metastasis (M). Similarly, tumor differentiation, percentage of tubular formation, nuclear pleomorphism and mitotic activity establish the grade of the disease. Staging system, grade assessment and analysis of biomarkers have prognostic and predictive value in BC [2, 5]. Table 1 summarizes BC classifications and their main characteristics [4, 9].
The diagnosis of BC is based on the triad of clinical assessment, imaging test and biopsy. The classic imaging test is mammography although it has low sensitivity (25–59%) in young women with dense breasts. In all cases, the diagnosis is confirmed by biopsy [4, 8].
Treatment of BC is based on four main strategies: surgery, radiotherapy, systemic therapy and immunotherapy. Radiotherapy can be used as adjuvant or palliative therapy. Systemic therapy is administered as adjuvant or neoadjuvant and includes chemotherapy, endocrine hormone therapy and biological or targeted therapy [2]. The choice of systemic therapy largely depends on the molecular subtype, stage and grade of BC (Table 1).
Early detection, determination of prognosis and prediction of response to treatment are essential tools for managing BC and reducing its incidence and mortality in the population, as well as promoting personalized therapy for patients [9]. Moreover, more than half of BC cases are not related to any known risk factor [10]. Thereby, research into new biomarkers for diagnostic, prognostic and predictive value has emerged in recent years. In addition, the severity of side effects caused by the existing treatments, and the development of resistance and relapse, highlights the need to develop new therapeutic agents or strategies that improve treatment efficacy.
Human microbiome
The human body harbors trillions of commensals, symbiotic and pathogenic microorganisms (including bacteria, archaea, viruses, fungi, and protozoa) that comprise the human microbiota. Although this term is commonly used as a synonym for microbiome, the latter encompasses the taxonomy and abundance of microorganisms present in a particular environment (microbiota), their genetic material and their metabolites [11]. The microbiome is a dynamic ecosystem that develops upon birth under the influence of maternal microbiota and environment and varies throughout life, both between and within individuals. The microbiota colonizes different habitats within the human body (gut, oral cavity, vagina, skin, etc.) and its structure widely differs depending on the niche it occupies [3]. Breast tissue hosts a community of bacteria that contributes to maintaining healthy breast tissue by stimulating resident immune cells. In the female mammary gland, milk has been shown to contain bacterial species, ostensibly reaching the ducts from the skin. The phylum with the highest abundance in breast tissue was Proteobacteria [12].
The perfect balance in this complex community is known as eubiosis. Alteration or imbalance in the composition of the microbiota (dysbiosis) can trigger harmful effects on human health, leading to a variety of pathological conditions, such as inflammatory bowel disease, diabetes, autoimmune diseases and even some types of cancer [13].
Gut microbiota (GM) is the most widely studied and best characterized in the human body. The main phyla comprising it are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria and Verrucomicrobia. Its distribution varies throughout the gastrointestinal tract depending on the environment [14]. GM composition is defined by various internal and external factors, such as age, race, diet, stress, maternal colonization, host genetics and exposure to antibiotics and xenobiotics [3].
In eubiosis, GM contributes to maintaining the body’s homeostasis and exerts a wide set of beneficial effects on human health. First, GM maintains the intestinal barrier function by strengthening the tight junctions between intestinal epithelial cells and stimulating mucus production. In addition, it stimulates the secretion of immunoglobulin A (sIgA) by the immune cells in the intestine. Second, it competes with pathogenic microorganisms for attachment to the intestinal mucosa (competitive exclusion) or directly prevents attachment of pathogens to the intestinal mucosa. Third, GM produces a wide variety of molecules with diverse biological activities, including short-chain fatty acids (SCFAs), such as acetate, propionate and butyrate, which act as an energy source for intestinal epithelial cells; vitamins, such as K, cobalamin, biotin and folic acid; hormones, such as catecholamines; and neurotransmitters, such as acetylcholine, serotonin, and dopamine. Additionally, some commensal microorganisms produce peptides with antimicrobial activity (bacteriocins such as lactin) and compounds with antifungal activity (such as benzoic acid). Finally, it modulates the immune system by interacting with antigen-presenting cells (such as dendritic cells) or by interacting with the toll-like receptor (TLR) signaling cascade, among other mechanisms. This pathway allows commensal microbiota to trigger a T cell-mediated immune response against cancer cells [15].
Microbiota and breast cancer
The role of microbiota in carcinogenesis
Only a percentage of women with genetic predisposition or exposure to known environmental risk factors develop BC, and more than half of cases are unrelated to known risk factors [10]. In this context, the growing evidence for the dual role of the human microbiome in human health and disease has prompted the investigation of the gut and breast microbiome in BC, hypothesizing that dysbiosis could be an additional risk factor.
Alterations in the structure of GM and the functions exerted by the microorganisms can affect the development and progression of BC through various mechanisms. Some bacteria are capable of directly inducing carcinogenesis. In this regard, Helicobacter pylori is considered the only direct carcinogenic bacterium in humans and is responsible for gastric adenocarcinomas. However, the potential of other bacteria (“oncomomicrobes”) to induce cancer via genotoxic-mediated mutagenesis through toxins and virulence factors has also been unraveled [14]. Regarding BC, Parida et al. [16] reported that intestinal colonization by enterotoxigenic Bacteroides fragilis, which secretes the B. fragilis toxin, affects epithelial hyperplasia in the mammary gland. Furthermore, in vitro treatment of MCF-7 cells with this toxin before cell injection into mice significantly increases the rate of tumor growth and metastasis.
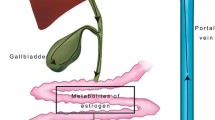
As mentioned above, hormone exposure is one of the major factors associated with BC development, especially in postmenopausal women. In this context, the estrobolome is particularly important, which refers to the set of microbial genes whose products are involved in estrogen metabolism, such as the enzymes glucuronidases, glucosidases and dehydrogenases [16]. Therefore, alterations in the microbiota/estrobolome can lead to elevated levels of circulating estrogens and their metabolites, increasing the risk of BC. Estrogen metabolism occurs in the liver, where are conjugated and excreted into the gastrointestinal lumen through the bile. There, a fraction is deconjugated by the action of bacterial β-glucuronidase and finally reabsorbed as free estrogens through the enterohepatic circulation, being distributed to organs such as the breast (Fig. 1). Additionally, estrogen-like metabolites that could have carcinogenic potential can be produced in the intestine. Moreover, bacterial β-glucuronidases could be involved in the deconjugation of xenobiotics and/or xenoestrogens, leading to their reuptake through the enterohepatic pathway, thereby increasing their half-life and availability in the organism. Bacteria with β-glucuronidase enzymes are found in two dominant subgroups, the Clostridium leptum cluster and the C. coccoides cluster, which belong to phylum Firmicutes. Moreover, the Escherichia/Shigella bacterial group, member of the phylum Proteobacteria, also possesses β-glucuronidases [3, 10, 15].
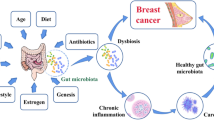
The immune system plays a key role preventing carcinogenesis and activation of the immune system has been observed upon treatment, especially in HER2+ and triple-negative BC [17, 18]. On the one hand, CD8+ T cells can directly kill cancer cells and their presence is associated with better prognosis. However, FOXP3+ CD4+ regulatory T cells mediate immunotolerance and correlate with poor prognosis. In BC, the proportion of regulatory T cells (Treg) increases with the disease stage and is associated with relapse and decreased survival. Due to the immunomodulatory capacity of the microbiota, changes in the abundance of specific bacteria could likely lead to increased production of Treg cells (Fig. 2). Furthermore, in animal models, some bacterial metabolites, such as butyrate and propionate, reduce inflammation via altering colonic regulatory T cells [10, 19]. Therefore, the microbiota and its metabolites can modulate the local immune microenvironment.
Microbiota can contribute to the development of BC by inducing a state of chronic inflammation. When the integrity of the intestinal barrier is lost, gut bacteria can upregulate TLRs and activate the nuclear factor-κB (NF-κB) pathway, which regulates inflammation and has been implicated in cancer. Pathogen-associated molecular patterns (PAMPs) are recognized by innate immune cells through pattern recognition receptors (PRRs), such as TLRs and NOD-like receptors (NLRs). These PAMPs are essential components of pathogens, such as lipopolysaccharide, flagellin, lipoteichoic acid and peptidoglycans. When TLRs recognize PAMPs, they activate the production of proinflammatory cytokines. Chronic TLR activation promotes tumor cell proliferation and enhances the mechanisms of invasion and metastasis through the regulation of cytokines, metalloproteinases and pro-inflammatory integrins [10, 20]. Consequently, chronic inflammation affects both the initiation and the progression of BC due to the constant presence of inflammatory cytokines and the recruitment of immune system cells, such as Tregs, which, as mentioned above, decrease the immune response promoting immune evasion by the tumor (Fig. 2).
Furthermore, changes in epigenetic marks and dietary patterns also affect microbiota composition and influence carcinogenesis. Modifications of epigenetic marks, such as those inducing the inactivation of tumor suppressor genes, are observed in patients with BC. In this line, the gut microbiome can contribute to this dysregulation through several mechanisms. Specific bacterial metabolites, such as SCFAs (butyrate and propionate), folates and biotins, can epigenetically modify gene expression. Similarly, microbiota synthesizes enzymes that induce epigenetic changes and contribute to the absorption of minerals that act as cofactors for these enzymes. In addition, the content and quality of the diet is particularly related to BC and is an important modulator of the diversity and structure of the microbiota [10]. Data from two large cohort studies showed that consuming more polyunsaturated and vegetable fats was linked to a decreased risk of hepatocarcinoma. This was related to the replacement of animal or dairy fats with vegetable fats or replacing saturated fats with monounsaturated or polyunsaturated fats [21].
The role of microbiota in breast cancer therapeutic approaches
Treatments against BC, such as radiotherapy, systemic therapy or immunotherapy, can alter the microbiota of patients, which, in turn, may affect the efficacy and side effects of treatments. On the other hand, GM can modulate cancer progression through the synthesis of antitumor compounds and the regulation of immune response and inflammatory pathways of the host [15]. Thus, the combination of these mechanisms may explain the impact of the microbiota on the efficacy of different cancer therapies.
Indeed, it is known that GM can participate in the metabolism of a wide range of drugs used in chemotherapy, thus modulating the response to treatment. For example, gemcitabine is a pyrimidine antagonist whose antitumor activity is based on its intracellular activation and subsequent degradation to an inactive compound by the enzyme cytidine deaminase. Studies in mice has shown that resistance to gemcitabine may be due to increased metabolic degradation of the drug due to the expression of an isoform of the bacterial enzyme cytidine deaminase, observed mainly in the Gammaproteobacteria class. Hence, the combination of gemcitabine with ciprofloxacin favors the antitumor activity of the drug; this synergism is caused by the bacterial inhibition exerted by the antibiotic. Furthermore, 5-fluorouracil is a thymidylate synthase inhibitor whose therapeutic use is limited due to the development of resistance and its gastrointestinal side effects. Preclinical trials in mice have shown that administration of 5-fluorouracil in combination with a cocktail of antibiotics decreases antitumor efficacy, while supplementation with probiotics significantly enhances anti-cancer effects [15].
Endocrine hormone therapy is used against hormone receptor-positive BC and it target the ER directly or the estrogen synthesis. The main types of endocrine therapy are selective ER modulators, selective modulators ER degraders, and aromatase inhibitors (AI) [22]. Nevertheless, there are few works addressing the role of GM in hormone therapies. In this regard, Lasagna et al. [23] carried out a monocenter observational case–control study in which they discovered that postmenopausal BC women who respond to AI had different fecal microbiota abundance than those resistant to AI therapy. In particular, Veillonella genus were enriched in the GM of patients resistant to AI. Although its implication in BC remains undetermined, the presence of Veillonella species in the GM of patients treated with CAR T cells have been associated with poor prognosis [24].
Immunotherapy is based on the use of immune checkpoint inhibitors (ICIs), molecules that block specific immune regulatory pathways to enhance the antitumor immune response. ICIs are monoclonal antibodies that target receptor molecules on the surface of T cells, such as cytotoxic T cell antigen 4 (CTLA-4) and the programmed cell death 1 (PD-1) receptor or PD-1 ligands (PD-L1 and PD-L2). By dysregulating the immune system, these molecules cause a wide range of side effects. Moreover, some patients do not benefit from treatment (primary resistance) or show no improvement in disease progression (secondary resistance). Furthermore, there is evidence that in some cases ICIs can favor tumor development. Consequently, studies have been carried out to identify predictive factors of the efficacy of these treatments, as well as strategies to overcome treatment resistance. Some of them have shown that the GM composition modulates the activity, efficacy and toxicity of ICIs. For example, anti-PD-L1 antibody shows more efficacy for the treatment of melanoma in mice when GM is enriched in Bifidobacterium species. Oral administration to patients of a bacterial cocktail of these species together with the anti-PD-L1 antibody specifically increases the T cell response and blocks the growth of melanoma, whereas if the treatment is combined with antibiotics, the survival rate is reduced [15].
Probiotics, prebiotics and breast cancer
Probiotics are defined as “strictly selected live strains of microorganisms that, when administered in adequate amounts, confer beneficial effects on the health of the host” [25]. Many studies have been carried out in mouse models to investigate the effects of probiotics on BC. Most studies are based on the administration of strains of the genus Lactobacillus, such as L. helveticus R389, L. acidophilus and L. reuteria, and their ability to prevent and control cancer progression is related to the modulation of the host immune system. However, clinical studies of probiotics in BC patients are very limited [10].
A prebiotic is defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [25]. They are mainly indigestible dietary fiber compounds that, when combined with harmful and carcinogenic substances in the intestine, promote their breakdown and the growth of probiotics, inhibiting the proliferation of pathogenic bacteria and the production of carcinogens. The effect of different prebiotics has been investigated in the context of BC, such as plant-based lignans, SCFAs or polyphenols. GM transforms plant-based lignans (present in soybeans, flax and sesame seeds, etc.) into phytoestrogens, estrogen-like compounds. Phytoestrogens act against BC at concentrations above 10 µM by inhibiting the synthesis and metabolism of estrogens and inducing antiangiogenic, antimetastatic and epigenetic effects. SCFAs produced by bacterial fermentation of dietary fiber also have anticancer effects, especially butyrate. Butyrate can reduce the viability of MCF-7 tumor cells. Finally, dietary polyphenols are bio transformed by GM into derivatives with increased bioavailability. In addition, dietary polyphenols can modulate the composition of the gut microbial community, inhibiting the proliferation of pathogenic bacteria and stimulating the activity and proliferation of beneficial bacterial species [10].
Rationale and hypotheses
There is an increasing number of studies characterizing the GM of BC patients and healthy women to describe specific microbial signatures of BC. Broadly, most studies define the microbiota based on three characteristics: (1) alpha (α) diversity, which refers to the diversity within a community of microorganisms and includes parameters, such as richness and uniformity; (2) beta (β) diversity, which refers to the differences between communities; and (3) taxonomic composition (measured in operational taxonomic units, OTUs), which reports on the abundance (absolute or relative) of specific members of the community [13]. Recently, recent studies have also analyzed breast microbiota composition.
Objective
Considering all of the above information, the general aim of this review is to describe specific microbiome patterns that could be associated with BC. The specific aims of the review are (1) to describe the differences between the GM of women with BC and healthy controls, (2) to define possible microbial profiles that could be used as non-invasive biomarkers of BC, (3) to provide insight into the influence of the GM profile in the treatment of BC and (4) to investigate the potential application of microorganisms as probiotics.
Materials and methods
Search strategy
A literature search has been carried out using the PubMed database. To use appropriate English vocabulary and terminology, the following MeSH terms from the MeSH medical database were used: “Breast Neoplasms” and “Gastrointestinal Microbiome”. To optimize and refine the search, the above terms were combined with the Boolean operator “AND” to return all results containing the specified terms.
Inclusion criteria
The following eligibility criteria were applied to the literature search: (1) articles published between 2016 and 2022 to accomplish an updated literature review, (2) documents with full text available, and (3) articles published in English.
Study selection and information collection
The literature search using the mentioned MeSH terms combined with the Boolean operator “AND” provided an initial result of 75 publications (Fig. 3). Application of the aforementioned inclusion criteria reduced the search to 71 articles. The selection of publications that met the inclusion criteria was carried out by two independent researchers by reviewing the title and abstract, to discard those articles that did not contain relevant information to achieve the proposed objectives. Thus, 15 articles were discarded. To ensure that only publications that provided information of interest were included, an exhaustive reading of full-text selected articles was carried out. This assessment allowed us to finally include 29 publications.
Results and discussion
A large proportion of the articles selected have in common the aim of characterizing the gut microbiome or breast tissue of BC patients. The selected studies analyze patients with different molecular types, stages, and grade of cancer, as well as specific groups of patients: premenopausal, postmenopausal, overweight, or obese, undergoing chemotherapy, with benign breast lesions, with non-malignant breast disease or with benign breast disease [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. We also include a group of studies in animal models [26, 36, 43,44,45,46,47,48,49,50,51,52] and cell cultures [28, 33, 40, 53, 54] that focused on the impact of the GM on various treatments against BC, as well as on determining the effect of the GM or its metabolites on tumor cells.
Nowadays, the two most extensively used metagenome sequencing strategies are shotgun and the 16S rRNA. Both are being used to catalog the human microbiome in health and disease and to study microbial communities of medical, pharmaceutical, or biotechnological relevance [55]. However, it is noteworthy that we have not found any works using full-length sequencing. This technique is performed to determine the complete sequence of the protein-coding as well as the non‐coding parts of the mRNA which allows us to reach species and regions to genus [56].
For instance, a comparative analysis by Durazzi et al. [57] showed that the use of 16S rRNA technique sequenced partly the GM community detected by shotgun and that genera detected by shotgun sequencing are biologically meaningful even when less abundant.
All the included studies, their main characteristics and findings are summarized in Tables 2, 3 and 4.
Human studies
In human studies (Table 2), BC patients and controls were in all cases matched or similar in terms of age, body mass index (BMI), age at menarche and cancer grade and stage. Characterization of the microbiota in human trials was performed mainly from stool samples [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] although in some cases breast tissue was analyzed [41, 42]. Microorganisms were identified by sequencing regions of the 16S rRNA (ribosomal ribonucleic acid) gene [26,27,28, 30, 31, 33,34,35,36,37,38,39,40] or by shotgun metagenomic sequencing [32, 36]. Specifically, the 16S rRNA gene regions sequenced were V3–V4 [26,27,28, 30, 33, 34, 38], V4–V6 [42] or V4 [31, 35, 37, 39].
Studies characterizing GM mainly found that the microbial composition of BC patients differs from that of healthy controls [27, 28, 31,32,33,34,35, 37,38,39]. Therefore, GM dysbiosis should be associated with BC (Table 2). However, GM is not the only one that seems to play a role in this disease. Lately, the importance of the breast microbiota in BC is being investigated and, analogously to GM, studies performed on breast tissue samples found differences in the microbial composition in tumor tissue compared to adjacent healthy tissue [42] (Table 2). These findings are consistent with other studies [58,59,60,61] and a recent meta-analysis [62]. Moreover, GM can also be related to other breast lesions and its composition may differ from BC (Table 2). In this regard, the GM of patients with benign breast lesions and non-malignant breast disease was different from controls [38, 39]. In addition, the composition also varied between patients with BC and patients with benign breast lesions [38] or benign breast disease [40]. However, no differences were observed between patients with BC and non-malignant breast disease [39].
Measurement of α- and β-diversity showed controversial results (Table 2). Several studies found lower α-diversity in the GM of BC patients compared to controls [27, 31, 34, 38, 39], while others found increased α-diversity [28, 32] or no significant differences [33,34,35, 37]. α-Diversity was also lower in tumor tissue compared to adjacent healthy tissue [42], in patients with benign breast lesions and non-malignant breast disease compared to controls [38, 39] and in BC patients compared to patients with benign breast disease [40]. A decrease in GM diversity has been associated with a variety of pathological conditions, such as obesity, inflammatory bowel disease and autism [63, 64], and it has also been found in several types of cancer, such as colorectal cancer [65, 66]. Regarding β-diversity, changes in GM β-diversity allowed discrimination between BC patients and controls [32, 34, 35, 38, 39], patients with benign breast lesions, non-malignant breast disease and controls [38, 39], and even between BC patients and patients with benign breast lesions [38]. Nevertheless, Bilenduke et al. [37] and Bobin-Dubigeon et al. [27] found no significant differences in the β-diversity of the microbiota of patients when compared to controls. In the same line, Byrd et al. [39] did not find significant differences between BC patients and those with non-malignant breast disease and Esposito et al. [42] were unable to discriminate between tumor and adjacent healthy tissue based on microbiota β-diversity.
Contradictory data increase when comparing the results obtained in the studies that analyze the GM of specific patients (Table 2). In this regard, it should be noted that menopausal status is an important factor to take into account in BC since the disease is more aggressive and has a worse prognosis in premenopausal patients [67] and the risk of BC is increased in postmenopausal women because of the accumulation of endogenous estrogens. When investigating the GM of premenopausal women with BC, J. Zhu et al. [32] found no significant differences between the microbial species of patients compared to controls. However, Hou et al. [34] and He et al. [33] demonstrated the existence of dysbiosis in premenopausal patients although the GM imbalance was different in their population samples. Hou et al. [34] described an enrichment of Anaerostipes and Bacteroides fragilis and a reduction of Bifidobacterium longum, B. bifidum and B. adolescentis in the GM of patients. He et al. [33] observed an increased Firmicutes/Bacteroidetes ratio, as well as higher abundance of Allisonella, Megasphaera, Pediococcus, Abiotrophia, Granulicatella, Clostridium_sensu_stricto, Serratia, Enhydrobacter, Fusobacterium and Slackia genus, and lower abundance of Clostridium_IV, Eubacterium, Terrisporobacter, Turicibacter, Intestinibacter, Butyricicoccus, Romboutsia, Providencia, Desulfovellario and Desulfovibrio. Dysbiosis clearly characterized BC during postmenopause [31, 32, 34]. In spite of this, patients’ GM composition was highly variable. For instance, J. Zhu et al. [32] found significant differences in the relative abundance of 45 species, including increases in Escherichia coli, Klebsiella sp_1_1_55, Prevotella amnii, Enterococcus gallinarum, Actinomyces sp. HPA0247, Shewanella putrefaciens, Erwinia amylovora and Acinetobacter radioresistens and decreases in Eubacterium eligens and Lactobacillus vaginalis. Notably, A. radioresistens and E. gallinarum were weakly positively correlated with C-reactive protein expression and S. putrefaciens and E. amylovora correlated with estradiol levels, whereas Actinomyces sp. HPA0247 was weakly negatively correlated with the number of T CD3+ and CD8+ cells. Hou et al. [34] observed an enrichment of Proteobacteria and Klebsiella pneumoniae and a decrease of Akkermansia muciniphila and Phascolarctobacterium. It is worth noting that Hou et al. [34] demonstrated the existence of differences in the GM of premenopausal and postmenopausal patients of BC. Based on this fact, they suggested that changes in the relative abundance of specific bacterial species could be used as potential universal BC biomarkers (high abundance of Sutterella and Haemophilus parainfluenzae and low abundance of Faecalibacterium prausnitzii, Ruminococcus gnavus and Rothia mucilaginosa), premenopause-specific BC biomarkers (increased abundance of Anaerostipes and B. fragilis and decreased abundance of B. longum, B. bifidum and B. adolescentis) or postmenopause-specific BC biomarkers (increased abundance of Proteobacteria and K. pneumoniae and decreased abundance of A. muciniphila and Phascolarctobacterium).
Among these potential universal BC biomarkers, Sutterella and H. parainfluenzae are pathogenic bacteria associated with autism, ulcerative colitis and oropharyngeal cancer [68,69,70]; F. prausnitzii is an important producer of SCFAs and its decrease serves as an indicator of dysbiosis [71], and decreased abundance of R. gnavus and R. mucilaginosa has also been found in other cancers [68, 72]. For premenopause-specific BC biomarkers, Anaerostipes has been linked to endometrial cancer, hepatocellular carcinoma and thyroid cancer [73,74,75]; B. fragilis contributes to the development of colorectal cancer [76]. Furthermore, B. fragilis is a resident bacterium in breast tissue and is able to colonize the gut to promote breast tumorigenesis and metastatic progression [16]. In addition, Bifidobacterium is a well-known probiotic. Finally, regarding postmenopause-specific BC biomarkers, Proteobacteria is a phylum enriched in pathogenic bacteria; K. pneumoniae is a pathogen that produces colibactin, a toxin that promotes the development of colorectal cancer [77]; the abundance of A. muciniphila is reduced in patients with metabolic diseases, such as obesity, diabetes and hypertension [78, 79] and Phascolarctobacterium, can produce SCFAs and is reduced in many types of cancer [73, 80].
The analysis of GM by He et al. [33] suggested that the enrichment in Desulfovibrio accompanied by a decrease in Pediococcus could have diagnostic value for premenopausal BC. Desulfovibrio genus has been associated with colon-related tumors [81] and it may contribute to inflammation and cardio-metabolic risk in BC [82], whereas Pediococcus exerts an anti-inflammatory role in the gut and has antiproliferative and anticancer activity on cervical and colon cancer cells [83, 84]. Moreover, Pediococcus is a genus that produces SCFAs. These metabolites seem to improve health status and their intestinal levels have also been reduced in premenopausal patients [33].
Interestingly, the GM of BC patients varied according to BC type, stage and grade, as well as based on other characteristics, such as BMI, percentage of total body fat, physical activity or age at menarche [29, 30, 39] (Table 2). For instance, Wu et al. [30] associated HER2-positive BC, overweight, obesity, increased total body fat, early menarche (less than or equal to 11 years) and a sedentary lifestyle with a decrease in α-diversity of GM and a different composition at phylum and genus level in BC patients. HER2-positive compared to HER2-negative patients were characterized by a decrease in Firmicutes (such as Clostridium, Blautia, Coprococcus and Ruminococcus) and an increase in Bacteroidetes. Early menarche compared to late menarche (greater than or equal to 12 years) was associated with a lower abundance of Firmicutes and a higher grade or stage of tumor correlated with enrichment of Clostridium and Veillonella and decreased abundance of Erysipelotrichaecea. Luu et al. [29] observed a similar pattern in overweight and obese patients. In this case, the GM of patients with high BMI was characterized by a decrease in Firmicutes, F. prausnitzii, Blautia sp. and Eggerthella lenta. Notably, F. prausnitzii exerts anti-inflammatory effects through the production of butyrate [85]. Thus, since both obesity and BC are associated with an inflammatory state, a significant decrease in this species could contribute to the development of the disease. The study also assessed the differences at the phylum, genus and species level depending on the stage and grade of BC. Patients in stage II/III had a higher total number of Bacteroidetes, Clostridium coccoides cluster, C. leptum cluster, F. prausnitzii and Blautia sp. compared to stage 0/I. Both Clostridium coccoides cluster and C. leptum cluster express β-glucuronidases which, as mentioned above, could contribute to the increase in systemic levels of free estrogens and favor the development of more severe clinical stages in patients with hormone-dependent BC. Finally, Luu et al. [29] found that an increased percentage of Blautia sp. correlated with a higher tumor grade, so this genus could be associated with poor prognosis. Based on these results, the GM profile could be used to define the molecular type, stage, and grade of BC. In addition, the relationship between this disease and GM dysbiosis suggests that GM alteration may be one of the mechanisms by which some risk factors contribute to the development of BC.
BC treatments, such as chemotherapy, affect the composition of the microbiome and, in turn, the microbiota may influence the effects of chemotherapy (Table 2). Terrisse et al. [36] described an association between GM β-diversity and tumor stage and grade, axillary node involvement and neurological side effects of chemotherapy. They also found different microbial composition and functional pathways depending on whether the patients had a favorable or unfavorable prognosis. Unfavorable prognosis was associated with Streptococcus, Lachnospiraceae (Blautia wexlerae), Veillonella (V. parvula), Bacteroides spp. (B. uniformis), E. ramosum, Enterobacteriaceae (Klebsiella spp.) and Clostridiaceae (C. spiroforme, C. asparagiforme, C. boltae); whereas a favorable prognosis was associated with Eubacteriaceae (E. rectale), A. muciniphila, Defulfovibrio piger, Coprococcus (C. comes, C. catus), Collinsella, B. vulgatus and Ruminococcaceae. The existence of a different microbial profile depending on the prognosis of the patients may indicate that the GM can be a predictive factor for the response to chemotherapy in BC patients. Furthermore, Terrisse et al. [36] demonstrated that the treatment was able to increase α-diversity and shift the microbial profile of patients with an unfavorable prognosis toward a microbial profile associated with a favorable prognosis. Thus, GM modulation could be used to promote an effective response to BC therapies. However, there is a need to determine which chemotherapeutic agent affect the microbiota and to validate its ability to inhibit unfavorable bacteria or enhance favorable commensals in future clinical trials. Similarly, Bilenduke et al. [37] found an association between cognitive impairment and depression symptoms with differences in the microbial composition of BC patients treated with chemotherapy. Specifically, they described a decrease in Odoribacter and an increase in Clostridium, Eggerthella and Erysipelotrichi. In this regard, certain drugs used in chemotherapy can contribute to a state of systemic inflammation, but these effects can also be attributed to various microorganisms. Therefore, taking into account that chemotherapy is capable of altering GM, it could potentiate the inflammatory effects of therapies too. For instance, Odoribacter is a butyrate-producing genus (anti-inflammatory SCFA), while Erysipelotrichi is associated with intestinal inflammation and activation of inflammatory pathways [86, 87]. Accordingly, Bilenduke et al. [37] found a large decrease in the abundance of the genus Akkermancia in BC patients. Low abundance of this genus is related to pathologies such as irritable bowel syndrome and is associated with loss of intestinal barrier integrity and inflammation [88].
The results obtained when characterizing the composition of the GM largely differ among the different studies included (Table 2), as previously described for specific groups of patients. Q. Zhu et al. [28] assessed GM alterations in patients compared to controls and observed in patients an increase in the relative abundance of Rothia, Actinomyces, Lautropia, Centipeda, Corynebacterium, Anaeroglobus, Selenomonas, Fretibacterium and Tannerella, whereas a decrease Subdoligranulum. The abundance of the Actinomyces and Rothia genus has been linked to the development of some cancers, such as colorectal cancer for Actinomyces [89] and squamous cell lung carcinoma for Rothia [90]. Moreover, both Actinomyces and Rothia genus have been negatively associated with levels of L-norvaline, a metabolite that combined with DOX could counteract carcinogenesis [91, 92]. Bobin-Dubigeon et al. [27] found that the GM of patients with early-stage BC was characterized by a relative enrichment in Firmicutes, Clostridium cluster IV and cluster XIVa, Blautia, Clostridium XVIII and Lachnospira; as well as a decreasse in Bacteroidetes, Bifidobacterium sp., Odoribacter sp., Butyricimonas sp. and Coprococcus sp. Clostridium cluster IV and Clostridium cluster XIVa express β-glucuronidases which, as previously mentioned, are enzymes that contribute to increased serum levels of free estrogens and, consequently, BC risk. Similarly, Coprococcus sp., Butyricimonas sp. and Odoribacter sp. are SCFA-producing genera, and their decrease has also been observed in colorectal cancer [93], non-Hodgkin lymphoma [94] and colorectal cancer [95], respectively. Bobin-Dubigeon et al. [27] also found a decrease in Bifidobacterium sp. (a genus that typically includes probiotic microorganisms related to the maintenance of human health status) in patients. The analysis of obese and overweight patients by Smith et al. [35] revealed higher levels of the genus Allobaculum and lower levels of Lysobacter in the GM of patients, while Agrobacterium was the predominant genus in the GM of controls. Saud Hussein et al. [41] compared the composition of the breast microbiota and observed growth of E. coli and Staphylococcus aureus in the breast tissue of BC patients but were unable to detect growth in samples from women with benign breast lesions. Nevertheless, Esposito et al. [42] detected an increased abundance of Proteobacteria and Firmicutes and a decrease in Actinobacteria (especially Propionibacterium acnes) in tumor breast tissue. These results are in agreement with the study by Thompson et al. [96]. Propionibacterium acnes is an opportunistic pathogen whose role in human health has not yet been established. Some authors support its antitumor effect in BC [97], while others suggest that it is involved in implant-associated infections [98]. Unlike the tumor tissue, Propionibacterium and Pseudomonas predominated in healthy adjacent tissue [42].
Finally, regarding other breast lesions (Table 2), Z. Ma et al. [38] investigated the composition of the GM in patients with BC and benign breast lesions. The authors established the increase of Porphyromonas, Prevotella, Peptoniphilus and Megamonas as an indicator of BC and the increase of Escherichia, Lactobacillus and Coprobacillus levels were associated with benign breast lesions. Porphyromonas and Prevotella have also been identified as potential biomarkers of postmenopausal BC [32, 99] and have been associated with colorectal cancer and precancerous adenomas [100, 101]. Similarly, Peptoniphilus genus was enriched in HER2-positive and triple-negative subtypes of BC [102]. Additionally, within the genus Escherichia, E. coli is capable of producing DNA mutagens, such as colibactin genotoxin, and inducing tumorigenesis [103]. Although Byrd et al. [39] found no significant differences between the microbiota of patients with BC and non-malignant breast disease, BC was positively associated with Bacteroides and Ruminococcaceae and negatively associated with Romboutsia, Coprococcus and Faecalibacterium. J. Ma et al. [40] identified 59 members of the microbiota that differed in abundance between patients with BC and benign breast disease. BC patients displayed a lower relative abundance of Firmicutes (Faecalibacterium) and Bacteroidetes and increased abundance of Verrucomicrobia, Proteobacteria, Actinobacteria, Bacillus, Enterobacter and Staphylococcus. Considering that many breast lesions can give rise to precursors of BC or be risk markers for the disease, the identification of microbial profiles associated with these lesions could contribute to early detection and help prevent BC development.
The entire gut microbiome was altered in BC patients (Table 2). Several studies included in this review have demonstrated that diverse metabolic pathways and particular metabolites can distinguish the microbiota of different patient groups. Zhu et al. [28] found a relationship between differences in the composition of the GM and its metabolites in patients and controls. For example, enrichment of Rothia and Actinomyces in patients was positively associated with the presence of 4-methylcatechol and guaiacol, but negatively associated with norvaline. Byrd et al. [39] observed an association between BC and non-malignant breast disease with taxa involved in estrogen metabolism and immune homeostasis. Similarly, J. Ma et al. [40] identified 26 metabolic pathways that differed between the microbiota of BC patients or benign breast disease. Additionally, the abundance of some genes and the activation of metabolic pathways were also related to the GM composition of premenopausal and postmenopausal BC patients. In this regard, Zhu et al. [32] identified an increase in genes related to PTS, secretion, vitamin B12 transport and manganese/iron systems in premenopausal and postmenopausal patients compared to controls. In addition, postmenopausal patients showed higher expression of several genes involved in lipopolysaccharide biosynthesis and lower expression of genes related to butyrate synthesis. Nevertheless, Hou et al. [34] revealed that premenopausal patients had a microbiota enriched in bacteria involved in steroid-related and oncogenic pathways, whereas the microbiota of postmenopausal patients was mainly composed of bacteria involved in chemical carcinogenesis and aldosterone-related pathways. He et al. [33] assessed premenopausal patients and observed a lower proportion of SCFA-producing bacteria and a decrease in the levels of these metabolites, especially butyrate. The microbiota of postmenopausal patients was characterized by an enrichment of metabolic pathways of immune diseases based on the study by Goedert et al. [31].
Taken together, BC is associated with an imbalance in the gut and breast microbiome. This dysbiosis affects both the diversity and abundance of specific microorganisms and their metabolites and varies according to clinical features (such as stage, grade, and molecular subtype of BC) and host factors (such as menopausal status, age at menarche, overweight, obesity and physical activity). In addition, the interaction between microorganisms and the development and progression of cancer is very complex, and a variety of bacteria or their metabolites can promote or inhibit the development of BC. In addition to this fact, the heterogeneity of the results reported does not allow a specific microbial profile to be associated with BC. In general, it seems that BC is related to a lower microbial diversity, an enrichment of genera with harmful effects (associated with various types of cancer, pathologies and inflammation or producers of β-glucuronidases and toxins), such as Clostridium and Bacteroides, and decreased in genera beneficial to health, such as Faecalibacterium, Bifidobacterium and Akkermansia. Furthermore, this disease can be related to a significant decrease in SCFA-producing bacteria and lower levels of these metabolites.
Animal model studies
Modulation of GM could be a useful strategy to enhance the efficacy of therapies against BC. This review includes some studies in animal models that support this hypothesis (Table 3). Shi et al. [45] highlights the implications of combined therapy of transforming growth factor-β (TGF-β) inhibitors, such as galunisertib, with Escherichia coli Nissle 1917 in the prevention and treatment of BC. Oral administration of E. coli Nissle 1917, a probiotic with beneficial effects on intestinal immune homeostasis, enhanced the effect of galunisertib by modulating the GM and the tumor immune microenvironment. Specifically, E. coli Nissle 1917 caused an increase in the abundance of Alistipes shahii, A. muciniphila, Bacteroides thetaiotaomicron, B. acidifaciens and Lactobacillus johnsonii; and a decrease in the abundance of Clostridium spp.
Di Modica et al. [26] determined that certain commensal bacteria may contribute to the efficacy of trastuzumab through modification of the tumor microenvironment. In their model of HER2-positive BC, antibiotic administration reduced the efficacy of trastuzumab by altering the GM by reducing the abundance of Clostridiales (Lachnospiraceae), Actinobacteria (Coriobacteriaceae), Turicibacteraceae and Bacteroidetes (Prevotellaceae). In addition, the GM composition of HER2-positive patients had an impact on the activity of trastuzumab, as the transfer of fecal microbiota from patients with different treatment responses to mice recapitulated the results observed in patients. Furthermore, Terrisse et al. [36] discovered that GM composition influences the progression of BC and the anticancer effects of cyclophosphamide (CTX) since mice displayed different microbial compositions depending on tumor development and CTX activity. The GM of mice with slow tumor progression was enriched on species present in the feces of patients in stage I or no axillary node involvement pre- or post-chemotherapy (Eubacterium rectale, E. eligens, A. muciniphila, B. longum, C. aerofaciens and Alispites shahii). On the contrary, mice that received fecal microbiota transplantation from patients associated with an unfavorable prognosis (B. uniformis, B. xylanivolvens, B. intestinalis) showed rapid disease progression. In addition, the tumor-killing activity of CTX was lower in mice with fecal microbiota transplant from patients compared to mice with fecal microbiota transplant from controls. These studies suggest that some microbial populations could serve as biomarkers to predict treatment response.
Dietary intervention may represent a promising strategy for the prevention and treatment of BC, as the intake of a wide range of compounds is able to regulate GM homeostasis and influence tumor progression in animal models (Table 3). Paul et al. [43] found that genistein, an isoflavone derived from soy products with anticancer properties, could increase tumor latency and reduce tumor growth in ER-negative mice by modulating GM. In particular, the GM of genistein-treated mice was characterized by an increase in Lactococcus and Eubacterium genus, members of Lachnospiraceae and Ruminococcaceae family and Verrucomicrobia phylum (such as A. muniphila). Daucosterol linolenate (DLA), daucosterol linoleate (DL) and daucosterol palmitate (DP), three phytosterols present in sweet potato, inhibited tumor growth in MCF-7 cell xenograft nude mice by altering the expression of tumor markers and cancer-related proteins, modulating GM and production of SCFAs. In particular, the three phytosterols reversed tumor-induced dysbiosis by increasing Bacteroidetes richness and decreasing Firmicutes richness, modulating GM diversity at family and genus level, and promoting butyric or acetic and butyric acid production by DL and DP, respectively [48]. Fucoidan, a complex sulphated polysaccharide obtained from brown algae, favored the development of a more diverse gut microbiome through an increase in the Bacteroidetes/Firmicutes ratio and an increase in SCFAs producers such as Prevotella in a rat model of BC. Furthermore, fucoidan repaired intestinal barrier function by promoting the expression of tight junction proteins (Zonula occludens-1, claudin-1 and claudin-8) and the levels of phosphorylated p38 MAPK and ERK1/2 [44]. The administration of Poria cocos fungus extracts exerted a similar effect in triple-negative BC mice. In this case, the change in the structure of the GM resulted in an increase in beneficial bacteria, such as Lactobacillus, Bifidobacterium and Blautia, and a decrease in sulfate-reducing bacteria, such as Desulfovibrio and bacteria, associated with inflammation, such as Mucispirillum, S24-7 and Staphylococcus [47]. Finally, Li et al. [49] determined that maternal n-3 polyunsaturated fatty acids reduced the risk of BC in the offspring by modulating the GM and reducing levels of the proinflammatory factors interleukin 1β (IL-1β), IL-6 and tumor necrosis factor α (TNF-α). In particular, n-3 polyunsaturated fatty acids caused an increase in α-diversity and relative abundance of Akkermansia, Lactobacillus and Mucispirillum. Mucispirillum was also positively associated with IL-10 levels, whereas Akkermansia was negatively associated with IL-6.
Although bacteria are the most studied members of the human microbiota, they are not the only microorganisms that can be involved in BC and its treatment. Shiao et al. [50] discovered that the mycobiome plays an opposite role to that of the bacteriome in the efficacy of radiotherapy against BC, by modulating the antitumor immune response (Table 3). While the decrease in gut fungi favors the efficacy of radiotherapy, the decrease in bacteria reduces the response to radiotherapy and is related to the overgrowth of commensal fungi.
Several studies in mouse models have addressed the link between alterations in GM in obesity associated with BC, the existence of previous dysbiosis and its relation with cancer prognosis and the effects of the tumor on the microbiota and intestinal barrier function (Table 3). Hossain et al. [51] used an immunocompetent mouse model of triple-negative BC to conclude that there is an association between Western diet-induced obesity and increased tumor growth, which is consistent with previous studies, suggesting that obesity favors the risk of this subtype of BC [104]. Similarly, obesity was associated with a loss of microbial diversity, a decrease in the Bacteroides/Firmicutes ratio (particularly Alistipes) and an alteration in bacterial metabolic pathways. This decrease in microbial diversity is also consistent with compelling data available in the existing literature [105]. Finally, Hossain et al. [51] demonstrated that the similarity of bacterial communities based on taxonomic profiles and the variability of functional profiles depended on obesity, although the latter was also explained to a lesser extent by the presence of the tumor and the obesity–tumor interaction.
Loman et al. [52] discovered that orthotopic mammary tumors compromise the intestinal barrier function by altering the microbiome, highlighting a lower abundance of Lactobacillus and increased Bacteroides. Consequently, they increase the translocation of enteric bacteria and cause systemic inflammation including splenomegaly, increased splenic bacterial load and proinflammatory cerebral and splenic cytokines. This may explain the gastrointestinal (diarrhea and nausea) and cognitive (anxiety and depression) symptoms associated with the tumor. In this context, Lactobacillus supplementation could help to alleviate these symptoms, as this probiotic genus is known to improve intestinal barrier function, regulate the immune system and influence intestinal motility [106, 107]. Furthermore, Rosean et al. [46] identified pre-existing dysbiosis of the commensal microbiota as a host-intrinsic regulator of tissue inflammation, myeloid recruitment, fibrosis, and tumor cell dissemination in ER/PR-positive BC. Therefore, these authors demonstrated that commensal dysbiosis contributes to the spread and aggressiveness of BC and that dysbiosis could be a biomarker or therapeutic target to reduce inflammation within the tissue microenvironment.
GM characterization in animal models was performed on stool samples and microbial composition was determined mainly by sequencing regions of the 16S rRNA gene [26, 43,44,45,46,47,48,49,50,51,52] or by shotgun metagenomic sequencing [36]. Sequenced 16S rRNA gene regions were V3-V4 [26, 44, 45, 47,48,49] or V4 [43, 51, 52]. In addition, in the case of the mycobiome, the composition of gut fungi was determined by sequencing the ITS1 gene [50].
In vitro studies
In vitro studies with BC cell lines (Table 4) highlight the potential therapeutic use of specific members of the microbiota or their metabolites in BC treatment. Ma et al. [40] discovered that Faecalibacterium prausnitzii prevents MCF-7 cells growth by inhibiting the IL-6/STAT3 pathway. This signaling pathway is hyperactivated in many cancers and its hyper-activation is associated with poor prognosis. In addition, in the tumor microenvironment, the IL-6/STAT3 pathway promotes tumor cell proliferation, survival, invasiveness and metastasis, while suppressing the antitumor immune response [108]. Consequently, F. prausnitzii could act as a probiotic in BC by inhibiting tumor growth and stimulating antitumor immunity. In the same cell line, An et al. [54] determined that extracellular vesicles derived from Klebsiella pneumoniae potentiate the antihormonal effect of tamoxifen by downregulating cyclin E2, p-ERK and p21. Furthermore, various metabolites produced by the GM also showed inhibitory effects on BC cell lines. Zhu et al. [28] found that L-norvaline can inhibit BC cell proliferation when combined with DOX. Moreover, He et al. [33] demonstrated that sodium propionate and sodium butyrate inhibit the activity of SKBR3 and MCF-7 cells in a dose-dependent manner.
Finally, some studies in BC cell models suggest that disruption of lipid rafts may be a key factor in BC cell proliferation and apoptosis. Bobin-Dubigeon et al. [53] found that GM interacts with the lipid metabolism of enteric cells and influences the behavior of MCF-7 cells. Since lipid metabolites can reach mammary cells through systemic circulation, they could affect BC. The authors used a basolateral medium of Caco-2 cells preincubated with patients or control fecal fluid and concluded that preincubation with patient or control fluid differentially affected MCF-7 cells viability. Fecal water valerate, a SCFA, was independently associated with a decreased ability of the Caco-2 cell medium to induce MCF-7 cell proliferation. In addition, MCF-7 cells viability was positively related to the percentage of Bifidobacterium sp. in the fecal water incubated with Caco-2 cells. Finally, regarding the expression of genes related to lipid metabolism, they found a positive relationship between the expression of the Apo AIV gene and acetate, butyrate, propionate, and the percentage of Bacteroidetes. Moreover, the percentage of Blautia sp. was negatively correlated with the expression of the LXR (liver X receptor) gen. Apo AIV and LXR genes regulate the synthesis of apolipoproteins, proteins that are involved in the release of cholesterol from BC cells, which is associated with reduced cell viability. Considering the above, a relationship has been found between the abundance of Blautia sp. and the severity of BC, this genus may influence tumor development through its negative effect on the LXR gene [53].
Limitations of the study
This literature review has some limitations. The bibliographic search was performed in a single database, which limited the incorporation of publications. Several of the included trials were carried out with a small number of samples and assessed the GM in patients with different characteristics, which limits the robustness of the conclusions. Additionally, the reported results show great heterogeneity, possibly due to biological factors (such as age, individual genetic variation, ethnic origin, geographic location, and dietary habits) that influence the composition of the microbiota, and the variability of analysis methods (hypervariable regions of the 16S rRNA gene, type and sequencing platform, bioinformatics tools, etc.). Finally, most of the articles identify the members of the GM at the genus level or higher levels, which precludes the establishment of a link of specific species with BC.
Finally, we are aware of recent changes in bacterial taxonomy and nomenclature. However, we have kept the nomenclature used by the authors for better traceability of the works mentioned in this review.
Conclusions
Based on the literature included in this work, some clear conclusions can be drawn. GM differs between BC patients and healthy women. Therefore, BC may be associated with microbiota dysbiosis. This imbalance affects microbial diversity and the abundance of particular microorganisms and their metabolites. Similarly, it varies depending on the molecular type, stage and grade of cancer, as well as based on the state of menopause, age at menarche, BMI and physical activity of the patients.
BC seems to be characterized by a loss of microbial diversity, an enrichment of genera with deleterious effects (related to cancer, pathologies and inflammation or producers of β-glucuronidases and toxins), such as Clostridium and Bacteroides, and a decrease in genera beneficial for the health as Faecalibacterium, Bifidobacterium and Akkermansia. In addition, BC can be related to a decrease in SCFA-producing bacteria and the levels of these metabolites. However, it has not been possible to identify a specific microbial profile as a non-invasive biomarker.
Studies in humans, animal models and in vitro indicate that GM may influence the efficacy of BC therapies through modulation of the tumor immune microenvironment, probably also being partly responsible for the treatment side effects.
Studies performed on animal models and in vitro cell cultures demonstrate the potential use of various microorganisms (such as Escherichia coli Nissle 1917 and Faecalibacterium prausnitzii) as probiotics to promote the efficacy of BC therapies or directly affect tumor development. Moreover, a variety of microbial metabolites (such as SCFA, such as butyrate, propionate and valerate) have been proposed as prebiotics.
Large-scale studies, especially clinical trials with standardized protocols, and the application of proper mathematical approaches to calculate adequately the microbiome abundances are necessary to confirm the association between microbiota and BC and to discover the potential clinical applications of the microbiota in the prevention and early diagnosis of BC, as well as therapeutic interventions [109].
The existence of biomarkers based on the composition of the human microbiota would provide a new method for the diagnosis of non-invasive BC. Furthermore, the ability of the microbiota to modulate therapies would allow the development of more effective therapeutic strategies, ultimately contributing to reducing mortality and prevalence of BC. Nevertheless, further research is needed to shed light on the relevance of microbiota modulation for BC treatment.
Availability of data and material
Not applicable.
Abbreviations
- DNA:
-
Deoxyribonucleic acid
- SCFA:
-
Short-chain fatty acids
- rRNA:
-
Ribosomal ribonucleic acid
- BC:
-
Breast cancer
- CTLA-4:
-
Cytotoxic T cell antigen 4
- CTX:
-
Cyclophosphamide
- DLA:
-
Daucosterol linolenate
- DL:
-
Daucosterol linoleate
- DP:
-
Daucosterol palmitate
- DOX:
-
Doxorubicin hydrochloride
- HER2:
-
Human epidermal growth factor receptor 2
- ICI:
-
Immune checkpoint inhibitors
- IL:
-
Interleukin
- BMI:
-
Body mass index
- GM:
-
Gut microbiota
- NF-κB:
-
Nuclear factor kappa B
- NLR:
-
NOD-like receptors
- PAMP:
-
Pathogen-associated molecular pattern
- PD-1:
-
Programmed cell death receptor 1
- PD-L1:
-
Programmed cell death ligand 1
- PD-L2:
-
Programmed cell death ligand 2
- PRR:
-
Pattern recognition receptors
- qPCR:
-
Quantitative polymerase chain reaction
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- sIgA:
-
Immunoglobulin A secretory
- TGF-β:
-
Transforming growth factor beta
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor alpha
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers. 2021;13:4287.
Fernández MF, Reina-Pérez I, Astorga JM, Rodríguez-Carrillo A, Plaza-Díaz J, Fontana L. Breast cancer and its relationship with the microbiota. Int J Environ Res Public Health. 2018;15:1747.
Fernández JÁ, Ozores PP, López VC, Mosquera AC, López RL. Breast cancer. Medicine. 2021;13:1506–17.
Barzaman K, Karami J, Zarei Z, Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, et al. Breast cancer: biology, biomarkers, and treatments. Int Immunopharmacol. 2020;84:106535.
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers. 2019;5:66.
Perou CM, Sørile T, Eisen MB, Van De Rijn M, Jeffrey SS, Ress CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Afzal S, Hassan M, Ullah S, Abbas H, Tawakkal F, Khan MA. Breast cancer; discovery of novel diagnostic biomarkers, drug resistance, and therapeutic implications. Front Mol Biosci. 2022;9: 783450.
Sarhangi N, Hajjari S, Heydari SF, Ganjizadeh M, Rouhollah F, Hasanzad M. Breast cancer in the era of precision medicine. Mol Biol Rep. 2022;49:10023–37.
Laborda-Illanes A, Sanchez-Alcoholado L, Dominguez-Recio ME, Jimenez-Rodriguez B, Lavado R, Comino-Méndez I, et al. Breast and gut microbiota action mechanisms in breast cancer pathogenesis and treatment. Cancers. 2020;12:1–27.
Young VB. The role of the microbiome in human health and disease: an introduction for clinicians. BMJ. 2017;356: j831.
Urbaniak C, Cummins J, Brackstone M, Macklaim JM, Gloor GB, Baban CK, et al. Microbiota of human breast tissue. Appl Environ Microbiol. 2014;80:3007–14.
Papakonstantinou A, Nuciforo P, Borrell M, Zamora E, Pimentel I, Saura C, et al. The conundrum of breast cancer and microbiome – a comprehensive review of the current evidence. Cancer Treat Rev. 2022;111: 102470.
Di Modica M, Arlotta V, Sfondrini L, Tagliabue E, Triulzi T. The Link between the microbiota and HER2+ breast cancer: the new challenge of precision medicine. Front Oncol. 2022;12: 947188.
Álvarez-Mercado AI, del Valle CA, Fernández MF, Fontana L. Gut microbiota and breast cancer: the dual role of microbes. Cancers. 2023;15:443.
Parida S, Wu S, Siddharth S, Wang G, Muniraj N, Nagalingam A, et al. A procarcinogenic colon microbe promotes breast tumorigenesis and metastatic progression and concomitantly activates notch and b-catenin axes. Cancer Discov. 2021;11:1138–57.
Bianchini G, Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014;15:e58–68.
Loizides S, Constantinidou A. Triple negative breast cancer: immunogenicity, tumor microenvironment, and immunotherapy. Front Genet. 2023;13:1095839.
Plaza-Diaz J, Álvarez-Mercado AI. The interplay between microbiota and chemotherapy-derived metabolites in breast cancer. Metabolites. 2023;13:703.
Pandey S, Singh S, Anang V, Bhatt AN, Natarajan K, Dwarakanath BS. Pattern recognition receptors in cancer progression and metastasis. Cancer Growth Metastasis. 2015;8:25.
Yang W, Sui J, Ma Y, Simon TG, Petrick JL, Lai M, et al. High Dietary intake of vegetable or polyunsaturated fats is associated with reduced risk of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2020;18:2775–83.
Burguin A, Diorio C, Durocher F. Breast cancer treatments: updates and new challenges. J Pers Med. 2021;11:808.
Lasagna A, De Amici M, Rossi C, Zuccaro V, Corbella M, Petazzoni G, et al. The bio-diversity and the role of gut microbiota in postmenopausal women with luminal breast cancer treated with aromatase inhibitors: an observational cohort study. Pathogens. 2022;11:1421.
Smith M, Dai A, Ghilardi G, Amelsberg KV, Devlin SM, Pajarillo R, et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat Med. 2022;28:713–23.
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502.
Di Modica M, Gargari G, Regondi V, Bonizzi A, Arioli S, Belmonte B, et al. Gut microbiota condition the therapeutic efficacy of trastuzumab in HER2-positive breast cancer. Cancer Res. 2021;81:2195–206.
Bobin-Dubigeon C, Luu HT, Leuillet S, Lavergne SN, Carton T, Le Vacon F, et al. Faecal microbiota composition varies between patients with breast cancer and healthy women: a comparative case-control study. Nutrients. 2021;13:2705.
Zhu Q, Zai H, Zhang K, Zhang X, Luo N, Li X, et al. L-norvaline affects the proliferation of breast cancer cells based on the microbiome and metabolome analysis. J Appl Microbiol. 2022;133:1014–26.
Luu TH, Michel C, Bard JM, Dravet F, Nazih H, Bobin-Dubigeon C. Intestinal proportion of Blautia sp. is associated with clinical stage and histoprognostic grade in patients with early-stage breast cancer. Nutr Cancer. 2017;69:267–75.
Wu AH, Tseng C, Vigen C, Yu Y, Cozen W, Garcia AA, et al. Gut microbiome associations with breast cancer risk factors and tumor characteristics: a pilot study. Breast Cancer Res Treat. 2020;182:451.
Goedert JJ, Hua X, Bielecka A, Okayasu I, Milne GL, Jones GS, et al. Postmenopausal breast cancer and oestrogen associations with the IgA-coated and IgA-noncoated faecal microbiota. Br J Cancer. 2018;118:471–9.
Zhu J, Liao M, Yao Z, Liang W, Li Q, Liu J, et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome. 2018;6:136.
He C, Liu Y, Ye S, Yin S, Gu J. Changes of intestinal microflora of breast cancer in premenopausal women. Eur J Clin Microbiol Infect Dis. 2021;40:503–13.
Hou MF, Ou-Yang F, Li CL, Chen FM, Chuang CH, Kan JY, et al. Comprehensive profiles and diagnostic value of menopausal-specific gut microbiota in premenopausal breast cancer. Exp Mol Med. 2021;53:1636–46.
Smith KS, Frugé AD, van der Pol W, Caston NE, Morrow CD, Demark-Wahnefried W, et al. Gut microbial differences in breast and prostate cancer cases from two randomised controlled trials compared to matched cancer-free controls. Benef Microbes. 2021;12:239–48.
Terrisse S, Derosa L, Iebba V, Ghiringhelli F, Vaz-Luis I, Kroemer G, et al. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 2021;28:2778–96.
Bilenduke E, Sterrett JD, Ranby KW, Borges VF, Grigsby J, Carr AL, et al. Impacts of breast cancer and chemotherapy on gut microbiome, cognitive functioning, and mood relative to healthy controls. Sci Rep. 2022;12:1–19.
Ma Z, Qu M, Wang X. Analysis of gut microbiota in patients with breast cancer and benign breast lesions. Pol J Microbiol. 2022;71:217–26.
Byrd DA, Vogtmann E, Wu Z, Han Y, Wan Y, Clegg-Lamptey JN, et al. Associations of fecal microbial profiles with breast cancer and nonmalignant breast disease in the Ghana Breast Health Study. Int J Cancer. 2021;148:2712–23.
Ma J, Sun L, Liu Y, Ren H, Shen Y, Bi F, et al. Alter between gut bacteria and blood metabolites and the anti-tumor effects of Faecalibacterium prausnitzii in breast cancer. BMC Microbiol. 2020;20:1–19.
Saud Hussein A, Ibraheem Salih N, Hashim SI. Effect of microbiota in the development of breast cancer. Arch Razi Inst. 2021;76:751–8.
Esposito MV, Fosso B, Nunziato M, Casaburi G, D’Argenio V, Calabrese A, et al. Microbiome composition indicate dysbiosis and lower richness in tumor breast tissues compared to healthy adjacent paired tissue, within the same women. BMC Cancer. 2022;22:1–11.
Paul B, Royston KJ, Li Y, Stoll ML, Skibola CF, Wilson LS, et al. Impact of genistein on the gut microbiome of humanized mice and its role in breast tumor inhibition. PLoS ONE. 2017;12: e0189756.
Xue M, Ji X, Liang H, Liu Y, Wang B, Sun L, et al. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer. Food Funct. 2018;9:1214–23.
Shi L, Sheng J, Wang M, Luo H, Zhu J, Zhang B, et al. Combination therapy of TGF-β blockade and commensal-derived probiotics provides enhanced antitumor immune response and tumor suppression. Theranostics. 2019;9:4115–29.
Rosean CB, Bostic RR, Ferey JCM, Feng TY, Azar FN, Tung KS, et al. Preexisting commensal dysbiosis is a host-intrinsic regulator of tissue inflammation and tumor cell dissemination in hormone receptor-positive breast cancer. Cancer Res. 2019;79:3662–75.
Jiang Y, Fan L. The effect of Poria cocos ethanol extract on the intestinal barrier function and intestinal microbiota in mice with breast cancer. J Ethnopharmacol. 2021;266: 113456.
Han B, Jiang P, Jiang L, Li X, Ye X. Three phytosterols from sweet potato inhibit MCF7-xenograft-tumor growth through modulating gut microbiota homeostasis and SCFAs secretion. Food Res Int. 2021;141: 110147.
Li J, Wan Y, Zheng Z, Zhang H, Li Y, Guo X, et al. Maternal n-3 polyunsaturated fatty acids restructure gut microbiota of offspring mice and decrease their susceptibility to mammary gland cancer. Food Funct. 2021;12:8154–68.
Shiao SL, Kershaw KM, Limon JJ, You S, Yoon J, Ko EY, et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell. 2021;39:1202-1213.e6.
Hossain F, Majumder S, David J, Bunnell BA, Miele L. Obesity modulates the gut microbiome in triple-negative breast cancer. Nutrients. 2021;13:3656.
Loman BR, Russart KLG, Grant CV, Lynch AJ, Bailey MT, Pyter LM. Mammary tumors alter the fecal bacteriome and permit enteric bacterial translocation. BMC Cancer. 2022;22:1–13.
Bobin-Dubigeon C, Bard JM, Luu TH, Le VF, Nazih H. Basolateral secretion from Caco-2 cells pretreated with fecal waters from breast cancer patients affects MCF7 cell viability. Nutrients. 2020;13:1–12.
An J, Kim JB, Yang EY, Kim HO, Lee WH, Yang J, et al. Bacterial extracellular vesicles affect endocrine therapy in MCF7 cells. Medicine. 2021;100: e25835.
Fritz A, Hofmann P, Majda S, Dahms E, Dröge J, Fiedler J, et al. CAMISIM: simulating metagenomes and microbial communities. Microbiome. 2019;7:1–12.
Wiemann S, Albert R, Moosmayer P, Schupp I. Full‐length cDNA sequencing. In: Encyclopedic reference of genomics and proteomics in molecular medicine. Berlin: Springer; 2005, pp 614–617.
Durazzi F, Sala C, Castellani G, Manfreda G, Remondini D, De Cesare A. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci Rep. 2021;11:1–10.
Goedert JJ, Jones G, Hua X, Xu X, Yu G, Flores R, et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. JNCI. 2015;107:147.
Frugé AD, Van der Pol W, Rogers LQ, Morrow CD, Tsuruta Y, Demark-Wahnefried W. Fecal Akkermansia muciniphila is associated with body composition and microbiota diversity in overweight and obese women with breast cancer participating in a presurgical weight loss trial. J Acad Nutr Diet. 2020;120:650–9.
Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368:973–80.
Costantini L, Magno S, Albanese D, Donati C, Molinari R, Filippone A, et al. Characterization of human breast tissue microbiota from core needle biopsies through the analysis of multi hypervariable 16S-rRNA gene regions. Sci Rep. 2018;8:1–9.
Wang N, Yang J, Han W, Han M, Liu X, Jiang L, et al. Identifying distinctive tissue and fecal microbial signatures and the tumor-promoting effects of deoxycholic acid on breast cancer. Front Cell Infect Microbiol. 2022;12:1029905.
Kang DW, Park JG, Ilhan ZE, Wallstrom G, LaBaer J, Adams JB, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE. 2013;8: e68322.
Sha S, Xu B, Wang X, Zhang Y, Wang H, Kong X, et al. The biodiversity and composition of the dominant fecal microbiota in patients with inflammatory bowel disease. Diagn Microbiol Infect Dis. 2013;75:245–51.
Huybrechts I, Zouiouich S, Loobuyck A, Vandenbulcke Z, Vogtmann E, Pisanu S, et al. The human microbiome in relation to cancer risk: a systematic review of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2020;29:1856–68.
Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105:1907–11.
Cheng SH, Tsou MH, Liu MC, Jian JJ, Cheng JCH, Leu SY, et al. Unique features of breast cancer in Taiwan. Breast Cancer Res Treat. 2000;63:213–23.
Panda M, Rai AK, Rahman T, Das A, Das R, Sarma A, et al. Alterations of salivary microbial community associated with oropharyngeal and hypopharyngeal squamous cell carcinoma patients. Arch Microbiol. 2020;202:785–805.
Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. 2013;4:42.
Sun M, Du B, Shi Y, Lu Y, Zhou Y, Liu B. Combined signature of the fecal microbiome and plasma metabolome in patients with ulcerative colitis. Med Sci Monit. 2019;25:3303.
Iebba V, Totino V, Gagliardi A, Santangelo F, Cacciotti F, Trancassini M, et al. Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol. 2016;39:1–12.
Li N, Bai C, Zhao L, Ge Y, Li X. Characterization of the fecal microbiota in gastrointestinal cancer patients and healthy people. Clin Transl Oncol. 2022;24:1134–47.
Walther-António MRS, Chen J, Multinu F, Hokenstad A, Distad TJ, Cheek EH, et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016;8:122.
Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2019;68:1014–23.
Zhang J, Zhang F, Zhao C, Xu Q, Liang C, Yang Y, et al. Dysbiosis of the gut microbiome is associated with thyroid cancer and thyroid nodules and correlated with clinical index of thyroid function. Endocrine. 2019;64:564–74.
Cheng WT, Kantilal HK, Davamani F. The mechanism of Bacteroides fragilis toxin contributes to colon cancer formation. Malays J Med Sci. 2020;27:9–21.
Strakova N, Korena K, Karpiskova R. Klebsiella pneumoniae producing bacterial toxin colibactin as a risk of colorectal cancer development – a systematic review. Toxicon. 2021;197:126–35.
Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol. 2017;8:1765.
Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103.
Wang Z, Wang Q, Zhao J, Gong L, Zhang Y, Wang X, et al. Altered diversity and composition of the gut microbiome in patients with cervical cancer. AMB Express. 2019;9:40.
Song H, Wang W, Shen B, Jia H, Hou Z, Chen P, et al. Pretreatment with probiotic Bifico ameliorates colitis-associated cancer in mice: transcriptome and gut flora profiling. Cancer Sci. 2018;109:666–77.
Fu BC, Hullar MAJ, Randolph TW, Franke AA, Monroe KR, Cheng I, et al. Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am J Clin Nutr. 2020;111:1226–34.
Shukla R, Goyal A. Novel dextran from Pediococcus pentosaceus CRAG3 isolated from fermented cucumber with anti-cancer properties. Int J Biol Macromol. 2013;62:352–7.
An BC, Ryu Y, Yoon YS, Choi O, Park HJ, Kim TY, et al. Colorectal cancer therapy using a Pediococcus pentosaceus SL4 drug delivery system secreting lactic acid bacteria-derived protein p8. Mol Cells. 2019;42:755–62.
Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–6.
Palm NW, De Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–10.
Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2015;211:19–27.
Geerlings SY, Kostopoulos I, de Vos WM, Belzer C. Akkermansia muciniphila in the human gastrointestinal tract: when, where, and how? Microorganisms. 2018;6:75.
Peters BA, Dominianni C, Shapiro JA, Church TR, Wu J, Miller G, et al. The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome. 2016;4:69.
Huang D, Su X, Yuan M, Zhang S, He J, Deng Q, et al. The characterization of lung microbiome in lung cancer patients with different clinicopathology. Am J Cancer Res. 2019;9:2047.
Gao L, Zhang JH, Chen XX, Ren HL, Feng XL, Wang JL, et al. Combination of L-Arginine and L-Norvaline protects against pulmonary fibrosis progression induced by bleomycin in mice. Biomed Pharmacother. 2019;113: 108768.
Ren X, Wang N, Zhou Y, Song A, Jin G, Li Z, et al. An injectable hydrogel using an immunomodulating gelator for amplified tumor immunotherapy by blocking the arginase pathway. Acta Biomater. 2021;124:179–90.
Shen XJ, Rawls JF, Randall T, Burcal L, Mpande CN, Jenkins N, et al. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes. 2010;1:138–47.
Montassier E, Al-Ghalith GA, Ward T, Corvec S, Gastinne T, Potel G, et al. Pretreatment gut microbiome predicts chemotherapy-related bloodstream infection. Genome Med. 2016;8:1–11.
Thomas AM, Jesus EC, Lopes A, Aguiar S, Begnami MD, Rocha RM, et al. Tissue-associated bacterial alterations in rectal carcinoma patients revealed by 16S rRNA community profiling. Front Cell Infect Microbiol. 2016;6:179.
Thompson KJ, Ingle JN, Tang X, Chia N, Jeraldo PR, Walther-Antonio MR, et al. A comprehensive analysis of breast cancer microbiota and host gene expression. PLoS ONE. 2017;12: e0188873.
Talib WH, Saleh S. Propionibacterium acnes augments antitumor, anti-angiogenesis and immunomodulatory effects of melatonin on breast cancer implanted in mice. PLoS ONE. 2015;10: e0124384.
Portillo ME, Corvec S, Borens O, Trampuz A. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. Biomed Res Int. 2013;2013: 804391.
Amanatullah DF, Tamaresis JS, Chu P, Bachmann MH, Hoang NM, Collyar D, et al. Local estrogen axis in the human bone microenvironment regulates estrogen receptor-positive breast cancer cells. Breast Cancer Res. 2017;19:121.
Warren RL, Freeman DJ, Pleasance S, Watson P, Moore RA, Cochrane K, et al. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome. 2013;1:16.
Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol. 2016;17:230–40.
Banerjee S, Tian T, Wei Z, Shih N, Feldman MD, Peck KN, et al. Distinct microbial signatures associated with different breast cancer types. Front Microbiol. 2018;9:951.
Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–3.
Tiwari P, Blank A, Cui C, Schoenfelt KQ, Zhou G, Xu Y, et al. Metabolically activated adipose tissue macrophages link obesity to triple-negative breast cancer. J Exp Med. 2019;216:1345–58.
Stanislawski MA, Dabelea D, Lange LA, Wagner BD, Lozupone CA. Gut microbiota phenotypes of obesity. NPJ Biofilms Microbiomes. 2019;5:18.
Wang J, Ji H, Wang S, Liu H, Zhang W, Zhang D, et al. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front Microbiol. 2018;9:1953.
Ding YH, Qian LY, Pang J, Lin JY, Xu Q, Wang LH, et al. The regulation of immune cells by Lactobacilli: a potential therapeutic target for anti-atherosclerosis therapy. Oncotarget. 2017;8:59915.
Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–48.
Rivera-Pinto J, Egozcue JJ, Pawlowsky-Glahn V, Paredes R, Noguera-Julian M, Calle ML. Balances: a new perspective for microbiome analysis. mSystems. 2018;3:e00053-18.
Acknowledgements
Ana I. Álvarez-Mercado was awarded a grant by the Regional Ministry of Health and Families (Andalucía, Spain), CSyF 2021 (RPS 24665).
Funding
Funding for open access publishing: Universidad de Granada/CBUA. This work has not received external funding.
Author information
Authors and Affiliations
Contributions
A.A.D.C. drafted the manuscript, T.R.T helped to draft and A.I.Á.M. conceived the work, drafted and revised the manuscript. All authors collaborated to carry out the presented work and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate
Not applicable.
Research involving human participants and/or animals and Informed consent
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amaro-da-Cruz, A., Rubio-Tomás, T. & Álvarez-Mercado, A.I. Specific microbiome patterns and their association with breast cancer: the intestinal microbiota as a potential biomarker and therapeutic strategy. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03554-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03554-w