Abstract
Background
The optimal chemotherapy backbone for HER2-negative advanced esophagogastric cancer, either in combination with targeted therapies or as a comparator in clinical trials, is uncertain. The subtle yet crucial differences in platinum-based regimens' safety and synergy with combination treatments need consideration.
Methods
We analyzed cases from the AGAMENON–SEOM Spanish registry of HER2-negative advanced esophagogastric adenocarcinoma treated with platinum and fluoropyrimidine from 2008 to 2021. This study focused exclusively on patients receiving one of the four regimens: FOLFOX (5-FU and oxaliplatin), CAPOX (capecitabine and oxaliplatin), CP (capecitabine and cisplatin) and FP (5-FU and cisplatin). The aim was to determine the most effective and tolerable platinum and fluoropyrimidine-based chemotherapy regimen and to identify any prognostic factors.
Results
Among 1293 patients, 36% received either FOLFOX (n = 468) or CAPOX (n = 466), 20% CP (n = 252), and 8% FP (n = 107). FOLFOX significantly increased PFS (progression free survival) compared to CP, with a hazard ratio of 0.73 (95% CI 0.58–0.92, p = 0.009). The duration of treatment was similar across all groups. Survival outcomes among regimens were similar, but analysis revealed worse ECOG–PS (Eastern Cooperative Oncology Group–Performance Status), > 2 metastatic sites, bone metastases, hypoalbuminemia, higher NLR (neutrophil-to-lymphocyte ratio), and CP regimen as predictors of poor PFS. Fatigue was common in all treatments, with the highest incidence in FOLFOX (77%), followed by FP (72%), CAPOX (68%), and CP (60%). Other notable toxicities included neuropathy (FOLFOX 69%, CAPOX 62%), neutropenia (FOLFOX 52%, FP 55%), hand–foot syndrome in CP (46%), and thromboembolic events (FP 12%, CP 11%).
Conclusions
FOLFOX shown better PFS than CP. Adverse effects varied: neuropathy was more common with oxaliplatin, while thromboembolism was more frequent with cisplatin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer ranks as the fifth most common neoplasm and the fourth leading cause of cancer-related deaths worldwide. Despite a reduction in incidence in recent years, along with advancements in diagnostic methods and surgical techniques, about 30% of patients with resected gastric cancer undergo relapse and subsequently require systemic treatment [1]. This is particularly concerning considering that at diagnosis, 50% of gastric cancer patients present with an unresectable disease—10% with locally advanced and 40% with metastatic stages [2].
Chemotherapy enhances overall survival (OS) in advanced disease by approximately 6–7 months compared to best supportive care, leading to median survival times of 9–11 months, even in patients with good functional status [3]. Now that chemotherapy combinations with new therapies, such as immunotherapy and anti-claudin agents, are emerging, it is worthwhile to briefly recall the complex historical development of various chemotherapy regimens to understand how we have arrived at the current state of treatment. In the 1990s, two-phase 3 randomized clinical trials (RCTs) showed greater efficacy and less toxicity with ECF [epirubicin, cisplatin, and 5-fluorouracil (5-FU)] [4] or FP4w (5-FU and cisplatin) [5] compared to the traditional FAM (5-FU, adriamycin, and mitomycin) and FAMTX (5-FU, adriamycin, and methotrexate) regimens. ECF became the reference standard in Europe, while FP4w was predominantly adopted in Asia and the United States. Notably, these two reference standards were never directly compared. In 2008, the British REAL-2 non-inferiority RCT demonstrated comparable OS between capecitabine and 5-FU, and between cisplatin and oxaliplatin, when combined with epirubicin [6]. Regarding toxicity, capecitabine and fluorouracil showed similar profiles, while oxaliplatin was linked to lower rates of grade 3–4 neutropenia, alopecia, renal toxicity, and thromboembolism compared to cisplatin, albeit with slightly increased incidences of grade 3–4 diarrhea and neuropathy. This study included patients with locally advanced disease (24.3%), esophageal neoplasia (34.2%), and squamous histology (12.1%), limiting the extrapolation of its data. Conversely, the international non-inferiority RCT ML17032 showed that the combination of 5-FU or capecitabine with cisplatin (FP or CP) were equivalent in activity with different toxicity profile [7]. Vomiting and mucositis were more frequent with FP, whereas hand–foot syndrome and anemia were more common with CP. Various RCTs have indicated that irinotecan and fluoropyrimidine display comparable [8, 9] or superior [10] efficacy to cisplatin and fluoropyrimidine. However, current clinical guidelines place it as an alternative option for patient’s intolerant to platinum [11].
In 2007, the international RCT V325, stood out as the first phase 3 study to show the superior efficacy and quality of life when docetaxel was added to the conventional FP regimen (DCF) [12]. DCF led to a slight increase in response and survival rates, albeit with a significant rise in hematological toxicity. These outcomes were observed in a carefully selected patient group, with 64% having a Karnofsky performance status (PS) of 90–100% and 76% being under 65 years of age. This raised concerns about the broader applicability of DCF beyond the perioperative context [13, 14]. In 2023, data from the French phase 3 PRODIGE 51–GASTFOX RCT revealed that mFLOT/TFOX significantly improved survival compared to FOLFOX (5-FU and oxaliplatin), but with increased incidences of grade 3–4 neuropathy, neutropenia, diarrhea, and fatigue [15]. Subgroup analysis particularly highlighted the benefit of the mFLOT/TFOX triplet in patients with an ECOG–PS of 0 and those with diffuse-type Lauren's cancer. In 2022, the non-inferiority Chinese EXELOX trial established that capecitabine and oxaliplatin (CAPOX) was as effective as the epirubicin, oxaliplatin and capecitabine (EOX) triplet, offered a better safety profile, and enhanced quality of life, thereby challenging the standard practice of adding anthracycline [14, 16]. In a 2017 Cochrane review that included 64 RCTs, the authors conclude that the magnitude of the observed survival benefits with the three-drug regimens is not large enough to be clinically meaningful [3].
Therefore, this background explains why we apparently now have multiple equally efficacious first-line treatment choices for advanced esophagogastric adenocarcinoma, which have not been adequately compared with each other, each characterized by its unique side effect profile. Several systematic reviews have compared alternative regimens to those based on platinum and fluoropyrimidine [14, 17, 18]. However, given the absence of a phase 3 RCT and despite the inherent limitations in such reviews, cisplatin and fluoropyrimidine continues, in the opinion of many, to be the standard treatment and reference regimen in phase 3 trials that include novel molecules, as evidenced by the RCT TOGA with trastuzumab, which precisely used this combination [19]. Contrary to this established paradigm, phase 3 RCTs involving immunotherapy, such as the CHECKMATE-649 [20] combined nivolumab with oxaliplatin and fluoropyrimidine, while the KEYNOTE-859 [21] allowed regimens with either cisplatin or oxaliplatin. Furthermore, the European Medicines Agency (EMA) has approved the use of nivolumab and pembrolizumab for the first-line treatment of advanced HER2-negative esophagogastric adenocarcinoma in combination with a platinum and fluoropyrimidine [20, 22].
Against this backdrop, we have analyzed a national gastric cancer registry to glean insights relevant to this matter. Consequently, our research is focused on assessing and comparing the efficacy and toxicity of four dual-agent chemotherapy regimens, each comprising either cisplatin or oxaliplatin combined with 5-FU or capecitabine. Our aim is to identify the most suitable regimen for broader application in forthcoming RCTs [23].
Materials and methods
Study design and population
This study integrated cases from the national, observational, AGAMENON–SEOM registry of the Spanish Society of Medical Oncology, sourced from 40 hospitals. Patients eligible were adults with histologically confirmed advanced, unresectable, or recurrent adenocarcinoma of the distal esophagus, gastroesophageal junction (GEJ), and stomach, without HER2 overexpression who had received first-line chemotherapy doublet with a platinum and a fluoropyrimidine, between 2008 and 2021. Patients who received targeted therapy or immunotherapy in addition to their chemotherapy, or patients with recurrent cancer who had undergone perioperative or adjuvant treatment in the past 6 months were excluded.
This research was conducted in accordance with the Good Clinical Practice guidelines and the Helsinki Declaration and received approval from the Research Ethics Committees of all participating hospitals.
Therapeutic regimen and variables
The therapeutic regimen, chosen by the medical oncologist, was administered according to the standard clinical practice of the center. Chemotherapy was categorized based on specific platinum and fluoropyrimidine compounds, encompassing combinations such as FOLFOX, FP, CAPOX, and CP. The rationale for selecting each treatment regimen was documented. The Relative Dose Intensity (RDI) was quantified in percentages, defined as the administered dose intensity (drug amount per unit of time, expressed as mg/m2 per week) relative to the planned dose for each regimen. In addition, the study collects data on several key parameters: the number of treatment cycles, reasons for discontinuing treatment, and the highest level of toxicity observed, all classified according to the CTCAE v 4.0 (Common Terminology Criteria for Adverse Events).
Baseline variables encompassed patient characteristics (age, sex, ECOG–PS, Charlson comorbidities), tumor characteristics (cancer-related serious complications at diagnosis, location, grade, Lauren classification, presence of signet ring cells, unresectable or metastatic stage, location of metastases and tumor burden), laboratory data and tumor markers [(albumin, bilirubin, alkaline phosphatase, lactate dehydrogenase, hemoglobin, neutrophil-to-lymphocyte ratio (NLR), and carcinoembryonic antigen (CEA)] and whether the primary cancer had been resected.
Treatment effectiveness was assessed using OS and PFS (progression-free survival), defined as the period (months) from the start of the first line until death from any cause (OS), or until progression (PFS), censoring subjects without any event at the last follow-up. The overall response rate (ORR) and disease control rate (DCR), comprising complete response, partial response, and stable disease, were determined based on the RECIST 1.1 criteria through local evaluation.
Data were collected from patients' medical records and recorded via a web tool (http://www.agamenonstudy.com/), which is equipped with filters and enables simultaneous online and telephone-based monitoring.
Statistical analysis
A basic descriptive analysis was conducted using standard estimators such as means, medians, standard deviations, and percentages. Categorical variables were compared using Chi-square tests. Survival was assessed using the Kaplan–Meier estimator, and survival functions were compared using the log-rank test. Toxicity was evaluated using Amit plots, which include relative risks and their 95% confidence intervals. To analyze prognostic factors, a multivariable Cox proportional hazards (PH) model was utilized. Covariates were selected based on factors previously considered in studies from the AGAMENON–SEOM registry [24, 25]. The PH assumption was verified. Subgroup effects were evaluated using the method proposed by Hahn et al. [26]. According to this author, the estimate in each subgroup is tested against a region of indifference. A fixed sample size approach was employed, conditioned on the number of available patients. This necessitates consideration of the confidence interval magnitudes. A 5% significance level (two-tailed tests) was used for statistical analysis. Statistical analysis was conducted with Stata (version 14.2).
Results
Baseline characteristics
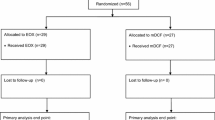
At the data cutoff date of December 2021, 4133 patients were registered, 1293 of whom met the inclusion criteria as detailed in Supplementary Fig. 1’s flowchart. Table 1 outlines these patients' baseline characteristics, grouped according to the administered chemotherapy regimen. In this cohort, FOLFOX and CAPOX were each administered to a nearly equal number of patients, together representing 72% of the entire set. Among the cisplatin regimens, the combination with capecitabine was more frequent than with 5-FU, accounting for 20% compared to 8% of the total, respectively.
The median age across all patients was 66 years, with the CAPOX group showing a slightly higher median age of 69, and 63% being 65 years or older. The cohort had twice as many men as women (864 vs. 429). 64% had an ECOG–PS of 1. Among those treated with FOLFOX, 22% (n = 114) had an ECOG–PS ≥ 2, compared to only 7.48% (n = 8) with FP. Most tumors were in the stomach (78.42%), followed by the GEJ (11.45%) and esophagus (8.82%). 40% (n = 519) had a histological grade 3.
Overall, 47% of the patients presented with peritoneal metastases, 35% with hepatic metastases, 24% had ascites, 13% experienced pulmonary metastases, and 10% had bone metastases. In general, patients treated with FOLFOX exhibited a more challenging clinical profile, characterized by a higher prevalence of diffuse and high-grade tumors, poorer functional status, and inferior nutritional parameters.
Selection and duration of chemotherapy regimen
The most common reason for choosing a chemotherapy regimen was compliance with local protocol (59%) and clinicians' experience (18%). Notably, FOLFOX was the preferred regimen when aiming to maximize the response rate in symptomatic disease (56%, n = 24), while CAPOX was predominantly chosen when patient quality of life was a concern (50%, n = 48).
The median duration of treatment with cisplatin was 4.14 and 4.17 months for FP and CP regimens, respectively, whereas for oxaliplatin, it was 4.50 and 4.12 months for FOLFOX and CAPOX, respectively. Regarding capecitabine, it was administered for 4.62 months in CP and 4.60 months in CAPOX; conversely, 5-FU was given for 4.27 months in FP and 5.40 months in FOLFOX.
64% of patients (n = 827) received at least 80% of the RDI of fluoropyrimidine, 55% and 72% for capecitabine regimens (CP and CAPOX, respectively), and 60% and 73% for 5-FU regimens (FOLFOX and FP, respectively). Regarding platinum-based therapies, 60% (n = 780) reached 80% of RDI, with 48% and 66% for cisplatin (CP and FP, respectively) and 56% and 71% for oxaliplatin (FOLFOX and CAPOX, respectively).
Only 20% of the patients (n = 253) underwent platinum treatment for more than 180 days, with the distribution being 5% for cisplatin (FP), 5% for cisplatin (CP), 9% for oxaliplatin (CAPOX) and 26% for oxaliplatin (FOLFOX). Conversely, 35% (n = 451) exceeded 180 days of fluoropyrimidine treatment, 16% and 41% with 5FU (FP and FOLFOX, respectively), and 31% and 35% with capecitabine (CP and CAPOX, respectively).
The primary reason for discontinuing platinum was cancer progression, accounting for 43% and 49% for cisplatin (FP and CP, respectively) and 42% and 43% for oxaliplatin (CAPOX and FOLFOX, respectively). Completion of the treatment plan was the next most common cause for discontinuing cisplatin (28% for CP and 39% for FP), while for oxaliplatin, it was toxicity (27% for CAPOX and 29% for FOLFOX). Regarding fluoropyrimidines, cancer progression was the main reason for discontinuation both for capecitabine (66% and 71%, CAPOX and CP, respectively) and for 5-FU (56% and 60%, FP and FOLFOX, respectively). These data are detailed for each drug according to the regimen used in Supplementary Table 1.
Efficacy
At the time of this analysis, there were 1,167 recorded progression events, representing 90% of cases, with a median PFS of 6.01 months (95% CI 5.72–6.30). In addition, there were 1,075 death events, accounting for 83% of cases, with a median OS of 10.67 months (95% CI 10.18–11.27). Kaplan–Meier curves, categorized by treatment regimen, are depicted in Fig. 1. The log-rank test, when applied to these stratified data, failed to reject the null hypothesis of no significant differences between regimens (log-rank test, p value = 0.23 for PFS, and 0.51 for OS, respectively). To clarify comparative therapeutic efficacy, we fitted Cox proportional hazards (PH) regression models for PFS and OS, considering the treatment regimens. The model for PFS identified several factors associated with a poorer prognosis, including worse ECOG–PS, presence of more than two metastatic sites, bone metastases, hypoalbuminemia, and increased NLR (Table 2). Notably, the use of FOLFOX was associated with improved PFS (HR 0.73; 95% CI 0.58–0.92, p = 0.009). However, its impact on OS was more limited, and the estimate was noisy, not allowing for the rejection of the null hypothesis of no effect (HR 0.90; 95% CI 0.71–1.15, p = 0.402, as shown in Supplementary Table 2) compared to CP. Similarly, in a sensitivity analysis focusing on regimens containing oxaliplatin (namely CAPOX or FOLFOX), the null hypothesis (that oxaliplatin regimens are equivalent to cisplatin regimens in terms of OS or PFS) also could not be rejected, possibly due to the relatively modest effect of CAPOX treatments (HR 0.85, 95% CI 0.71–1.01, p value = 0.077, as shown in Supplementary Table 3). Subgroup analysis revealed no statistical evidence of heterogeneous effects, indicating a consistent benefit with FOLFOX across all analyzed strata (Fig. 2). In addition, no interactions were observed when grouping by chemotherapy regimen, nor among platinum-based regimens (Supplementary Table 4 and 5). Regarding cisplatin regimens, the data do not allow for the rejection of the null hypothesis that CP and FP are similar in terms of PFS. Although there were indications that FP might be superior to CP in patients with an ECOG–PS of 0, the evidence was inconclusive. The interaction between the therapeutic effect and time was tested, finding no evidence that the year of initiation of first-line therapy altered the comparative effect of FOLFOX.
Forest plot of hazard ratio (HR) estimates for PFS in subgroup interactions by scheme FOLFOX (A), CAPOX (B) and FP (C). The red dotted vertical line highlights the overall treatment effect point (or main effect) for every scheme compared to CP (reference). The area shaded in gray represents the ‘indifference zone’ for the overall treatment effect, assuming that treatment effects between 80 and 125% of the 95% CI for the main effect do not represent clinically meaningful differences between each subgroup and the main effect. All subgroups with 95% CI that are only compatible with values within the indifference zone show treatment effect homogeneity. Subgroups with 95% CI that do not overlap with the red dotted vertical line (main effect) show evidence of treatment effect heterogeneity. All other subgroups are inconclusive. The 95% CI for the overall treatment effect HR is 0.5803–0.9243 corresponding to an indifference zone (shaded in grey) of 0.4642–1.1554. Abbreviations: ECOG–PS, Eastern Cooperative Oncology Group Performance Status; NLR, neutrophil-to-lymphocyte ratio; CI confidence interval. The 95% CI for the overall treatment effect HR is 0.6875–1.0715 corresponding to an indifference zone (shaded in grey) of 0.55–1.3394. Abbreviations: ECOG–PS, Eastern Cooperative Oncology Group Performance Status; NLR, neutrophil-to-lymphocyte ratio; CI confidence interval. The 95% CI for the overall treatment effect HR is 0.5792–1.1191 corresponding to an indifference zone (shaded in grey) of 0.4634–1.3989. Abbreviations: ECOG–PS, Eastern Cooperative Oncology Group Performance Status; NLR, neutrophil-to-lymphocyte ratio; CI confidence interval
The ORR across the entire sample was 31.56% and the DCR was 79.82%. These rates were consistent across the various chemotherapy regimens (p = 0.181 for ORR and p = 0.127 for DCR, respectively, as detailed in Table 3).
Toxicity
Common adverse events observed across all grades in all regimens included fatigue, emesis, anemia, and neutropenia, with neuropathy specifically noted in oxaliplatin-based regimens (Table 4). In terms of grade 3–4 toxicity, neutropenia was most common: FP (28.04%), FOLFOX (27.11%), CP (16.27%), and CAPOX (7.39%). Higher instances of grade 3–4 renal toxicity were observed in cisplatin regimens, with FP at 2.80% and CP at 1.59%, compared to CAPOX (0.43%) and FOLFOX (0.00%). Conversely, grade 3–4 neuropathy was lower in CP (0.00%) and FP (0.93%) than in CAPOX (4.13%) and FOLFOX (7.16%). The incidence of hand–foot syndrome was more prevalent in regimens containing capecitabine, with CP at 45.63% and CAPOX at 32.26%, vs. 5-FU in FOLFOX (11.28%) and FP (7.48%), although the occurrence of grade 3–4 hand–foot syndrome was less than 2% in all regimens. FP showed a higher rate of grade 3–4 mucositis (4.76%), and CP had a higher incidence of grade 3–4 thrombosis (5.95%) compared to other regimens. The rates of hospitalization due to toxicity were as follows: FP (24.04%), FOLFOX (20.14%), CAPOX (19.81%), and CP (17.48%).
Considering the nuanced differences in toxicity profiles across the various drug regimens, a direct comparison was undertaken (refer to Amit plot in Supplementary Fig. 2). Patients receiving oxaliplatin were found to have a lower risk of thrombotic events (RR = 0.64, 95% CI 0.44–0.95, p = 0.026), renal toxicity (RR = 0.39, 95% CI 0.17–0.89, p = 0.021), and neutropenia (RR = 0.78, 95% CI 0.66–0.92, p = 0.004) compared to those treated with cisplatin. On the other hand, oxaliplatin was associated with a higher risk of peripheral neuropathy (RR = 6.50; 95% CI 3.91–10.78; p < 0.001), thrombocytopenia (RR = 1.75; 95% CI 1.10–2.80; p = 0.016), and diarrhea (RR = 1.40; 95% CI 1.02–1.91; p = 0.035). Anemia (RR = 0.76; 95% CI 0.63–0.91; p = 0.003), stomatitis (RR = 0.60, 95% CI 0.42–0.87, p = 0.016), and neutropenia (RR = 0.57; 95% CI 0.48–0.67; p < 0.001) were less prevalent in patients treated with capecitabine compared with 5-FU, while hand–foot syndrome (HFS) was more common in those receiving capecitabine (RR = 5.13; 95% CI 2.75–9.62; p < 0.001).
Discussion
The cisplatin–5FU regimen, initially established in the 1980s [27], and subsequently refined over time, has emerged as the benchmark for treating advanced esophagogastric adenocarcinoma, a status affirmed by numerous randomized controlled trials (RCTs) [20, 22, 28,29,30,31,32,33]. The integration of novel agents into this classical regimen is an active area of research, emphasizing the importance of investigating combined efficacy, synergies, and additive toxicities. This knowledge is crucial for optimizing treatment protocols and enhancing clinical outcomes.
In our research, we assessed the efficacy and toxicity profiles of four platinum and fluoropyrimidine-based doublet chemotherapy regimens in first-line treatment for advanced HER2-negative esophagogastric adenocarcinoma, drawing upon data from the AGAMENON–SEOM national esophagogastric cancer registry. The results demonstrate comparable activity in terms of ORR across all chemotherapy schemes. FOLFOX was associated with improved PFS of 6.67 months (HR 0.73; 95% CI 0.58–0.92, p = 0.009) but not OS of 10.61 months (HR 0.90; 95% CI 0.71–1.15, p = 0.045) compared to CP, with PFS of 5.52 months and OS of 10.38 months, showing a consistent effect across all subgroups. This difference was not found between the two oxaliplatin-based regimens, FOLFOX and CAPOX, nor between the two cisplatin-based regimens, FP and CP. Each regimen was associated with specific toxicity profiles: FOLFOX had higher rates of asthenia (77%) and neuropathy (69%); neutropenia was significant in FOLFOX and FP (52% and 55%, respectively); hand–foot syndrome was prominent in CP (46%); and more thromboembolic events were noted in cisplatin-based schemes, FP and CP (12% and 11%, respectively). There were no differences in emesis, and diarrhea was slightly lower with CP. These findings suggest that selecting a chemotherapy regimen should consider its toxicity profile to improve treatment tolerance, continuity, and patient quality of life.
Our data align with previous literature in indicating that regimens containing oxaliplatin, particularly FOLFOX, outperform those with cisplatin, and contribute to reinforcing this evolving hypothesis [34]. In a study by the German AIO group, FOLFOX was found to have lower toxicity compared to FP, suggesting enhanced efficacy in older adults [35]. Further supporting this, the REAL2 RCT's secondary analysis showed EOX outperforming ECF in OS, 11.2 vs. 9.9 months (HR 0.80, 95% CI 0.66–0.97; p = 0.02), without significant differences in PFS and ORR among the regimens [6]. Notably, compared to cisplatin, oxaliplatin was associated with less frequent occurrences of severe neutropenia, alopecia, renal toxicity, and thromboembolism, though it showed a modest increase in severe diarrhea and neuropathy. Consistently, the Serbian Oncology and Radiology Institute's RCT comparing FOLFOX with FP favored the oxaliplatin regimen in terms ORR and OS, with a longer time to progression. However, the result was borderline significant (p = 0.073) [36]. This RCT also reported higher rates of severe hematologic and gastrointestinal toxicities with FP. Taken together, the data point to the superiority of oxaliplatin, with a meta-analysis including these three studies, all from 2008, highlighting a modest survival advantage over cisplatin (HR for death 0.88; 95% CI 0.78–0.99).
[18]. An earlier analysis of the AGAMENON–SEOM registry, focusing on HER2-positive tumors, found comparable results with ToGA and CAPOX–trastuzumab regimens, while suggesting a potential advantage of FOLFOX–trastuzumab, particularly for those subtypes that typically exhibit less sensitivity to trastuzumab. However, this potential benefit requires further validation through RCTs [37].
From 2010 to 2019, several RCTs attempted to improve the effectiveness of platinum–fluoropyrimidine doublets by incorporating targeted drugs, but these efforts did not yield favorable results [28,29,30,31]. In contemporary trials, there's a notable trend of equating all platinum and fluoropyrimidine doublets under the assumption that any differences between existing options are thought to be minimal. As a result, the selection of a chemotherapy regimen has commonly been based on toxicity, considering the profile of the experimental drug [31, 38] the potential interaction with the immune system, or dosing schedules (bi-weekly or tri-weekly) to enhance efficacy and tolerance when combined with new drugs.
In the phase 3 RCT CHECKMATE-649, oxaliplatin-based regimens, FOLFOX and CAPOX, were tested alongside nivolumab administered either bi-weekly or tri-weekly [20]. Oxaliplatin was preferred over cisplatin due to its potentially better toxicity profile. Among patients with PD-L1 ≥ 5, there was no significant difference in OS when comparing different chemotherapy combinations. Specifically, OS was 14.3 months for FOLFOX with nivolumab versus 11.3 months for FOLFOX alone (HR 0.71, 0.57–0.88), and 15 months for CAPOX with nivolumab versus 11 months for CAPOX alone (HR 0.69, 0.55–0.85), p = 0.9. The phase 3 RCT KEYNOTE-859 compared tri-weekly chemotherapy regimens, CAPOX (86% of the sample) or FP, each combined with pembrolizumab, finding no differences in activity between both in subgroup analysis [21]. Finally, a network meta-analysis involving eight phase 3 RCTs examined the efficacy and safety of PD-1 inhibitors combined with either oxaliplatin- or cisplatin-based chemotherapy as first-line treatment for advanced gastric cancer [39]. In tumors with a CPS ≥ 1, oxaliplatin combinations showed enhanced efficacy (HR 0.75, 0.57–0.99). PFS was more prolonged with oxaliplatin compared to cisplatin (HR 0.72, 0.53–0.99), with no significant difference in ORR (RR 1.09, 0.74–1.61). This clinical observation was suggested to potentially stem from the stronger immunogenic cell death effect of oxaliplatin. The rate of severe side effects was comparable between both regimens (RR 0.86, 0.66–1.12). However, these results are preliminary and primarily hypothesis-generating, as only two of the RCTs in the meta-analysis were international, and out of 5723 patients studied, only 250 were treated with cisplatin in these trials.
The phase 3 SPOTLIGHT and GLOW RCTs combined oxaliplatin-based regimens, FOLFOX and CAPOX, with zolbetuximab (anti-CLDN18.2 +) [33]. This choice could be due to zolbetuximab's high emetogenic profile, making its combination with cisplatin less advisable. Our analysis validates this approach, revealing a higher incidence of emesis with cisplatin-based regimens compared to those with oxaliplatin. The SPOTLIGHT RCT showed an OS of 18.23 months with the addition of zolbetuximab versus 15.54 months with FOLFOX (HR 0.75, p = 0.0053). The GLOW RCT reported an OS of 14.39 months in the zolbetuximab plus CAPOX group, compared to 12.16 months with CAPOX alone (HR 0.77, p = 0.0118). While not directly comparable studies, FOLFOX achieved better results both in monotherapy and combined with zolbetuximab.
The trends shown here occur while controlling for multiple covariates with already elucidated prognostic effects. An ECOG–PS > 1, more than two metastatic locations, the presence of ascites, bone metastases, hypoalbuminemia, and a raised NLR were identified as poor prognostic factors without distinct subgroup effects linked to any covariate among the studied chemotherapy regimens. These factors have been previously described in works from this registry [24], with a particular emphasis on their significance in HER2-positive tumors [37], a conclusion that is supported by prior meta-analyses in this field [40].
Other relevant factors for future research consideration are infusion times and the direct and indirect costs of different regimens. Protocols that include capecitabine do not require hospital day time due to oral administration, and oxaliplatin has shorter administration time than cisplatin, avoiding the pre- and post-hydration needed to prevent tubulopathy. Furthermore, the economic impact of toxicity, such as costs from hospitalizations or emergency visits due to adverse events, can influence the overall cost of each regimen. In our analysis, around 20% of patients in each group were hospitalized due to toxicity, with the FP regimen showing the highest rate (24%) and CP the lowest (17%).
The most notable limitation of this study is its retrospective nature. Progression and mortality are endpoints often reliably recorded in medical records, whereas toxicity is a more nuanced endpoint. It is subject to greater uncertainty in retrospective studies and may be influenced by variability in data collection among different investigators and centers. Second, the choice of chemotherapy treatment and the timing of computed tomography scans were based on individual center criteria. Finally, it is crucial to note that our series does not include HER2-positive cases or chemotherapy combinations with other agents. Therefore, we cannot assess how our findings might compare with regimens combined with trastuzumab, immunotherapy, or a biological agent.
In conclusion, the AGAMENON–SEOM series data, encompassing 1293 patients with advanced HER2-negative esophagogastric adenocarcinoma, reveals that FOLFOX is superior in PFS compared to CP. The adverse effect profiles of the platinum-based regimens differ, with neuropathy more prevalent in oxaliplatin and thromboembolic events more common in cisplatin.
Data availability
Available upon request to the authors.
References
Martín-Richard M, Carmona-Bayonas A, Custodio AB, Gallego J, Jiménez-Fonseca P, Reina JJ, et al. SEOM clinical guideline for the diagnosis and treatment of gastric cancer (GC) and gastroesophageal junction adenocarcinoma (GEJA). Clin Transl Oncol. 2019;22(2):236–44.
Cancer Stat Facts Stomach Cancer. In: SEER. Bethesda (MD): National Cancer Institute. https://seer.cancer.gov/statfacts/html/stomach.html. Accessed 22 Dec 2023.
Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;29:8.
Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et al. Randomized Trial Comparing Epirrubicin, Cisplatin, and Fluorouracil versus Fluorouracil, Doxorrubicin, and Methotrexate in Advanced Esofagogastric Cancer. J Clin Oncol. 1997;15:261–7.
Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18(14):2648–57.
Cunningham D, Okines AFC, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2010;362(9):858–9.
Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20(4):666–73.
Moehler M, Kanzler S, Geissler M, Raedle J, Ebert MP, Daum S, et al. A randomized multicenter phase II study comparing capecitabine with irinotecan or cisplatin in metastatic adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2010;21(1):71–7.
Dank M, Zaluski J, Barone C, Valvere V, Yalcin S, Peschel C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19(8):1450–7.
Bouché O, Raoul JL, Bonnetain F, Giovannini M, Etienne PL, Lledo G, et al. Randomized Multicenter Phase II Trial of a Biweekly Regimen of Fluorouracil and Leucovorin (LV5FU2), LV5FU2 Plus Cisplatin, or LV5FU2 Plus Irinotecan in Patients With Previously Untreated Metastatic Gastric Cancer: A Fédération Francophone de Cancérologie Digestive Group Study—FFCD 9803. J Clin Oncol. 2004;22(21):4319–28.
ESMO Guidelines Committee, Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Electronic address: clinicalguidelines@esmoorg Gastric cancer ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–20.
Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Quality of Life With Docetaxel Plus Cisplatin and Fluorouracil Compared With Cisplatin and Fluorouracil From a Phase III Trial for Advanced Gastric or Gastroesophageal Adenocarcinoma: The V-325 Study Group. J Clin Oncol. 2007;25(22):3210–6.
Roth AD, Fazio N, Stupp R, Falk S, Bernhard J, Saletti P, et al. Docetaxel, Cisplatin, and Fluorouracil; Docetaxel and Cisplatin; and Epirubicin, Cisplatin, and Fluorouracil As Systemic Treatment for Advanced Gastric Carcinoma: A Randomized Phase II Trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007;25(22):3217–23.
ter Veer E, Haj Mohammad N, van Valkenhoef G, Ngai LL, Mali RMA, Anderegg MC, et al. The Efficacy and Safety of First-line Chemotherapy in Advanced Esophagogastric Cancer: A Network Meta-analysis. J Natl Cancer Inst. 2016;30(10):108.
Zaanan A, Bouche O, de la Fouchardiere C, Samalin-Scalzi E, Le Malicot K, Pernot S, et al. LBA77 5-fluorouracil and oxaliplatin with or without docetaxel in the first-line treatment of HER2 negative locally advanced (LA) unresectable or metastatic gastric or gastro-esophageal junction (GEJ) adenocarcinoma (GASTFOX-PRODIGE 51): A randomized phase III trial sponsored by the FFCD. Ann Oncol. 2023;34:S1318.
Zhu X, Huang M, Wang Y, Feng W, Chen Z, He Y, et al. XELOX doublet regimen versus EOX triplet regimen as first-line treatment for advanced gastric cancer: An open-labeled, multicenter, randomized, prospective phase III trial (EXELOX). Cancer Commun. 2022;42(4):314–26.
Petrelli F, Zaniboni A, Coinu A, Cabiddu M, Ghilardi M, Sgroi G, et al. Cisplatin or Not in Advanced Gastric Cancer: A Systematic Review and Meta-Analysis. PLoS One. 2013;8(12):e83022.
Montagnani F, Turrisi G, Marinozzi C, Aliberti C, Fiorentini G. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2011;14(1):50–5.
Bang YJ, van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40.
Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(11):1181–95.
Rha SY, Wyrwicz L, Yanez Weber PE, Bai Y, Ryu MH, Lee J, et al. KEYNOTE-859 study of pembrolizumab plus chemotherapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer Outcomes in the protocol-specified PD-L1–selected populations. J Clin Oncol. 2023;1:4014–4014.
Petracci F, Ghai C, Pangilinan A, Suarez LA, Uehara R, Ghosn M. Use of real-world evidence for oncology clinical decision making in emerging economies. Future Oncol. 2021;17(22):2951–60.
Custodio A, Carmona-Bayonas A, Jiménez-Fonseca P, Sánchez ML, Viudez A, Hernández R, et al. Nomogram-based prediction of survival in patients with advanced oesophagogastric adenocarcinoma receiving first-line chemotherapy: a multicenter prospective study in the era of trastuzumab. Br J Cancer. 2017;116(12):1526–35.
Alvarez-Manceñido F, Jimenez-Fonseca P, Carmona-Bayonas A, Arrazubi V, Hernandez R, Cano JM, et al. Is advanced esophageal adenocarcinoma a distinct entity from intestinal subtype gastric cancer? Data from the AGAMENON-SEOM Registry. Gastric Cancer. 2021;24(4):926–36.
Hahn AW, Dizman N, Msaouel P. Missing the trees for the forest: most subgroup analyses using forest plots at the ASCO annual meeting are inconclusive. Ther Adv Med Oncol. 2022;1(14):175883592211031.
Lacave AJ, Barón FJ, Antón LM, Estrada E, De Sande LM, Palacio I, Esteban E, Gracia JM, Buesa JM, Fernández OA, et al. Combination chemotherapy with cisplatin and 5-fluorouracil 5-day infusion in the therapy of advanced gastric cancer: a phase II trial. Ann Oncol. 1991;2(10):751–4.
Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in Combination With Chemotherapy As First-Line Therapy in Advanced Gastric Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase III Study. J Clin Oncol. 2011;29(30):3968–76.
Fuchs CS, Shitara K, Di Bartolomeo M, Lonardi S, Al-Batran SE, Van Cutsem E, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):420–35.
Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):490–9.
Shah MA, Bang YJ, Lordick F, Alsina M, Chen M, Hack SP, et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma. JAMA Oncol. 2017;3(5):620.
Shah MA, Shitara K, Ajani JA, Bang YJ, Enzinger P, Ilson D, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023;29(8):2133–41.
Shitara K, Lordick F, Bang YJ, Enzinger P, Ilson D, Shah MA, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN182-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2023;401(10389):1655–68.
Enzinger PC, Burtness BA, Niedzwiecki D, et al. CALGB 80403 (Alliance)/E1206: A Randomized Phase II Study of Three Chemotherapy Regimens Plus Cetuximab in Metastatic Esophageal and Gastroesophageal Junction Cancers. J Clin Oncol. 2016;34(23):2736–42.
Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26(9):1435–42.
Popov I, Radosevic-Jelic L, Jezdic S, Milovic M, Borojevic N, Stojanovic S, et al. Biweekly oxaliplatin, fluorouracil and leucovorin versus cisplatin, fluorouracil and leucovorin in patients with advanced gastric cancer. J BUON. 2008;13(4):505–11.
Jimenez-Fonseca P, Carmona-Bayonas A, Martinez-Torron A, Alsina M, Custodio A, Serra O, et al. External validity of clinical trials with diverse trastuzumab-based chemotherapy regimens in advanced gastroesophageal adenocarcinoma: data from the AGAMENON-SEOM registry. Ther Adv Med Oncol. 2021;17(13):175883592110196.
Shitara K, Xu RH, Moran DM, Guerrero A, Li R, Pavese J, et al. Global prevalence of CLDN18.2 in patients with locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma Biomarker analysis of two zolbetuximab phase 3 studies (SPOTLIGHT and GLOW). J Clin Oncol. 2023;41(16):4035–4035.
Guo X, Yang B, He L, Sun Y, Song Y, Qu X. PD-1 inhibitors plus oxaliplatin or cisplatin-based chemotherapy in first-line treatments for advanced gastric cancer: A network meta-analysis. Front Immunol. 2022;8(13):905651.
ter Veer E, Creemers A, de Waal L, van Oijen MGH, van Laarhoven HWM. Comparing cytotoxic backbones for first-line trastuzumab-containing regimens in human epidermal growth factor receptor 2-positive advanced oesophagogastric cancer: A meta-analysis. Int J Cancer. 2018;143(2):438–48.
Acknowledgement
The AGAMENON registry, a project of the Evaluation of Results and Clinical Practice Group within the Spanish Society of Medical Oncology (SEOM), values SEOM’s unwavering support. Our appreciation goes to Natalia Cateriano, Miguel Vaquero, and IRICOM S.A. for their assistance with the registry’s website. We’re grateful to all patients, centers, and investigators who participated. We extend our gratitude to the Pharmacy Doctoral Programme at the University of Granada, as this article forms a foundational component of A.A.M.’s doctoral research.
Funding
None to declare; this is an academic study. The study was supported by the authors themselves.
Author information
Authors and Affiliations
Contributions
AAM, PJF, FAM, ACB and JG developed the project, analyzed the data, and drafted the manuscript. The other authors recruited patients and provided clinical information, comments, and improvements to the manuscript. All authors participated in the interpretation and discussion of data, and the critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding the scope of this article.
Ethical approval and consent to participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. This observational, non-interventional study received approval from both the Research Ethics Committee of the Principality of Asturias and the Spanish Agency of Medicines and Medical Devices (AEMPS) under the identifier EFP-AGA-2014–01.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arias-Martinez, A., Martínez de Castro, E., Gallego, J. et al. Is there a preferred platinum and fluoropyrimidine regimen for advanced HER2-negative esophagogastric adenocarcinoma? Insights from 1293 patients in AGAMENON–SEOM registry. Clin Transl Oncol 26, 1674–1686 (2024). https://doi.org/10.1007/s12094-024-03388-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-024-03388-6