Abstract
Background
Advanced esophageal adenocarcinoma (EAC) is generally treated similarly to advanced gastroesophageal junction (GEJ-AC) and gastric (GAC) adenocarcinomas, although GAC clinical trials rarely include EAC. This work sought to compare clinical characteristics and treatment outcomes of advanced EAC with those of GEJ-AC and GAC and examine prognostic factors.
Patients and methods
Participants comprised patients with advanced EAC, intestinal GEJ-AC, and GAC treated with platin and fluoropyrimidine (plus trastuzumab when HER2 status was positive). Overall and progression-free survival were estimated using the Kaplan–Meier method. Cox proportional hazards regression gauged the prognostic value of the AGAMENON model.
Results
Between 2008 and 2019, 971 participants from the AGAMENON-SEOM registry were recruited at 35 centers. The sample included 67.3% GAC, 13.3% GEJ-AC, and 19.4% EAC. Pulmonary metastases were most common in EAC and peritoneal metastases in GAC. Median PFS and OS were 7.7 (95% CI 7.3–8.0) and 13.9 months (12.9–14.7). There was no difference in PFS or OS between HER2− and HER2+ tumors from the three locations (p > 0.05). Five covariates were found to be prognostic for the entire sample: ECOG-PS, histological grade, number of metastatic sites, NLR, and HER2+ tumors treated with trastuzumab. In EAC, the same variables were prognostic except for grade. The favorable prognosis for HER2+ cancers treated with trastuzumab was homogenous for all three subgroups (p = 0.351) and, after adjusting for the remaining covariates, no evidence supported primary tumor localization as a prognostic factor (p = 0.331).
Conclusion
Our study supports the hypothesis that EAC exhibits clinicopathological characteristics, prognostic factors, and treatment outcomes comparable to intestinal GEJ-AC and GAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Advanced esophageal adenocarcinoma (EAC) is usually treated in much the same way as gastroesophageal junction (GEJ-AC) and gastric adenocarcinoma (GAC). However, the distinction for advanced EAC treatment in clinical guidelines is based on very few specific randomized clinical trials (RCT) for this cancer subtype [1,2,3,4]. Most of the evidence available for the treatment of advanced EAC comes from GAC RCT in which esophageal cancer patients are included in an arbitrary, heterogeneous way. In first line, most phase III GAC RCT that examined various chemotherapy schemes have included GEJ tumors and only the REAL2 study included 34.2% of EAC [5,6,7,8,9,10]. While the phase III TOGA RCT, demonstrated the benefit of adding trastuzumab to chemotherapy for cancers that overexpress HER2 (HER2+), only 20% of the study population had GEJ-AC and 0 had EAC [11].
Therefore, we are treating individuals with EAC with no real insight into the true impact these treatments have on this disease. What does appear to be clear at present is that squamous cell carcinoma of the esophagus differs biologically from EAC and that both histological subtypes respond differently to immunotherapy and, probably, to chemotherapy as well [1, 2, 12, 13]. Consequently, future esophageal cancer RCTs should be designed to separate squamous-cell carcinoma from adenocarcinoma histologies.
EAC arises from gland cells present in the lower third of the esophagus when epithelial cells transform into a kind of intestinal cell, a condition known as Barrett’s esophagus [14]. The (TCGA) The Cancer Genome Atlas Research Network classification system, has recognized molecular features of EAC, which closely resemble those of chromosomal instable (CIN) gastric cancer, albeit with a gradient of molecular alterations throughout the upper gastrointestinal tract with as yet unknown clinical correlation and treatment implications [14,15,16,17,18]. Furthermore, Lauren’s diffuse subtype esophagogastric adenocarcinoma displays specific clinical, histopathological, molecular, and therapeutic characteristics, regardless of having been traditionally included indistinctly with intestinal-type adenocarcinoma [15, 19, 20].
In light of the afore and based on the AGAMENON-SEOM registry, we have compared the clinical characteristics and treatment outcomes of advanced EAC with those of intestinal-type GEJ-AC and GAC. Our aim has been to answer the question of whether we should consider EAC as a distinct entity or if there are arguments that justify its being integrated into the rest of non-diffuse esophagogastric adenocarcinoma with respect to its therapeutic approach.
Methods
Patient selection criteria
Cases belong to the ambispective AGAMENON-SEOM registry to which 35 Spanish centers contribute. This registry comprises adult patients (aged ≥ 18 years) with pathologically confirmed, unresectable, or metastatic gastric, gastroesophageal junction, or distal esophageal adenocarcinoma, who received at least one cycle of polychemotherapy [20, 21]. Eligibility criteria for this analysis included unresectable or metastatic, EAC, Lauren’s intestinal GAC and GEJ-AC who received first-line chemotherapy with a platin- and fluoropyrimidine-based regimen, associated with trastuzumab in the case of HER2+ tumors. The most relevant exclusion criteria were the absence of at least 3 months of follow-up (except for patients who were deceased prior to 3 months), fewer than 6 months since completing some kind of adjuvant or neoadjuvant therapy, and the presence of other synchronous tumors. Individuals with diffuse subtype, signet ring cells and Lauren mixed GEJ-AC or GAC were excluded for this analysis. Furthermore, given that only patients with HER2− gastric cancer tend to receive triple-drug schedule with anthracyclines and taxanes in regular clinical practice, these regimens were excluded from the analysis.
Variables
The data evaluated included basal demographic (age, sex), clinical (Eastern Cooperative Oncology Group performance status (ECOG-PS), comorbidities), tumor-related (stage, number, and site of metastases, location (esophagus, EGJ, stomach)), histopathological (histological grade, HER2 status), and laboratory (albumin, alkaline phosphatase, bilirubin, LDH, CEA, neutrophil–lymphocyte ratio (NLR), hemoglobin, platelets) variables.
The classification according to the location of the primary tumor was locally assessed as routinely performed at each center, and no centralized review was carried out. Regardless of the date of the cancer diagnosis, esophagogastric adenocarcinoma was staged by the 8th TNM classification that defines tumor site based on where its center is located, instead of where its proximal edge is. Tumours were classified as GEJ-AC when the epicentre was within the proximal 2 cm of the cardias (Siewert types I/II) and as GAC when the epicentre was more than 2 cm distal from the GEJ [22].
The prognostic factors contemplated were those of the AGAMENON nomogram; i.e., HER2+ tumors treated with trastuzumab, ECOG-PS, number of metastatic sites, bone metastases, ascites, histological grade, and neutrophil-to-lymphocyte ratio (NLR) [21].
Overall survival (OS) and progression-free survival (PFS) were defined from initiation of chemotherapy until demise, progression, or loss to follow-up.
Statistics
Qualitative variables and use of chemotherapy regimens are reported as percentages and were compared by means of Chi-square test. Quantitative variables are reported in terms of the mean and standard deviation or the median and confidence interval (CI) and were compared using ANOVA.
OS and PFS were estimated by the Kaplan–Meier method and compared using the log-rank test. Cox’s multivariate proportional hazards regression with AGAMENON model prognostic factors and tumor site as covariates were used [21]. Likewise, first-order interaction between tumor site and trastuzumab treatment effect was utilized in the regression model. Multiple imputation predictive mean matching by chained equations was chosen as the multiple imputation method for missing values [23]. All statistical assessments were two-sided and p values < 0.05 were deemed significant. Statistical analyses were performed using Stata 16.1.
Results
Baseline characteristics according to primary tumor site
At the time of this analysis (May 2020), the registry contained 4052 patients with advanced esophagogastric cancer of which 971 met the selection criteria for this analysis (Fig. 1); 31% were retrospective cases.
The primary tumor was located in the stomach in 67.3% of the cases (n = 654); in the GEJ in 13.3% (n = 129), and in the esophagus in 19.4% (n = 188). Baseline characteristics are displayed in Table 1. Being male was more common in all subtypes and sites, especially in EAC (93.6%), which had the largest proportion of younger patients (< 65 years, 61.7%). Performance status and comorbidities were similar across individuals with cancers of all three locations; similarly, the proportion of locally advanced unresectable disease, distribution of tumor grades, as well as the number of metastatic sites were comparable. Metastases were uniformly distributed except for pulmonary metastases, more common in EAC (31.4%) and GEJ (29.5%) than in GAC (15%), and peritoneal metastases, more frequent in GAC (34.7%) than in EAC (11.2%) and GEJ-AC (17.8%). Ascites was uncommon in EAC, eight subjects had mild and one had moderate-severe ascites. EGJ-AC was associated with more HER2+ cancers (45%), followed by EAC (33.5%) and GAC (30.1%). Biochemical and CEA values were similar in all subgroups. Anemia was present in more individuals with EAC (69.7%) at diagnosis than in those with EGJ-AC (36.4%) or GAC (57.6%).
Resection of the primary tumor was carried out in a similar proportion of the sample (25–29%); likewise, the use of chemotherapy regimens based on oxaliplatin (61–67%) and on cisplatin (33–39%) was comparable to treat cancers in all three locations.
Baseline characteristics according to localization and first-line treatment
Table 1 shows the patient distribution by primary tumor site and HER2 status. There was little difference between HER2+ and HER2− adenocarcinoma both overall and in each of the locations. The exception is the higher percentage of individuals < 65 years (71.4%) in the HER2+ EAC subgroup compared to 56.8% in HER2− EAC.
In all three primary locations, more grade 1 cancers were HER2+ vs HER2− (27% vs 16.8% in EAC, 27.6% vs 18.3% in GEJ-AC and 28.9% vs 16% in GAC) and more grade 3 tumors were HER2− vs HER2+ (27.2% vs 9.5% in EAC, 19.7% vs 3.4% in GEJ-AC, and 28% vs 15.2% in GAC). For all three sites, the pulmonary spread was more common in HER2+ vs HER2− adenocarcinomas (39.7% vs 27.2% in EAC, 36.2% vs 23.9% in GEJ-AC, and 19.8% vs 13.1% in GAC), whereas peritoneal extension was more frequent in HER2− vs HER2+ (23.9% vs 10.3% in GEJ-AC, and 36.8% vs 29.9% in GAC) except for EAC (8.8% vs 15.9%).
As for treatment, oxaliplatin outweighed cisplatin and capecitabine predominated over 5-FU, particularly in GAC. Oxaliplatin-based schemes were more frequently used to treat HER2− cancers, while cisplatin-based regimens were more common for HER2+ tumors irrespective of location. Chemotherapy schemes are summarized in Appendix Table 3. In all GAC and GEJ-AC and in HER2− EAC, the most widely used strategies were capecitabine and oxaliplatin (CAPOX) and 5-FU and oxaliplatin (FOLFOX), whereas in HER2+ EAC, capecitabine and cisplatin were prescribed more often, followed by CAPOX.
Effect of tumor site on first-line systemic treatment efficacy
With a median follow-up of 44.9 months, 771 patients (79.4%) had died. Median PFS and OS were 7.7 (95% CI 7.3–8.0) and 13.9 months (95% CI 12.9–14.7), respectively. In HER2− tumors, PFS was comparable across the three groups with medians of 6.9 (95% CI 6.2–8.3), 6.9 (4.9–7.8), and 6.9 months (6.4–7.6) for EAC, EGJ-AC, and GAC, respectively (p = 0.125). Likewise, in HER2 + tumors, PFS was comparable, with medians of 8.5 (95% CI 6.8–11.2), 9.0 (8.0–11.0), and 8.9 months (7.9–10.2) for EAC, EGJ-AC, and GAC, respectively (p = 0.874). Figure 2 illustrates Kaplan–Meier PFS curves.
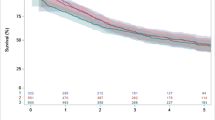
This study did not detect conclusive statistical evidence of dissimilar OS for these three locations. Thus, in HER2− cancers, median OS was 13.4 (95% CI 11.1–15.7), 10.7 (9.7–12.5), and 12.3 months (11.5–13.9) for EAC, GEJ-AC, and GAC, respectively (log-rank test, p = 0.190). In HER2 + cancers, median OS was 15.4 (95% CI, 11.7–19.8), 19.5 (16–28.3), and 16.4 months (14.3–20.4) for EAC, EGJ-AC, and GAC, respectively (p = 0.474). Figure 3 displays Kaplan–Meier OS curves.
Overall survival by location and HER2 status. a Overall survival by location in HER2− adenocarcinomas treated with chemotherapy. b Overall survival by location in HER2+ adenocarcinomas treated with chemotherapy and trastuzumab. Ct chemotherapy, E esophageal adenocarcinoma, GEJ gastroesophageal junction adenocarcinoma, G gastric adenocarcinoma, T trastuzumab
Prognostic factors for overall survival
Cox’s regression model for OS can be seen in Table 2. For the entire sample, five covariates of the AGAMENON nomogram were significantly associated with survival: ECOG-PS, number of metastatic sites, histological grade, NLR, and HER2+ tumors treated with trastuzumab. The favorable prognosis for HER2+ cancers treated with trastuzumab is homogenous across all three tumor sites with hazard ratio (HR) of 0.68 (95% CI 0.48–0.97), 0.47 (0.31–0.71), and 0.64 (0.53–0.78) for esophagus, GEJ, and stomach, respectively (p interaction = 0.351). After adjusting for the other covariates, there was no evidence that tumor localization was a prognostic factor (p = 0.331). In the specific case of EAC, estimates are uncertain due to the low numbers in some subgroups. Even so, the prognostic factors for EAC were ECOG-PS, number of metastatic sites, NLR, ascites, and HER2+ tumors treated with trastuzumab.
Discussion
This analysis based on cases from the AGAMENON-SEOM registry supports EAC is a similar entity to GEJ-AC and intestinal-type GAC that benefits comparably from platin-fluoropyrimidine-based chemotherapy schemes, associating trastuzumab in HER2+ cancers. PFS and OS medians for the series of 7.7 months (95% CI 7.3–8.0) and 13.9 months (12.9–14.7), respectively, are conditioned by the exclusion of Lauren diffuse-type adenocarcinomas, which are specific to gastric cancer and are associated with worse prognosis, as well as responding differently to chemotherapy. Given that diffuse adenocarcinoma is a different entity, it has not been factored into this analysis [15, 19, 20, 24]. Likewise, the exclusion of diffuse subtype cancers determines a high percentage of HER2+ neoplasms in this series: 45%, 33.5%, and 30.1% in GEJ-AC, EAC, and GAC, respectively, versus 15–20% in most reported studies that fail to differentiate by Lauren subtype [15, 25].
In this entire sample, the AGAMENON nomogram variables [21], initially developed for intestinal and diffuse tumors, that maintained their prognostic value were: ECOG-PS, histological grade, number of metastatic sites, NLR, and trastuzumab-treated HER2 + tumors. Even after excluding diffuse cancers with greater disposition toward peritoneal extension, moderate-severe ascites appears to determine a worse prognosis, HR 1.46 (95% CI 0.98–2.19, p = 0.065) as reported in earlier studies [26,27,28].
Notably, nothing evinced an interaction of tumor site with HER2 subtype or with the remaining covariates. This is especially pertinent because, at present, there are no EAC-specific RCT and it is underrepresented in GAC RCT. Additionally, the effect of adding trastuzumab to chemotherapy for HER2+ EAC is uncertain, as these tumors were not included in the TOGA study [16]. Several phase III RCT with chemotherapy and targeted drugs (bevacizumab, panitumumab, cetuximab, rilotumumab, onartuzumab, trastuzumab and pertuzumab) included GAC and GEJ-AC, but not EAC [29,30,31,32,33,34]. All these studies were negative. Only two phase III RCT compared chemotherapy alone against chemotherapy plus a targeted drug in esophagogastric adenocarcinoma. The REAL-3 study with panitumumab (38% EAC) and TRIO-013/LOGiC with lapatinib (5% EAC) were both negative and no site-based subgroup analyses are available [35, 36].
Meanwhile, although inroads into the understanding of the biology of esophagogastric cancer have been made, translation into clinical guidelines or an RCT remains inconsistent. While the 8th and latest TNM classification groups cancer of the esophagus and GEJ (Siewert I and II) as a joint entity [22], clinical guidelines combine epidermoid and adenocarcinoma of the esophagus, and GEJ-AC and GAC [1,2,3,4]. Esophageal cancer guidelines include advanced EAC treatment recommendations based on results of advanced GAC RCT with more restricted options due to the lower grade of evidence in this localization [2]. As such, it would seem more logical to group EAC with advanced GAC in clinical recommendations and develop others for advanced epidermoid carcinoma of the esophagus.
Advances in molecular knowledge will presumably guide us toward a more dynamic, specific treatment aimed at adenocarcinoma of each of these three primary tumor locations [14,15,16,17,18, 37]. At a molecular level, chromosomal instability subtype (CIN) is the most common in esophagogastric adenocarcinoma, especially in the West and in adenocarcinomas of the esophagus (100%, CIN), GEJ (74.6–100%, CIN), and proximal stomach (74.6%, CIN) [14].
This work has the following limitations. Firstly, it is an ambispective sample with 31% retrospective cases. Secondly, the sample was restricted to Lauren intestinal GEJ-AC and GAC and confirmed their similarity to EAC, although we cannot extrapolate the data to diffuse subtype AC. Thirdly, although AGAMENON-SEOM registry derived an extensive series, since esophagogastric adenocarcinoma located in the GEJ and esophagus are uncommon, as are HER2+ adenocarcinoma, the proportion of cases with HER2+ GEJ-AC and EAC is scant, which could compromise the accuracy of the measurements in these cancers. Nevertheless, to the best of our knowledge, this is the first study to compare the results of first-line treatment of cancers of the stomach, GEJ, and esophagus with RWD from a single population. Finally, the reader must be mindful that only dual-agent chemotherapy was administered; consequently, the introduction of additional agents to these schemes may influence the results. Also, the cohort does not contain any HER2+ cases that were not treated with trastuzumab; hence, what has been appraised is the prognostic effect of this variable.
The results of our analysis endorse a similar treatment approach in EAC, intestinal GAC, and GEJ-AC, as well as their inclusion in RCT of intestinal esophagogastric adenocarcinoma as a whole, albeit a basic requirement would be the exclusion of diffuse subtypes or stratification by Lauren type. This study also supports the recommendation of first-line treatment with fluoropyrimidine and platin for advanced EAC and the association of trastuzumab in HER2+ EAC.
In conclusion, our study supports the hypothesis that EAC exhibits clinicopathological characteristics, prognostic factors, and treatment outcomes comparable to intestinal GEJ-AC and GAC. Consequently, we believe it is important that future RCT combine esophagogastric adenocarcinomas and contemplate stratification by Lauren type.
Data availability
Available upon request to the authors.
References
Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D, On behalf of the ESMO Guidelines Committee clinicalguidelines@esmo org. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis treatment and follow-up. Ann Oncol. 2016;27:50–7.
Muro K, Lordick F, Tsushima T, Pentheroudakis G, Baba E, Lu Z, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic oesophageal cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS SSO and TOS. Ann Oncol. 2019;30:34–43.
Ajani JA, D Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. NCCN Guidelines Version 3.2020 Gastric Cancer [Internet]. 2020.
Martin-Richard M, Díaz Beveridge R, Arrazubi V, Alsina M, Galan Guzmán M, Custodio AB, et al. SEOM clinical guideline for the diagnosis and treatment of esophageal cancer (2016). Clin Transl Oncol. 2016;18:1179–86.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V25 study group. J Clin Oncol. 2006;24:4991–7.
Kang Y-K, Kang W-K, Shin D-B, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol Off J Eur Soc Med Oncol. 2009;20:666–73.
Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–42.
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46.
Dank M, Zaluski J, Barone C, Valvere V, Yalcin S, Peschel C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–7.
Guimbaud R, Louvet C, Ries P, Ychou M, Maillard E, André T, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Fédération Francophone de Cancérologie Digestive, Fédération Nationale des Centres de Lutte Contre le Cancer, and Groupe Coopérateur Multidisciplinaire en Oncologie) study. J Clin Oncol. 2014;32:3520–6.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506–17.
Shah MA, Adenis A, Enzinger PC, Kojima T, Muro K, Bennouna J, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase 3 KEYNOTE-181 study. J Clin Oncol. 2019;37:4010–4010.
Kim J, Bowlby R, Mungall AJ, Robertson AG, Odze RD, Cherniack AD, et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–74.
Nagaraja AK, Kikuchi O, Bass AJ. Genomics and targeted therapies in gastroesophageal adenocarcinoma. Cancer Discov. 2019;9(12):1656–72.
Wang Q, Liu G, Hu C. Molecular classification of gastric adenocarcinoma. Gastroenterol Res. 2019;12:275–82.
Testa U, Castelli G, Pelosi E. Esophageal cancer: genomic and molecular characterization, stem cell compartment and clonal evolution. Med. 2017;4:67.
Chia NY, Tan P. Molecular classification of gastric cancer. Ann Oncol. 2016;27(5):763–9.
Iyer P, Moslim M, Farma JM, Denlinger CS. Diffuse gastric cancer: histologic, molecular, and genetic basis of disease. Transl Gastroenterol Hepatol. 2020;5:52.
Jiménez Fonseca P, Carmona-Bayonas A, Hernández R, Custodio A, Cano JM, Lacalle A, et al. Lauren subtypes of advanced gastric cancer influence survival and response to chemotherapy: real-world data from the AGAMENON National Cancer Registry. Br J Cancer. 2017;117:775–82.
Custodio A, Carmona-Bayonas A, Jiménez-Fonseca P, Sánchez ML, Viudez A, Hernández R, et al. Nomogram-based prediction of survival in patients with advanced oesophagogastric adenocarcinoma receiving first-line chemotherapy: a multicenter prospective study in the era of trastuzumab. Br J Cancer. 2017;116:1526–35.
Brierley JD, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 8th ed. Oxford; 2017.
White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–99.
Ma J, Shen H, Kapesa L, Zeng S. Lauren classification and individualized chemotherapy in gastric cancer. Oncol Lett. 2016;11:2959–64.
Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, Jäger E, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA international collaborative analysis. Ann Oncol. 2012;23:2656–62.
Chau I, Ashley S, Cunningham D. Validation of the Royal Marsden Hospital prognostic index in advanced esophagogastric cancer using individual patient data from the REAL 2 study. J Clin Oncol. 2009;27(19):e3–4.
Kim JG, Ryoo BY, Park YH, Kim BS, Kim TY, Im YH, et al. Prognostic factors for survival of patients with advanced gastric cancer treated with cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2008;61:301–7.
Lee J, Lim T, Uhm JE, Park KW, Park SH, Lee SC, et al. Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol. 2007;18:886–91.
Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–76.
Fuchs CS, Shitara K, Di Bartolomeo M, Lonardi S, Al-Batran SE, Van Cutsem E, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:420–35.
Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–9.
Catenacci DVT, Tebbutt NC, Davidenko I, Murad AM, Al-Batran SE, Ilson DH, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1467–82.
Shah MA, Bang YJ, Lordick F, Alsina M, Chen M, Hack SP, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the MET gastric randomized clinical trial. JAMA Oncol. 2017;3:620–7.
Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19:1372–84.
Waddell T, Chau I, Cunningham D, Gonzalez D, Frances A, Okines C, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–9.
Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC-a randomized phase III trial. J Clin Oncol. 2016;34:443–51.
Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9.
Acknowledgements
AGAMENON registry is part of the Evaluation of Results and Clinical Practice Section included in the Spanish Society of Medical Oncology (SEOM); we are grateful to them for their logistical support of this project. Priscilla Chase Duran for editing the manuscript. Natalia Cateriano, Miguel Vaquero, and IRICOM S.A. for supporting the registry website. We are indebted to all patients, as well as to AGAMENON centers and investigators who participated in this research and made it possible.
Funding
None to declare, this is an academic study. The study was supported by the authors themselves.
Author information
Authors and Affiliations
Contributions
FAM, PJF, ACB and JG developed the project, analyzed the data and drafted the manuscript. The other authors recruited patients and provided clinical information, comments, and improvements to the manuscript. All authors participated in the interpretation and discussion of data, and the critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding the scope of this article.
Ethical approval and consent to participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients before they were included in the study. Ethics committee Hospital General Universitario José María Morales Meseguer approved the study (C.P.AGAMENON-C.I.EST:30/14, 26 November 2014).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Rights and permissions
About this article
Cite this article
Alvarez-Manceñido, F., Jimenez-Fonseca, P., Carmona-Bayonas, A. et al. Is advanced esophageal adenocarcinoma a distinct entity from intestinal subtype gastric cancer? Data from the AGAMENON-SEOM Registry. Gastric Cancer 24, 926–936 (2021). https://doi.org/10.1007/s10120-021-01169-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-021-01169-6