Abstract
Purpose
SMEK1, also known as PP4R3α, the regulatory subunit 3α of serine and threonine phosphatase PP4, participates in diversely critical biological processes such as the integration of centromere, deacetylation of histones, asymmetric divisions of neuroblast, and other crucial cellular activities. SMEK1 was formerly reported to play a part in carcinogenesis. This study aims to reveal the role of SMEK1 in lung adenocarcinoma and the underlying molecular mechanism.
Methods
Using immunohistochemical (IHC) staining, the protein level of SMEK1 in lung adenocarcinoma and adjacent non-tumor tissue was detected. The functional role of SMEK1 in cell proliferation and invasion was explored using cell counting kit-8 and Transwell assay, respectively. Xenograft tumor experiment was used to investigate the effect of SMEK1 on tumor growth in vivo. The alteration of Wnt/β-catenin signaling pathway was detected by Western blotting, quantitative PCR, and dual-luciferase reporter assays.
Results
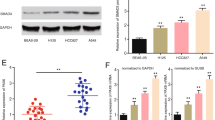
SMEK1 was highly expressed at the protein level in lung adenocarcinoma compared to the adjacent non-tumor tissue. In vitro, suppression of SMEK1 significantly decreased the proliferation, migration, and invasion of lung adenocarcinoma cell lines, while overexpression of SMEK1 enhanced above abilities. The xenograft model demonstrated that down-regulation of SMEK1 significantly inhibited tumor growth in vivo. In addition, we found that SMEK1 could positively regulate Wnt/β-catenin signaling in lung adenocarcinoma cell lines.
Conclusions
SMEK1 exerts a cancer-promoting effect in lung adenocarcinoma by activating Wnt/β-catenin signaling.





Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–87. https://doi.org/10.1016/j.ejca.2018.07.005.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. https://doi.org/10.3322/caac.21708.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. https://doi.org/10.3322/caac.21654.
Gillette MA, Satpathy S, Cao S, Dhanasekaran SM, Vasaikar SV, Krug K, et al. Proteogenomic characterization reveals therapeutic vulnerabilities in lung adenocarcinoma. Cell. 2020;182(1):200–25. https://doi.org/10.1016/j.cell.2020.06.013.
Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377(9):849–61. https://doi.org/10.1056/NEJMra1703413.
Lamberti G, Andrini E, Sisi M, Rizzo A, Parisi C, Di Federico A, et al. Beyond EGFR, ALK and ROS1: current evidence and future perspectives on newly targetable oncogenic drivers in lung adenocarcinoma. Crit Rev Oncol Hematol. 2020;156: 103119. https://doi.org/10.1016/j.critrevonc.2020.103119.
Yang SR, Schultheis AM, Yu H, Mandelker D, Ladanyi M, Buttner R. Precision medicine in non-small cell lung cancer: current applications and future directions. Semin Cancer Biol. 2020. https://doi.org/10.1016/j.semcancer.2020.07.009.
Park J, Lee D-H. Functional roles of protein phosphatase 4 in multiple aspects of cellular physiology: a friend and a foe. BMB Rep. 2020;53(4):181–90. https://doi.org/10.5483/BMBRep.2020.53.4.019.
Gingras AC, Caballero M, Zarske M, Sanchez A, Hazbun TR, Fields S, et al. A novel, evolutionarily conserved protein phosphatase complex involved in cisplatin sensitivity. Mol Cell Proteomics. 2005;4(11):1725–40. https://doi.org/10.1074/mcp.M500231-MCP200.
Mendoza MC, Du F, Iranfar N, Tang N, Ma H, Loomis WF, et al. Loss of SMEK, a novel, conserved protein, suppresses MEK1 null cell polarity, chemotaxis, and gene expression defects. Mol Cell Biol. 2005;25(17):7839–53. https://doi.org/10.1128/MCB.25.17.7839-7853.2005.
Su C, Li Z, Cheng J, Li L, Zhong S, Liu L, et al. The protein phosphatase 4 and SMEK1 complex dephosphorylates HYL1 to promote miRNA biogenesis by antagonizing the MAPK cascade in arabidopsis. Dev Cell. 2017;41(5):527–39. https://doi.org/10.1016/j.devcel.2017.05.008.
Sen I, Zhou X, Chernobrovkin A, Puerta-Cavanzo N, Kanno T, Salignon J, et al. DAF-16/FOXO requires protein Phosphatase 4 to initiate transcription of stress resistance and longevity promoting genes. Nat Commun. 2020;11(1):138. https://doi.org/10.1038/s41467-019-13931-7.
Sousa-Nunes R, Chia W, Somers WG. Protein phosphatase 4 mediates localization of the Miranda complex during Drosophila neuroblast asymmetric divisions. Genes Dev. 2009;23(3):359–72. https://doi.org/10.1101/gad.1723609.
Lipinszki Z, Lefevre S, Savoian MS, Singleton MR, Glover DM, Przewloka MR. Centromeric binding and activity of protein Phosphatase 4. Nat Commun. 2015;6(1):1. https://doi.org/10.1038/ncomms6894.
Lyu J, Jho E-H, Lu W. Smek promotes histone deacetylation to suppress transcription of Wnt target gene brachyury in pluripotent embryonic stem cells. Cell Res. 2011;21(6):911–21. https://doi.org/10.1038/cr.2011.47.
Chang WH, Choi SH, Moon BS, Cai M, Lyu J, Bai J, et al. Smek1/2 is a nuclear chaperone and cofactor for cleaved Wnt receptor Ryk, regulating cortical neurogenesis. Proc Natl Acad Sci USA. 2017;114(50):E10717–25. https://doi.org/10.1073/pnas.1715772114.
Yoon YS, Lee MW, Ryu D, Kim JH, Ma H, Seo WY, et al. Suppressor of MEK null (SMEK)/protein phosphatase 4 catalytic subunit (PP4C) is a key regulator of hepatic gluconeogenesis. Proc Natl Acad Sci USA. 2010;107(41):17704–9. https://doi.org/10.1073/pnas.1012665107.
Dong SM, Byun HJ, Kim BR, Lee SH, Trink B, Rho SB. Tumor suppressor BLU enhances pro-apoptotic activity of sMEK1 through physical interaction. Cell Signal. 2012;24(6):1208–14. https://doi.org/10.1016/j.cellsig.2012.02.002.
Byun HJ, Kim BR, Yoo R, Park SY, Rho SB. sMEK1 enhances gemcitabine anti-cancer activity through inhibition of phosphorylation of Akt/mTOR. Apoptosis. 2012;17(10):1095–103. https://doi.org/10.1007/s10495-012-0751-0.
Kim BR, Seo SH, Park MS, Lee SH, Kwon Y, Rho SB. sMEK1 inhibits endothelial cell proliferation by attenuating VEGFR-2-dependent-Akt/eNOS/HIF-1α signaling pathways. Oncotarget. 2015;6(31):31830–43. https://doi.org/10.18632/oncotarget.5570.
Wang Q, Wang G, Niu L, Zhao S, Li J, Zhang Z, et al. Exosomal MiR-1290 promotes angiogenesis of hepatocellular carcinoma via targeting SMEK1. J Oncol. 2021;2021:6617700. https://doi.org/10.1155/2021/6617700.
Wang B, Zhao A, Sun L, Zhong X, Zhong J, Wang H, et al. Protein phosphatase PP4 is overexpressed in human breast and lung tumors. Cell Res. 2008;18(9):974–7. https://doi.org/10.1038/cr.2008.274.
Zhu K, Dai Z, Pan Q, Wang Z, Yang GH, Yu L, et al. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2011;17(23):7294–302. https://doi.org/10.1158/1078-0432.CCR-11-1327.
Yuan J, Han B, Hu H, Qian Y, Liu Z, Wei Z, et al. CUL4B activates Wnt/beta-catenin signalling in hepatocellular carcinoma by repressing Wnt antagonists. J Pathol. 2015;235(5):784–95. https://doi.org/10.1002/path.4492.
Zhou J, Fan J, Tang Z, Dai Z, Luo R, Jia H, et al. Metadherin–PRMT5 complex enhances the metastasis of hepatocellular carcinoma through the WNT–β-catenin signaling pathway. Carcinogenesis. 2020;41(2):130–8. https://doi.org/10.1093/carcin/bgz065.
Wu HI, Brown JA, Dorie MJ, Lazzeroni L, Brown JM. Genome-wide identification of genes conferring resistance to the anticancer agents cisplatin, oxaliplatin, and mitomycin C. Cancer Res. 2004;64(11):3940–8. https://doi.org/10.1158/0008-5472.Can-03-3113.
Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50(4):649–57. https://doi.org/10.1016/0092-8674(87)90038-9.
Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–5. https://doi.org/10.1038/nature04108.
Behrens J, Jerchow BA, Würtele M, Grimm J, Asbrand C, Wirtz R, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280(5363):596–9. https://doi.org/10.1126/science.280.5363.596.
Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108(6):837–47. https://doi.org/10.1016/s0092-8674(02)00685-2.
Kim JK, Diehl JA. Nuclear cyclin D1: an oncogenic driver in human cancer. J Cell Physiol. 2009;220(2):292–6. https://doi.org/10.1002/jcp.21791.
Ramos-Garcia P, Gil-Montoya JA, Scully C, Ayen A, Gonzalez-Ruiz L, Navarro-Trivino FJ, et al. An update on the implications of cyclin D1 in oral carcinogenesis. Oral Dis. 2017;23(7):897–912. https://doi.org/10.1111/odi.12620.
Montalto FI, De Amicis F. Cyclin D1 in cancer: a molecular connection for cell cycle control, adhesion and invasion in tumor and stroma. Cells. 2020;9(12):1. https://doi.org/10.3390/cells9122648.
Gonzalez-Ruiz L, Gonzalez-Moles MA, Gonzalez-Ruiz I, Ruiz-Avila I, Ayen A, Ramos-Garcia P. An update on the implications of cyclin D1 in melanomas. Pigment Cell Melanoma Res. 2020;33(6):788–805. https://doi.org/10.1111/pcmr.12874.
Qie S, Diehl JA. Cyclin D degradation by E3 ligases in cancer progression and treatment. Semin Cancer Biol. 2020;67(Pt 2):159–70. https://doi.org/10.1016/j.semcancer.2020.01.012.
Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):165. https://doi.org/10.1186/s13045-020-00990-3.
Aguilera KY, Dawson DW. WNT ligand dependencies in pancreatic cancer. Front Cell Dev Biol. 2021;9:671022. https://doi.org/10.3389/fcell.2021.671022.
Zhong ZA, Michalski MN, Stevens PD, Sall EA, Williams BO. Regulation of Wnt receptor activity: implications for therapeutic development in colon cancer. J Biol Chem. 2021;14:100782. https://doi.org/10.1016/j.jbc.2021.100782.
Rapp J, Jaromi L, Kvell K, Miskei G, Pongracz JE. WNT signaling—lung cancer is no exception. Respir Res. 2017;18(1):167. https://doi.org/10.1186/s12931-017-0650-6.
Li XQ, Yang XL, Zhang G, Wu SP, Deng XB, Xiao SJ, et al. Nuclear β-catenin accumulation is associated with increased expression of Nanog protein and predicts poor prognosis of non-small cell lung cancer. J Transl Med. 2013;6(11):114. https://doi.org/10.1186/1479-5876-11-114.
Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138(1):51–62. https://doi.org/10.1016/j.cell.2009.04.030.
Pacheco-Pinedo EC, Durham AC, Stewart KM, Goss AM, Lu MM, Demayo FJ, et al. Wnt/β-catenin signaling accelerates mouse lung tumorigenesis by imposing an embryonic distal progenitor phenotype on lung epithelium. J Clin Invest. 2011;121(5):1935–45. https://doi.org/10.1172/jci44871.
Funding
This work was supported by the National Natural Science Foundation of China (81873878, 32070586, and 81671114) and Shandong Provincial Natural Science Foundation (ZR2020MH086, ZR2020LZL018).
Author information
Authors and Affiliations
Contributions
QJL, JSL, and DDC designed the study. DDC performed the experiments, analyzed the data, and drafted the manuscript. JSL revised the manuscript and helped with English editing. SG, FG, and AL helped with experiments. QJL and JXL contributed essential reagents and tools. QJL and JSL supervised the data collection and the analysis of the data.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethic Committee of Shandong University.
Informed consent
Written informed consent was obtained from participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, D., Gao, S., Gao, F. et al. SMEK1 promotes lung adenocarcinoma proliferation and invasion by activating Wnt/β-catenin signaling pathway. Clin Transl Oncol 25, 976–986 (2023). https://doi.org/10.1007/s12094-022-03001-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-03001-8