Abstract
Endometrial cancer (EC) is one of the most common tumors in the female reproductive system, which seriously threatens women's health, particularly in developed countries. 13% of the patients with EC have a poor prognosis due to recurrence and metastasis. Therefore, identifying good predictive biomarkers and therapeutic targets is critical to enable the early detection of metastasis and improve the prognosis. For decades, extensive studies had focused on glycans and glycoproteins in the progression of cancer. The types of glycans that are covalently attached to the polypeptide backbone, usually via nitrogen or oxygen linkages, are known as N‑glycans or O‑glycans, respectively. The degree of protein glycosylation and the aberrant changes in the carbohydrate structures have been implicated in the extent of tumorigenesis and reported to play a critical role in regulating tumor invasion, metabolism, and immunity. This review summarizes the essential biological role of glycosylation in EC, with a focus on the recent advances in glycomics and glycosylation markers, highlighting their implications in the diagnosis and treatment of EC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the global incidence and mortality of endometrial cancer (EC), regions, such as North America and Europe, rank at the front of global [1]. In the United States, endometrial cancer has been identified as the third most common cancer in women aged between 20 and 39, with annual incidence rates of about 15 to 25 per 100,000 women [2]. Approximately, 65,950 new cases of EC and 12,550 related deaths have been reported in 2021 [3]. But South Africa and several countries in Asia showing the largest increase, such as Japan, the Philippines, and Singapore[4], the age-standardized incidence rate ranges between 5.5 per 100,000 in Central Asia and 70.9 per 100,000 in East Asia. The age-standardized mortality rate ranges between 3.2 per 100,000 in Central Asia and 1.9 per 100,000 in East Asia, even though the death rate in East Asia is the most significant globally [5]. The five-year survival rate for EC patients following appropriate therapy is 80% [6], but the median survival time for stage III–IV EC patients is 9 to 10 months [7]. Patients with higher-stage EC are more likely to suffer from recurrence and mortality, making its prevention increasingly challenging [8]. Therefore, early diagnosis and prediction of prognosis for patients with EC are critical for improving women’s health globally.
Among the various post-translational modifications of proteins, glycosylation is a very important one, which directly interacts with the surroundings or indirectly changes the conformation, stability, and turnover of the proteins [9]. Glycoproteins are widely distributed, including membrane receptors, adhesion molecules, extracellular matrix proteins, intracellular kinases, and transcription factors [10]. With a deeper understanding of glycosylation and the continuous development of mass spectrometry (MS) technology, accumulating data implicates the indispensable role of protein glycosylation in health and disease [11]. High-throughput glycoproteomics technologies have enabled the analysis of thousands of proteins N-glycans in ovarian cancer (OC) [12], providing a platform for the study of glycosylation in EC. However, compared to other tumors, limited studies have investigated the role of protein glycosylation in EC. In this review, we focused on the recent advances in the literature related to glycosylation and glycoproteomics, to better illustrate their roles in the pathogenesis of EC, aiming to identify new tumor-associated glycosylated biomarkers and their clinical applications.
Overview of glycosylation
Definition of glycosylation
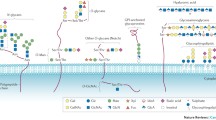
In eukaryotes, the vast majority of protein glycosylation in the cell occurs along the secretory pathway, under the regulation of glycosyltransferases and glycosidases. The carbohydrates are transferred to the amino acid residue on the protein forming a glycosidic bond. The initial synthesis of the peptide chain of the glycoprotein occurs in the ribosome, and most glycoproteins need to enter the endoplasmic reticulum for modification and folding, such as N‑glycans modification, while for the O-glycans, they need to enter the Golgi apparatus [13]. According to the nitrogen or oxygen linkages attached to the polypeptide backbone, the glycoproteins are usually defined as N‑glycans or O‑glycans, respectively. N-glycosylation refers to the amino acid residues of the asparagine side chain in a polypeptide chain that are connected to N-acetylglucosamine (GlcNAc) of the N-glycan chain [14]. While O-glycosylation is a type of glycosylation wherein a carbohydrate group forms an O-glycosidic bond with the hydroxyl group of an amino acid side chain in a peptide chain. The hydroxyl groups that can be used for bonding are mainly the alcoholic hydroxyl groups of serine and threonine, but in some instances, the hydroxyl groups of hydroxylysine and the phenolic hydroxyl group of tyrosine may also be involved. After N-glycosylation or O-glycosylation, a series of fucosylation and sialylation are required to complete the assembly. The addition of sialic acid or fucose moieties to the N-linked or O-linked glycoproteins is one of the most frequently occurring modifications in cancer [15]. The glycosidic bond is different from the above, such as in C-glycosylation, wherein the mannose is linked to the tryptophan through the carbon–carbon bond [16]. If the glycosidic bond modification site is cysteine, it is called S-glycosylation [17]. P-glycosylation involves the attachment of phosphorylated glycans to a serine or threonine and is only observed in lower eukaryotes [18].
Other major classes of glycoconjugates include proteoglycans and glycosphingolipids. Proteoglycan is a protein with a large number of glycosylation modifications and is an important component of the extracellular matrix. Proteoglycans are formed by the covalent attachment of the core protein to one or more glycosaminoglycan chains, and their carbohydrate content is usually higher than that of general glycoproteins. Glycosylphosphatidylinositol is a complex glycolipid composed of mannose, glucosamine, phosphoethanolamine, and inositol phospholipids, which can be covalently linked to the carboxyl terminus of some proteins, anchoring them to the cell membranes for a variety of biological functions [19].
In general, glycans have important biological functions due to their high proportion and wide distribution in cells [19, 20]. Figure 1 shows the synthetic routes for the different types of glycosylation.
Glycosylation changes in cancer and normal cells
Glycosylated proteins participate in various biological processes in the cell. Aberrant glycosylation is closely related to many pathological processes, such as tumorigenesis and inflammatory response [21]. Meanwhile, due to the complexity of glycosylation and substrate binding sites and the diversity of the structure of the carbohydrate chain itself, glycan modifications are protein-specific, site-specific, and cell-specific [22]. In general, several modifications are observed in the glycosylation pathway that occurs in cancer cells, including the aberrant expression of glycoproteins or glycosyl compounds, alterations in the sites and structures where glycans are linked to the amino acids, abnormal localization and expression of the corresponding glycosyltransferases and glycosidases during glycan synthesis, and somatic mutations [23, 24].
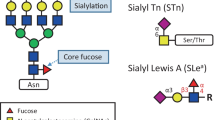
The most frequent changes in glycosylation in cancer are the abnormal sialylation and fucosylation, O-glycan truncation, and N/O-linked glycan branching. Altered sialylation and fucosylation are closely associated with the development and progression of cancer, with the altered sialyltransferase expression leading to the formation of specific sialylated structures [25]. Similar to sialylation modifications, the process of fucosylation relies on a series of fucosyltransferases (FUT1-11). Fucosylation is further divided into two categories, including terminal fucosylation, and core fucosylation. Fucosyltransferase 8 (FTU8) is the most important FTU in mammalian cells, which catalyzes the transfer of GDP-β-L-fucose to the N-sugar chain of Asn in the adjacent N-acetylglucosamine (GlcNAc) residues to form core fucose [26]. Altered expression of polypeptide GalNAc transferases results in the incomplete synthesis of O-glycans, known as truncated-O-glycans, and is observed in about 80% of cancers [27]. The disaccharide Thomsen–Friedenreich antigen (T antigen) and the monosaccharide GalNAc (Tn) and their sialylated forms (sT and sTn) are some of the truncated glycans [28]. What’s more, the frequently occurring N/O-linked glycan branching changes in cancer cells cause the overexpression of complex β1,6-branched N-glycans, as a result of the increased activity of β1,6 N-acetylglucosaminyltransferase V, which is regulated by the RAS/RAF/MAPK signaling pathway in cancer [29].
Research techniques related to glycosylation in gynecological oncology
Proteins may contain multiple glycan modification sites. The type of carbohydrate and occupancy rates at each site may be different, while a specific site may also contain multiple types of glycan structures [30]. Due to the complexity of glycosylation modifications, conventional experimental methods for gene and protein measurements, such as ELISA [31], immunohistochemistry (IHC) [32], and polymerase chain reaction (PCR) [33], are insufficient. The advances in MS-based methods have led to a gradual increase in glycosylation research in recent years, providing an effective and versatile tool for glycan and protein analysis [34]. The current research on glycoproteins is mainly based on three methods: the intact glycoproteins/glycopeptides; the glycopeptide after the glycoprotein is digested by enzyme; and the structure of the glycans released by chemical method or enzyme cleavage method [35]. Before subjecting the samples to MS, some of them undergo release, separation, and enrichment for glycans [36]. Some reviews have specifically described the technical approaches for preparing such samples [37, 38]. Due to the low abundance of glycoproteins and glycosylated peptides in the biological samples, a series of enrichment analyses are conducted before the analysis, such as lectin enrichment and hydrophilic affinity enrichment [39]. Commonly used techniques for characterizing glycans structure include capillary electrophoresis, high-performance liquid chromatography and MS technology, especially the matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF-MS), and electrospray ionization mass spectrometry (ESI/MS) [37, 40]. Although these methods can qualitatively and quantitatively assess the structure of glycosyl and glycopeptides, they lack information regarding the binding sites of glycans and glycopeptides. Therefore, the analysis of intact glycopeptides is more suitable [41]. The widely used tandem MS peptide fragmentation modes include collision-induced dissociation [42], high-energy-induced dissociation, and electron transfer dissociation [43]. The latest updates on the methodology used to detect glycosylation changes in gynecologic oncology were summarized and listed in Table 1.
Role of glycosylation in endometrial cancer
Glycans can alter protein conformation and structure, thereby modulating the functional activity of the protein. In this part, we discuss specific examples to highlight the diverse roles of glycosylation in EC. We also try to unravel the biological significance of glycan-based interactions to decipher the molecular mechanisms of tumorigenesis. The role of glycosylation in EC is presented in Fig. 2.
Glycosylation in tumor invasion and migration
Tumor invasion and metastasis are usually closely associated with the extracellular matrix [65]. Glycoproteins, glycolipids, and glucosamine are important components of the cell surface. The complex carbohydrates attached to the membrane proteins and extracellular matrix proteins, such as E-cadherin [66], integrins [67], Mucin1 (MUC1) [68], and CD44 [69], alter the structure and function of the glycoproteins, as well as intracellular signaling, to promote tumor metastasis. E-cadherin is a widely expressed transmembrane glycoprotein in cancer and is a specific indicator of the loss of epithelial integrity. The reduced expression of E-cadherin in EC associated with deep myometrial invasion and poor differentiation [70]. Kurita et al. showed that positive staining for GalNAc transferase 6 (GalNAc-T6) was significantly associated with positive staining of E-cadherin and advanced grade of EC. Furthermore, the overexpression of GalNAc-T6 enhanced the ability of cell–cell adhesion and the characteristic differentiation found in the early phase of EC invasion [71]. MUC1 is a transmembrane glycoprotein whose glycosylation is altered in the malignant cells, owing to the extracellular heavily glycosylated domain. It has been reported that glycosylation-modified MUC1 promotes tumor growth by regulating the epidermal growth factor receptor (EGFR) pathway in EC cells. Knockdown of MUC1 downregulated the expression of EGFR, and further suppressed EGFR-dependent proliferation, growth, and survival. Additionally, MUC1 knockout cells were more sensitive to lapatinib, an EGFR inhibitor [72]. Integrins showed reduced homogenous adhesion in tumor cells and they are the carriers of N-glycans, which plays a role in mutual recognition and adhesion between cells and the extracellular matrix. Tunicamycin is an N-linked glycosylation inhibitor that reduces MUC1 concentration and inhibits MUC1 glycosylation, and the downregulation of MUC1 increases the expression of α2ß1 integrin to promote cell adhesion [60]. CD44 is a complex transmembrane adhesion glycoprotein, and the adhesion between tumor cells and the host cell’s matrix promotes invasion and metastasis. The glycosylation inhibitor tunicamycin is known to inhibit the glycosylation of CD44 and inhibit the metastatic ability of OC cells [73, 74]. Moreover, the glycosylation modification of CD44 induced by transfection of α1,2-Fuc-T, was reported to enhance cell motility and tumorigenicity in rat carcinoma cells, suggesting similar effects in EC [75, 76].
Glycosylation modification affects the invasion and migration of tumor cells not only through the connection between the extracellular matrix and transmembrane proteins, but also through the regulation of metastasis-related molecular signaling pathways. N-Acetylgalactosaminyltransferase2 (GALNT2) is an enzyme that regulates the initial steps of O-glycosylation of mucin and regulates the malignancy of various cancers. It promotes the malignant characteristics of glioma by regulating the O-glycosylation and phosphorylation of EGFR, further modulating the PI3K/Akt/mTOR axis [77, 78]. Proteomics analysis also showed that GALNT2 was highly expressed in the endometrial hyperplasia group, and was closely related to the activation of the EGFR/Akt/ERK pathway [79]. Not only N-glycosylation and O-glycosylation, but also fucosylation and sialylation play an important role in tumor metastasis. FUT8 catalyzes the addition of fucose unit to the GlcNAc at the end of N-glycans to form core fucosylation, which promotes tumor invasion and migration by regulating downstream pathways, such as TGF-β, EGFR, and Wnt/β-catenin. Other studies reported similar findings in breast cancer [80], small cell lung cancer [81], and hepatocellular carcinoma [82]. Radhakrishnan et al. recently found that the aberrant expression of immature truncated O-glycans played a role in the early onset of cancer, wherein they promoted tumorigenesis by disrupting the basement membrane adhesion and increasing cancer cell proliferation [83]. sTn neo- or over-expression prevents cancer cell growth and adhesion to promote metastasis [28]. Increased levels of sialylated glycans were shown to upregulate the expression of tumor-associated antigens and increase cell detachment through electrostatic repulsion of the negative charges [84]. sTn also inhibits the recognition of cancer cells by the immune cells by preventing the mutual recognition of cell–cell or cell–matrix substances, such as selectins, siglecs, and galectins, thereby protecting the invasion and metastasis ability of tumor cells. The expression level of the T antigen is higher in breast cancer cell lines with higher metastatic ability. Treatment of cancer cells with the synthetic T antigen antagonist, lactulose-L-leucine, was found to decrease cancer cell adhesion [85], further verifying the crucial role of sialic acid glycosylation in tumor metastasis.
Glycosylation with sex hormone imbalance
Endometrial cancer is a hormone-related malignancy, whose pathogenesis is related to several hormone receptors. O- and N-glycosylated modifications are considered important ways to regulate hormone activity. O-linked glycosylation and N-linked glycosylation play roles in signal transduction, in receptor binding regulation and in glycoprotein hormones bioactivity alteration [86]. An estimated 40% of EC cases are related to obesity, due to increased conversion of androstenedione into estrone by the excess of adipose tissue, which exposes the endometrium to continuously high levels of estrogens. Furthermore, type II diabetes and insulin resistance are also known to be risk factors for Type I EC. Hyperinsulinemia and insulin resistance affect the level of sex hormones, promoting the onset and development of EC [87]. Fasting insulin levels, insulin resistance index, follicle-stimulating hormone, luteinizing hormone, and estrogen are the family members of heterodimeric glycoprotein hormones, all of which participate in the development of EC. Studies also revealed that people with higher levels of insulin resistance index, fasting insulin level, and estrogen are more susceptible to EC [88]. It has also been shown that the glycosylation of reproductive hormones is associated with tumorigenesis [89]. Recently, several groups had reported that human chorionic gonadotrophin-β promoted tumor development and progression [90]. Hyperglycosylated human chorionic gonadotrophin and human chorionic gonadotrophin-β had similar effects on the apoptosis of endometrial adenocarcinoma cells [91]. Therefore, the glycosylation of human chorionic gonadotrophin may be involved in the onset and development of EC [92]. Steroid 5 alpha-reductase 3, a highly expressed protein in human hepatocellular carcinoma and cervical cancer, plays a role in the earliest steps of N-linked glycosylation and steroid hormone formation, which may further help us in understanding the role of hormone glycosylation in EC [93].
Glycosylation modification with metabolism
Glucose metabolism is closely related to tumorigenesis and development [94]. Glucose metabolism affects glycosylase and further regulates the glycosylation modifications of protein and its biological functions [95]. 2-Deoxy-D-glucose, an inhibitor that targets glucose metabolism, inhibits the synthesis of N-glycosylation and promotes the apoptosis of tumor cells. Its combination with radiotherapy has synergistic anti-tumor effects [96]. As mentioned earlier, one of the pathogenic characteristics of EC is the increase in glucose metabolism [97]. Abnormal glucose metabolism affects the hexosamine biosynthesis pathway flux, which in turn affects processes such as O-glycosylation, and leads to cellular dysfunction, for example, subjecting EC cell lines to hyperglycemic conditions elevated the activities of the Wnt/β-catenin pathway [77]. Glucose metabolism disorders in diabetic patients were associated with an increased occurrence of EC [97]. Glucose metabolism indicators, such as the body mass index, waist-hip ratio, and insulin resistance index, are all associated with the occurrence of EC [98]. 80% of EC patients are estrogen-dependent type I, and are relatively young patients with accompanying metabolic syndromes, such as obesity, diabetes, hyperinsulinemia, and insulin resistance, further supporting the concept that glucose metabolic disorders promote the occurrence and development of EC [99].
Lipid metabolism disorders are also one of the high-risk factors for EC [94]. Apolipoprotein E (ApoE), an O-glycosylated glycoprotein and part of the high-density lipoprotein, showed antioxidant, anti-inflammatory, and anti-atherogenic properties [100]. The expression of ApoE was found to be altered in gynecological pathologies, such as breast cancer [101], choriocarcinoma [102], and endometrial adenocarcinoma [103], and OC [104]. Studies have reported that the content of ApoE in poorly differentiated EC is 13.1 and 9.7 times higher than that in moderately differentiated and well-differentiated EC, respectively [103]. The structure and degree of glycosylation of ApoE at different positions are different. For example, the degree of C-terminal glycosylation of ApoE in cerebrospinal fluid is elevated [105, 106]. It further affects metabolism by promoting the uptake of cholesterol and high-density lipoprotein [107], and remodeling the tumor microenvironment through extracellular matrix, further increasing the occurrence and development of tumors [108], which may be considered a differentiated factor in gynecological cancers [109].
Glycosylation and immune modulation
In the humoral immune system, almost all of the immunoglobulins and the complement components are glycosylated [110], suggesting that glycosylation plays an indispensable role in the innate and adaptive immune response. Glycan-binding receptors, also known as lectins, are present in the immune cells and participate in tumor invasion, metastasis, and immune escape [13]. The diversity in glycosylation modifications of proteins generates a range of different cancer-associated epitopes [111, 112]. The epitopes change as a result of the altered glycosylation patterns may be unique to cancer cells, and a multitude of monoclonal antibodies to these epitopes have been reported [113]. At the same time, the corresponding antibodies could also undergo glycosylation modifications to exert different biological effects. The glycosylation modification sites at the Fc end of the antibodies are usually the binding sites of Fc receptors and C1q [114]. The changes in glycosylation could increase the binding of Fc receptors and C1q to the antibody, thereby increasing their antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity activity toward the tumor cells [115, 116]. Moreover, researchers found that estradiol treatment elevated the levels of glycosylated epitopes of complement C3 in rat endometrial adenocarcinoma cell lines. Recent studies had demonstrated that sialoglycan-siglec glyco-immune checkpoint interacted with dendritic cells, inducing antibody-dependent cellular cytotoxicity [117, 118]. Siglec-9 inhibits T cell activation by modulating signaling of the T cell receptor [119]. It has also been reported that the increased sialylation of mucin-associated carbohydrates produced by cancer cells caused an asynchronous change in the expression of cyclooxygenase (COX)-2 [120]. The overexpression of Tn and sTn antigens were significantly associated with COX-2 overexpression, which in turn reduced the infiltration of CD8 + T cells and suppressed the host-immune function [120]. Therefore, glycosylation could affect the cytotoxic ability of the immune cells and the expression level of complement factors, which could promote the occurrence and development of EC.
Clinical application of glycosylation in endometrial cancer
Characterization of glycan biomarkers in endometrial cancer
The Cancer Genome Atlas proposed the classification of EC into four subtypes according to the types of gene mutation, including hyper-mutated DNA polymerase ε (POLE), microsatellite-instability high (MSI-H), copy-number low, and copy-number high [121]. Two molecular classification schemes, which are the Translational Research in Post-Operative Radiation Therapy in EC (TransPORTEC) molecular classification system [122] and the Proactive Molecular Risk Classifier for EC (ProMisE) [123], were established as the molecular tests for risk stratification. TransPORTEC and ProMisE systems stratified the risk according to the patients’ abnormality in the POLE and p53 genes, both of which showed the potential to be implemented as the standard practice for risk stratification of EC patients. However, both of them are still underdeveloped and need to be further confirmed and validated for their potential clinical relevance [124].
The above-mentioned genetic classification is complex, with the development of glycosylomics technology, glycoprotein biomarkers that carry certain specific glycans are showing increasing clinical potential [125]. Although the diagnosis and treatment of EC have now shifted from histological typing to molecular typing, given the extensiveness of glycosylation modifications, even small changes in glycosylation could contribute to the occurrence and development of tumors. Stratifying the risk helps with the early diagnosis of cancer and improves patient prognosis [123]. A series of glycoproteins used for the detection and monitoring of EC are enlisted in Table 2.
Glycoconjugated chemotherapy drugs and targeted cancer therapy
At present, anti-tumor drugs are mainly chemotherapeutic agents, which have limited specificity and cause substantial toxicity. However, glycosylated drugs have shown reduced drug toxicity [131]. Paclitaxel, for example, conjugated with monosaccharides has been reported to show promising anti-cancer effects [132]. Adriamycin conjugated with 2-amino-2-Deoxy-D-glucose and succinic acid had superior anti-cancer efficacy by targeting glucose transporter 1 than free adriamycin [133]. Similarly, the clinical application of geldanamycin (GA), an HSP90 inhibitor, is limited due to its strong toxicity, but the galactose and lactose modified GA was reduced by 40 times as compared to the Glucose-GA [134]. Besides, other studies have reported the antitumor effects of drugs modified by glycosylation, such as azomycin, ketoprofen, cadalene, docetaxel, chlorambucil, etc. [135,136,137,138]. Protein tyrosine kinases are a class of kinases that catalyze the transfer of phosphate groups on ATP to protein tyrosine residues. The function of tyrosine kinases is closely related to the occurrence, invasion, and metastasis of malignant tumors [139]. Tyrosine kinase inhibitors (TKIs), such as mTOR inhibitors, EGFR inhibitors, human epidermal growth factor receptor 2 inhibitors, and anti-angiogenic drugs, have shown promising clinical application in cancer patients. However, due to low patient response rates, the above TKIs are not clinically applicable in EC [140]. Therapeutic resistance to TKIs may develop through parallel or bypass mechanisms. It is worth noting that receptor protein tyrosine kinases and other highly complex cell surface signaling molecules are glycoproteins, which require post-translational modification by N-linked glycans to achieve appropriate confirmation, function, and distribution into specific cellular compartments [141]. A previous study performed sensitivity screening of 94 lung cancer cell lines against NGI-1, the targeted inhibitor of oligo-saccharyl-transferase (OST), and reported that mutant EGFR was more sensitive to the inhibitor, and OST inhibition caused cell cycle arrest and also inhibited the expression of other EGFR co-expressed receptors, such as the mesenchymal–epithelial transition factor, thereby inhibiting the growth of tumor cells [142]. Furthermore, OST inhibition in combination with radiation or cytotoxic chemotherapy showed synergistic antitumor effects in glioma [143]. FUT8 modifies the activities of both the hepatocyte growth factor receptor and EGFR and affects tumor growth and invasion. Studies have reported an enhanced therapeutic effect of temozolomide in glioblastoma cells upon suppressing FUT8 expression or using the fucosylation inhibitor 2F-peracetyl-fucose [144]. Besides, the expression of EFGR in head and neck squamous cell carcinoma patients is up-regulated, but the clinical response rate of EGFR monoclonal antibody, cetuximab, is less than 20%. The expression of the tumor-related immune antigen PD-L2 was up-regulated in head and neck squamous cell carcinoma, and FUT8, as a transcriptional target of STAT3, played a key role in the glycosylation of PD-L2. The study showed that inhibiting PD-L2 binding to FUT8, or using Stattic to inhibit STAT3, improved the response to cetuximab [145].
Glycan-based nano-therapies and cancer therapeutics
There are abundant polysaccharides in nature, such as chitosan, dextran, hyaluronic acid, chondroitin sulfate, and heparin, all of which have low toxicity, low immunogenicity, and are easy to be modified by physical or chemical means, enabling the rapid development of polysaccharide encapsulated drugs for cancer therapy. Such drugs may not just be chemotherapeutic drugs but also drugs enabling gene therapy and immunotherapy [146]. Drug-loaded nanoparticles generally improve the therapeutic effects by targeting specific receptors on the surface of tumor cells, including overexpressed antibody fragments [147], carbohydrates [148], peptides [149], and proteins [150]. Meanwhile, nanomaterials are highly permeable due to the enhanced permeability and retention effect [151]. Cisplatin has limited application in metastatic tumors due to its high toxicity and non-targeted delivery. Benefiting from the properties of polymeric nanogels, cisplatin encapsulated within polymeric nano-gels coated with TKH2 mAb targeting the sTn antigen was reported to have synergistic anti-cancer effects in an orthotopic mouse model of pancreatic cancer [152].
The biosynthetic process of glycosylation modification is complex and involves many vital enzymes [153]. Currently, the research focusing on glycosylase inhibitors is still in progress. Tunicamycin inhibits the formation of dolichol carriers that are necessary for the synthesis of N-glycans, therefore inhibiting the transfer of N-acetylglucosamine-1-phosphate to dolichol in the biosynthesis of glycoprotein sugar chains of asparagine [154]. BenzylN-acetyl-·-galactosaminide, a typical O-glycosylation inhibitor, prevents the elongation of O-glycans [60]. Treating Ishikawa cells with BenzylN-acetyl-·-galactosaminide and tunicamycin induced an increase in the adhesion ability of the cells, and reduced the binding of alpha2beta1 integrin and MUC1, inhibiting tumor growth and migration [28]. As sTn can be carried by different proteins as mentioned above, the sialylation modification of proteins may cause organ and tumor-specific reactions [155]. The sTn modified protein was shown to have a tumor-promoting effect [28]. The study reported sialic acid levels to be elevated during cancer development [156]. Of note, the well-known liver cancer marker alpha-fetoprotein, the prostate-specific antigen of prostate cancer, and thyroglobulin of thyroid cancer are all sialylated glycoproteins [157]. Changes in the expression of sialylase during sialylation are closely related to breast tumor stage and prognosis [158]. Therefore, targeting tumor sialylation has strong therapeutic prospects for EC, just as previous review have mentioned [159].
The application of glycans in the immunotherapy of endometrial cancer
Immunotherapy strategies based on glycosylation modifications are mainly divided into three categories, which are glycosyltransferase inhibitors, antibody-based immunotherapies, and vaccines against glycosylated antigens [160]. Some of the mAbs targeting glycosylation-related tumor-associated epitopes are specific for glycolipids, such as gangliosides (GM2, GM3, GD2, and GD3), while others bind to the carbohydrate haptens present on both glycolipids and glycoproteins, including Lex /Ley and SLex /SLea glycan hapten structures [161]. Glycoside-specific targeting of proteins had fewer reduced off-target effects and improved anti-tumor specificity [162]. Tumor-associated carbohydrate antigens, which are carbohydrates linked to immunologically active proteins, have been considered the principal targets for therapeutic anti-cancer vaccines [163, 164]. Examples include vaccines targeting the mucin-related antigens for suppression of breast cancer, the gangliosides GM2 and GD3 for treatment of melanoma, and the glycosphingolipid Globo-H for prostate cancer treatment [165, 166]. Dendritic cells are the core components of anti-tumor immunity, reporting the real-time dynamics through antigen cross-presentation to T cells, which possess cancer cell killing abilities. Cancer vaccines and immunotherapies are greatly compromised if the tumor-associated dendritic cells are defective in antigen cross-presentation. Therefore, stimulating dendritic cells to enable sustained antigen cross-presentation, and contribute to the anti-cancer immune response is of great significance. The Mannan–MUC1 fusion protein-mediated stimulation of DCs has been proven to be efficacious in phase I clinical trials [167,168,169]. For example, MUC1 is the O-glycosylated protein prevalent in breast carcinoma, and the MUC1 lysate-pulsed DCs promote the expression of MUC1-specific CD8 + T cells in breast cancer immunotherapy [170, 171]. The transformation of chimeric antigen receptor T cell immunotherapy (CAR-T) cells enhances their ability to recognize tumor-specific glycosylated antigens, improving their anti-tumor immunotherapeutic effect [161]. Some of the above studies are currently in different stages of clinical trials. However, the majority of the studies on cancer immunotherapy have mainly focused on cancers other than EC, providing a reference for the study of glycosylation targeted immunotherapy in EC.
Conclusion and future perspectives
Due to the complexity of glycosylation modifications and the unstable structure of the biological samples during the research process, the specificity and integrity of the glycosylation structure may not be fully guaranteed. The structural and functional analysis of glycans needs further development. A typical method is the mass spectrometry fragmentation technology electron transfer/high energy collisional dissociation, which combines high-energy-induced dissociation, and electron transfer dissociation, to effectively improve the identification throughput, coverage depth, and the accuracy of identification of the site of O-glycoproteome [172, 173]. Modified proteome researches with improved sequencing depth and breadth would be helpful for future studies.
In conclusion, the current research on glycosylation has certain limitations. Though few studies have investigated the glycosylation impacts on EC, they are still very valuable and exciting. Any minor modifications to glycosylation may affect the localization and stability of cell surface receptors and their sensitivity to signaling molecules, influencing cellular functions, which may support tumor growth and metastasis, as well as the immune response. Meanwhile, glycoconjugates are a new generation of therapeutic biomarkers, and the glycosidic form of the protein can provide more predictive information. Development of glycosyl-based cancer neo-antigens for cancer vaccines and targeted therapy, such as antibodies against these antigens, and CAR-T therapy has great therapeutic potential. Glycoside-specific targeting protein nanoparticles can limit off-target effects and enhance antitumor specificity. In short, glycosylation provides a new strategy for individualized and comprehensive treatment and also the experimental direction for future research in EC.
Abbreviations
- EC:
-
Endometrial cancer
- MS:
-
Mass spectrometry
- GlcNAc:
-
N-Acetylglucosamine
- FUT:
-
Fucosyltransferases
- GalNAc:
-
N-Acetylgalactosamine
- T antigen:
-
Thomsen-friedenreich antigen
- Tn:
-
Monosaccharide GalNAc
- sT:
-
Sialylated Thomsen-Friedenreich antigen
- sTn:
-
Sialylated monosaccharide GalNAc
- ELISA:
-
Enzyme-linked immunosorbent assay
- IHC:
-
Immunohistochemistry
- PCR:
-
Polymerase chain reaction
- MALDI-TOF-MS:
-
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry
- SELDI-TOF-MS:
-
Surface-enhanced laser desorption/ionization time-of-flight mass spectrometry
- HILIC-UPLC:
-
Hydrophilic interaction chromatography
- LC-MS/MS:
-
Liquid chromatography–electrospray tandem mass spectrometry
- MALDI-MSI:
-
Matrix-assisted laser desorption/ionization mass spectrometry imaging
- GalNAc-T6:
-
N-Acetylgalactosaminyl transferase 6
- OC:
-
Ovarian cancer
- MUC1:
-
Mucin1
- EGFR:
-
Epidermal growth factor receptor
- GALNT2:
-
Acetylgalactosaminyltransferase2
- ApoE:
-
Apolipoprotein E
- Gd and GdA:
-
Glycodelin and Glycodelin A
- COX:
-
Cyclooxygenase
- TKI:
-
Tyrosine kinase inhibitors
- GA:
-
Geldanamycin
References
Sung H, Ferlay J, Siegel RL ,et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a Cancer Journal For Clinicians. 2021; 71:209–249.
Ravegnini G, Gorini F, De Crescenzo E, et al. Can miRNAs be useful biomarkers in improving prognostic stratification in endometrial cancer patients? An update review. Int J Cancer. 2022;150:1077–90.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978–2013. J Natl Cancer Inst. 2018;110:354–61.
Gu B, Shang X, Yan M, et al. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990–2019. Gynecol Oncol. 2021;161:573–80.
Nicolaije KA, Ezendam NP, Vos MC, et al. Follow-up practice in endometrial cancer and the association with patient and hospital characteristics: a study from the population-based PROFILES registry. Gynecol Oncol. 2013;129:324–31.
Ang C, Bryant A, Barton DP, Pomel C, Naik R. Exenterative surgery for recurrent gynaecological malignancies. Cochrane Database Syst Rev. 2014:CD010449.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
de Vries T, Knegtel RM, Holmes EH. Macher BA. Fucosyltransferases: structure/function studies. Glycobiology. 2001;11:119R-128R.
Wong CH. Protein glycosylation: new challenges and opportunities. J Org Chem. 2005;70:4219–25.
Hutt S, Tailor A, Ellis P, Michael A, Butler-Manuel S, Chatterjee J. The role of biomarkers in endometrial cancer and hyperplasia: a literature review. Acta Oncol. 2019;58:342–52.
Briggs MT, Condina MR, Klingler-Hoffmann M, et al. Translating N-glycan analytical applications into clinical strategies for ovarian cancer. Proteomics Clin Appl. 2019;13: e1800099.
Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–42.
Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–55.
Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, Packer NH. Cell surface protein glycosylation in cancer. Proteomics. 2014;14:525–46.
Boehlich GJ, Schutzenmeister N. beta-Selective C-glycosylation and its application in the synthesis of scleropentaside A. Angew Chem Int Ed Engl. 2019;58:5110–3.
Qin W, Qin K, Zhang Y, et al. S-glycosylation-based cysteine profiling reveals regulation of glycolysis by itaconate. Nat Chem Biol. 2019;15:983–91.
Pavlikova L, Seres M, Imrichova D, et al. The expression of P-gp in leukemia cells is associated with cross-resistance to protein N-glycosylation inhibitor tunicamycin. Gen Physiol Biophys. 2016;35:497–510.
Jie X, Fong WP, Zhou R, et al. USP9X-mediated KDM4C deubiquitination promotes lung cancer radioresistance by epigenetically inducing TGF-beta2 transcription. Cell Death Differ. 2021;28:2095–111.
Eichler J. Protein glycosylation. Curr Biol. 2019;29:R229–31.
Ladenson RP, Schwartz SO, Ivy AC. Incidence of the blood groups and the secretor factor in patients with pernicious anemia and stomach carcinoma. Am J Med Sci. 1949;217:194–7.
Hanisch FG, Breloy I. Protein-specific glycosylation: signal patches and cis-controlling peptidic elements. Biol Chem. 2009;390:619–26.
Dhanisha SS, Guruvayoorappan C, Drishya S, Abeesh P. Mucins: Structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Crit Rev Oncol Hematol. 2018;122:98–122.
Mita Y, Aoyagi Y, Suda T, Asakura H. Plasma fucosyltransferase activity in patients with hepatocellular carcinoma, with special reference to correlation with fucosylated species of alpha-fetoprotein. J Hepatol. 2000;32:946–54.
Irazoqui FJ, Nores GA. Thomsen-friedenreich disaccharide immunogenicity. Curr Cancer Drug Targets. 2003;3:433–43.
Miyoshi E, Moriwaki K, Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem. 2008;143:725–9.
Kudelka MR, Ju T, Heimburg-Molinaro J, Cummings RD. Simple sugars to complex disease–mucin-type O-glycans in cancer. Adv Cancer Res. 2015;126:53–135.
Fu C, Zhao H, Wang Y, et al. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. HLA. 2016;88:275–86.
Dennis JW, Laferte S, Waghorne C, Breitman ML, Kerbel RS. Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987;236:582–5.
Rudd PM, Colominas C, Royle L, et al. A high-performance liquid chromatography based strategy for rapid, sensitive sequencing of N-linked oligosaccharide modifications to proteins in sodium dodecyl sulphate polyacrylamide electrophoresis gel bands. Proteomics. 2001;1:285–94.
Li X, Xu Y, Zhang L. Serum CA153 as biomarker for cancer and noncancer diseases. Prog Mol Biol Transl Sci. 2019;162:265–76.
Wen K-C, Sung P-L, Hsieh S-L, et al. α2,3-sialyltransferase type I regulates migration and peritoneal dissemination of ovarian cancer cells. Oncotarget. 2017;8:29013–27.
Hashiguchi Y, Kasai M, Fukuda T, Ichimura T, Yasui T, Sumi T. Serum Sialyl-Tn (STN) as a tumor marker in patients with endometrial cancer. Pathol Oncol Res. 2016;22:501–4.
Smith J, Mittermayr S, Váradi C, Bones J. Quantitative glycomics using liquid phase separations coupled to mass spectrometry. Analyst. 2017;142:700–20.
Cao L, Qu Y, Zhang Z, Wang Z, Prytkova I, Wu S. Intact glycopeptide characterization using mass spectrometry. Expert Rev Proteomics. 2016;13:513–22.
Xiao K, Han Y, Yang H, Lu H, Tian Z. Mass spectrometry-based qualitative and quantitative N-glycomics: An update of 2017–2018. Analytica Chimica Acta. 2019; 1091.
Han L, Costello CE. Mass spectrometry of glycans. Biochemistry. Biokhimiia. 2013;78:710–20.
Paton B, Suarez M, Herrero P, Canela N. Glycosylation Biomarkers Associated with Age-Related Diseases and Current Methods for Glycan Analysis. Int J Mol Sci 2021; 22.
Chen C-C, Su W-C, Huang B-Y, Chen Y-J, Tai H-C, Obena RP. Interaction modes and approaches to glycopeptide and glycoprotein enrichment. Analyst. 2014;139:688–704.
Şahar U, Deveci R. Profiling N-glycans of the egg jelly coat of the sea urchin Paracentrotus lividus by MALDI-TOF mass spectrometry and capillary liquid chromatography electrospray ionization-ion trap tandem mass spectrometry systems. Mol Reprod Dev. 2017;84:401–7.
Cao L, Tolić N, Qu Y ,et al. Characterization of intact N- and O-linked glycopeptides using higher energy collisional dissociation. Analytical Biochemistry. 2014; 452.
Scott NE, Parker BL, Connolly AM ,et al. Simultaneous glycan-peptide characterization using hydrophilic interaction chromatography and parallel fragmentation by CID, higher energy collisional dissociation, and electron transfer dissociation MS applied to the N-linked glycoproteome of Campylobacter jejuni. Mol Cell Proteomics. 2011; 10:M000031-MMCP000201.
Toghi Eshghi S, Yang W, Hu Y, et al. Classification of tandem mass spectra for identification of N- and O-linked glycopeptides. Sci Rep. 2016;6:37189.
Lin S, Wang Y, Wang X, Yan B, Lou W, Di W. Serum immunoglobulin G N-glycome: a potential biomarker in endometrial cancer. Ann Transl Med. 2020;8:748.
Wu J, Xie X, Nie S, Buckanovich RJ, Lubman DM. Altered expression of sialylated glycoproteins in ovarian cancer sera using lectin-based ELISA assay and quantitative glycoproteomics analysis. J Proteome Res. 2013;12:3342–52.
Alley WR Jr, Vasseur JA, Goetz JA, et al. N-linked glycan structures and their expressions change in the blood sera of ovarian cancer patients. J Proteome Res. 2012;11:2282–300.
Ruhaak LR, Kim K, Stroble C, et al. Protein-Specific Differential Glycosylation of Immunoglobulins in Serum of Ovarian Cancer Patients. J Proteome Res. 2016;15:1002–10.
Mittal P, Briggs M, Klingler-Hoffmann M, et al. Altered N-linked glycosylation in endometrial cancer. Anal Bioanal Chem. 2021;413:2721–33.
Wen KC, Sung PL, Hsieh SL, et al. alpha2,3-sialyltransferase type I regulates migration and peritoneal dissemination of ovarian cancer cells. Oncotarget. 2017;8:29013–27.
Wu X, Zhao J, Ruan Y, Sun L, Xu C, Jiang H. Sialyltransferase ST3GAL1 promotes cell migration, invasion, and TGF-beta1-induced EMT and confers paclitaxel resistance in ovarian cancer. Cell Death Dis. 2018;9:1102.
Wang PH, Lee WL, Juang CM, et al. Altered mRNA expressions of sialyltransferases in ovarian cancers. Gynecol Oncol. 2005;99:631–9.
Wang JW, Ambros RA, Weber PB, Rosano TG. Fucosyltransferase and alpha-L-fucosidase activities and fucose levels in normal and malignant endometrial tissue. Cancer Res. 1995;55:3654–8.
Mu AK, Lim BK, Aminudin N, Hashim OH, Shuib AS. Application of SELDI-TOF in N-glycopeptides profiling of the urine from patients with endometrial, ovarian and cervical cancer. Arch Physiol Biochem. 2016;122:111–6.
Ye B, Skates S, Mok SC, et al. Proteomic-based discovery and characterization of glycosylated eosinophil-derived neurotoxin and COOH-terminal osteopontin fragments for ovarian cancer in urine. Clin Cancer Res. 2006;12:432–41.
Mu AK, Lim BK, Hashim OH, Shuib AS. Detection of differential levels of proteins in the urine of patients with endometrial cancer: analysis using two-dimensional gel electrophoresis and o-glycan binding lectin. Int J Mol Sci. 2012;13:9489–501.
Hautala LC, Pang PC, Antonopoulos A, et al. Altered glycosylation of glycodelin in endometrial carcinoma. Lab Invest,. 2020;100:1014–25.
Greville G, Llop E, Huang C, et al. Hypoxia alters epigenetic and N-Glycosylation profiles of ovarian and breast cancer cell lines in-vitro. Front Oncol. 2020;10:1218.
Wu Y, Chen X, Dong W, et al. ST3Gal IV mediates the growth and proliferation of cervical cancer cells in vitro and in vivo via the Notch/p21/CDKs pathway. Front Oncol. 2020;10: 540332.
Qi F, Isaji T, Duan C, et al. ST3GAL3, ST3GAL4, and ST3GAL6 differ in their regulation of biological functions via the specificities for the alpha2,3-sialylation of target proteins. FASEB J. 2020;34:881–97.
Paszkiewicz-Gadek A, Porowska H, Lemancewicz D, Wolczynski S, Gindzienski A. The influence of N- and O-glycosylation inhibitors on the glycosylation profile of cellular membrane proteins and adhesive properties of carcinoma cell lines. Int J Mol Med. 2006;17:669–74.
Miyamoto S, Ruhaak LR, Stroble C, et al. Glycoproteomic analysis of malignant ovarian cancer ascites fluid identifies unusual glycopeptides. J Proteome Res. 2016;15:3358–76.
Biskup K, Braicu EI, Sehouli J, Tauber R, Blanchard V. The ascites N-glycome of epithelial ovarian cancer patients. J Proteomics. 2017;157:33–9.
Kuzmanov U, Musrap N, Kosanam H, et al. Glycoproteomic identification of potential glycoprotein biomarkers in ovarian cancer proximal fluids. Clin Chem Lab Med. 2013;51:1467–76.
Gomes J, Gomes-Alves P, Carvalho SB, et al. Extracellular vesicles from ovarian carcinoma cells display specific glycosignatures. Biomolecules. 2015;5:1741–61.
Eble JA, Niland S. The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis. 2019;36:171–98.
Ohtsubo K, Takamatsu S, Minowa MT, Yoshida A, Takeuchi M, Marth JD. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell. 2005;123:1307–21.
Partridge EA, Le Roy C, Di Guglielmo GM, et al. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306:120–4.
Schachter H. The search for glycan function: fucosylation of the TGF-beta1 receptor is required for receptor activation. Proc Natl Acad Sci U S A. 2005;102:15721–2.
Liu YC, Yen HY, Chen CY, et al. Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. Proc Natl Acad Sci U S A. 2011;108:11332–7.
Pinho SS, Seruca R, Gartner F, et al. Modulation of E-cadherin function and dysfunction by N-glycosylation. Cell Mol Life Sci. 2011;68:1011–20.
Kurita T, Thi TN, Koi C, et al. Expression of N-acetylgalactosaminyltransferase-6 Is related to expression of cell adhesion molecules in endometrial cancer. Anticancer Res. 2017;37:3905–10.
Ratan C, Cicily KDD, Nair B, Nath LR. MUC Glycoproteins: potential biomarkers and molecular targets for cancer therapy. Curr Cancer Drug Targets. 2021;21:132–52.
Catterall JB, Jones LM, Turner GA. Membrane protein glycosylation and CD44 content in the adhesion of human ovarian cancer cells to hyaluronan. Clin Exp Metastasis. 1999;17:583–91.
Katoh S, Zheng Z, Oritani K, Shimozato T, Kincade PW. Glycosylation of CD44 negatively regulates its recognition of hyaluronan. J Exp Med. 1995;182:419–29.
Goupille C, Hallouin F, Meflah K, Le Pendu J. Increase of rat colon carcinoma cells tumorigenicity by alpha(1–2) fucosyltransferase gene transfection. Glycobiology. 1997;7:221–9.
Makrydimas G, Zagorianakou N, Zagorianakou P, Agnantis NJ. CD44 family and gynaecological cancer. In Vivo. 2003;17:633–40.
Zhou F, Huo J, Liu Y, et al. Elevated glucose levels impair the WNT/beta-catenin pathway via the activation of the hexosamine biosynthesis pathway in endometrial cancer. J Steroid Biochem Mol Biol. 2016;159:19–25.
Sun Z, Xue H, Wei Y, et al. Mucin O-glycosylating enzyme GALNT2 facilitates the malignant character of glioma by activating the EGFR/PI3K/Akt/mTOR axis. Clin Sci (Lond). 2019;133:1167–84.
Zhou X, Xu Y, Yin D, et al. Type 2 diabetes mellitus facilitates endometrial hyperplasia progression by activating the proliferative function of mucin O-glycosylating enzyme GALNT2. Biomed Pharmacother. 2020;131: 110764.
Tu C-F, Wu M-Y, Lin Y-C, Kannagi R, Yang R-B. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res. 2017;19:111.
Chen C-Y, Jan Y-H, Juan Y-H, et al. Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proc Natl Acad Sci USA. 2013;110:630–5.
Jia L, Li J, Li P, et al. Site-specific glycoproteomic analysis revealing increased core-fucosylation on FOLR1 enhances folate uptake capacity of HCC cells to promote EMT. Theranostics. 2021;11:6905–21.
Radhakrishnan P, Dabelsteen S, Madsen FB, et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc Natl Acad Sci U S A. 2014;111:E4066-4075.
Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–206.
Munkley J. The Role of Sialyl-Tn in Cancer. Int J Mol Sci. 2016;17:275.
Fares F. The role of O-linked and N-linked oligosaccharides on the structure-function of glycoprotein hormones: development of agonists and antagonists. Biochim Biophys Acta. 2006;1760:560–7.
Sivalingam VN, Myers J, Nicholas S, Balen AH, Crosbie EJ. Metformin in reproductive health, pregnancy and gynaecological cancer: established and emerging indications. Hum Reprod Update. 2014;20:853–68.
Lai Y, Sun C. Association of abnormal glucose metabolism and insulin resistance in patients with atypical and typical endometrial cancer. Oncol Lett. 2018;15:2173–8.
Willey KP. An elusive role for glycosylation in the structure and function of reproductive hormones. Hum Reprod Update. 1999;5:330–55.
Li J, Yin M, Song W, et al. B subunit of human chorionic gonadotropin promotes tumor invasion and predicts poor prognosis of early-stage colorectal cancer. Cell Physiol Biochem. 2018;45:237–49.
Cole LA, Butler S. Hyperglycosylated hCG, hCGβ and Hyperglycosylated hCGβ: interchangeable cancer promoters. Mol Cell Endocrinol. 2012;349:232–8.
Cole LA, Butler S. Hyperglycosylated hCG, hCGbeta and Hyperglycosylated hCGbeta: interchangeable cancer promoters. Mol Cell Endocrinol. 2012;349:232–8.
Mai Q, Sheng D, Chen C, et al. Steroid 5 alpha-reductase 3 (SRD5A3) promotes tumor growth and predicts poor survival of human hepatocellular carcinoma (HCC). Aging (Albany NY). 2020;12:25395–411.
Cai Y, Wang B, Xu W, et al. Endometrial cancer: genetic, metabolic characteristics, therapeutic strategies and nanomedicine. Curr Med Chem. 2021;28:8755–81.
Adeva-Andany MM, Perez-Felpete N, Fernandez-Fernandez C, Donapetry-Garcia C, Pazos-Garcia C. Liver glucose metabolism in humans. Biosci Rep. 2016; 36.
Zhang D, Li J, Wang F, Hu J, Wang S, Sun Y. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014;355:176–83.
Lambe M, Wigertz A, Garmo H, Walldius G, Jungner I, Hammar N. Impaired glucose metabolism and diabetes and the risk of breast, endometrial, and ovarian cancer. Cancer Causes Control. 2011;22:1163–71.
Mitsuhashi A, Uehara T, Hanawa S, Shozu M. Prospective evaluation of abnormal glucose metabolism and insulin resistance in patients with atypical endometrial hyperplasia and endometrial cancer. Support Care Cancer. 2017;25:1495–501.
Yang X, Li X, Dong Y, et al. Effects of metabolic syndrome and its components on the prognosis of endometrial cancer. Front Endocrinol. 2021;12: 780769.
Yamazaki Y, Liu C-C, Yamazaki A ,et al. Vascular ApoE4 Impairs Behavior by Modulating Gliovascular Function. Neuron. 2021; 109.
Moysich KB, Freudenheim JL, Baker JA, et al. Apolipoprotein E genetic polymorphism, serum lipoproteins, and breast cancer risk. Mol Carcinog. 2000;27:2–9.
Rindler MJ, Traber MG, Esterman AL, Bersinger NA, Dancis J. Synthesis and secretion of apolipoprotein E by human placenta and choriocarcinoma cell lines. Placenta. 1991;12:615–24.
Huvila J, Brandt A, Rojas CR, et al. Gene expression profiling of endometrial adenocarcinomas reveals increased apolipoprotein E expression in poorly differentiated tumors. Int J Gynecol Cancer. 2009;19:1226–31.
Kacperczyk M, Kmieciak A, Kratz EM. The Role of ApoE Expression and Variability of Its Glycosylation in Human Reproductive Health in the Light of Current Information. Int J Mol Sci. 2021; 22.
Lee Y, Kockx M, Raftery MJ, Jessup W, Griffith R, Kritharides L. Glycosylation and sialylation of macrophage-derived human apolipoprotein E analyzed by SDS-PAGE and mass spectrometry: evidence for a novel site of glycosylation on Ser290. Mol Cell Proteomics. 2010;9:1968–81.
Flowers SA, Grant OC, Woods RJ, Rebeck GW. O-glycosylation on cerebrospinal fluid and plasma apolipoprotein E differs in the lipid-binding domain. Glycobiology. 2020;30:74–85.
Liu H-W, Zhang F, Fan P, Bai H, Zhang J-X, Wang Y. Effects of apolipoprotein E genotypes on metabolic profile and oxidative stress in southwest Chinese women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;170:146–51.
Lai H, Zhao X, Qin Y, et al. FAK-ERK activation in cell/matrix adhesion induced by the loss of apolipoprotein E stimulates the malignant progression of ovarian cancer. J Exp Clin Cancer Res. 2018;37:32.
Zunarelli E, Nicoll JA, Migaldi M, Trentini GP. Apolipoprotein E polymorphism and breast carcinoma: correlation with cell proliferation indices and clinical outcome. Breast Cancer Res Treat. 2000;63:193–8.
Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–6.
Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci U S A. 2002;99:10231–3.
Dube DH, Bertozzi CR. Glycans in cancer and inflammation–potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–88.
Heczey A, Louis CU, Savoldo B, et al. CAR T Cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Ther. 2017;25:2214–24.
Lu LL, Das J, Grace PS, Fortune SM, Restrepo BI, Alter G. Antibody Fc glycosylation discriminates between latent and active tuberculosis. J Infect Dis. 2020;222:2093–102.
Ragupathi G, Liu NX, Musselli C, Powell S, Lloyd K, Livingston PO. Antibodies against tumor cell glycolipids and proteins, but not mucins, mediate complement-dependent cytotoxicity. J Immunol. 2005;174:5706–12.
Sawada R, Sun SM, Wu X ,et al. Human monoclonal antibodies to sialyl-Lewis (CA19.9) with potent CDC, ADCC, and antitumor activity. Clin Cancer Res. 2011; 17:1024–1032.
Lavrsen K, Madsen CB, Rasch MG, et al. Aberrantly glycosylated MUC1 is expressed on the surface of breast cancer cells and a target for antibody-dependent cell-mediated cytotoxicity. Glycoconj J. 2013;30:227–36.
Saeland E, van Vliet SJ, Backstrom M, et al. The C-type lectin MGL expressed by dendritic cells detects glycan changes on MUC1 in colon carcinoma. Cancer Immunol Immunother. 2007;56:1225–36.
Laubli H, Borsig L. Altered Cell Adhesion and Glycosylation Promote Cancer Immune Suppression and Metastasis. Front Immunol. 2019;10:2120.
Ohno S, Ohno Y, Nakada H, Suzuki N, Soma G, Inoue M. Expression of Tn and sialyl-Tn antigens in endometrial cancer: its relationship with tumor-produced cyclooxygenase-2, tumor-infiltrated lymphocytes and patient prognosis. Anticancer Res. 2006;26:4047–53.
Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15:e268-278.
Stelloo E, Bosse T, Nout RA, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol. 2015;28:836–44.
Talhouk A, McConechy MK, Leung S, et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer. 2017;123:802–13.
Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer. 2019;19:510–21.
Drake RR. Glycosylation and cancer: moving glycomics to the forefront. Adv Cancer Res. 2015;126:1–10.
Liu DT. EMMPRIN in gynecologic cancers: pathologic and therapeutic aspects. Tumour Biol. 2015;36:4883–8.
Lenhard M, Heublein S, Kunert-Keil C, et al. Immunosuppressive Glycodelin A is an independent marker for poor prognosis in endometrial cancer. BMC Cancer. 2013;13:616.
Memarzadeh S, Kozak KR, Chang L, et al. Urokinase plasminogen activator receptor: Prognostic biomarker for endometrial cancer. Proc Natl Acad Sci U S A. 2002;99:10647–52.
Miyamoto T, Suzuki A, Asaka R, et al. Immunohistochemical expression of core 2 beta1,6-N-acetylglucosaminyl transferase 1 (C2GnT1) in endometrioid-type endometrial carcinoma: a novel potential prognostic factor. Histopathology. 2013;62:986–93.
Nguyen TT, Kurita T, Koi C, et al. GalNAc-T6 in the relationship with invasion ability of endometrial carcinomas and prognostic significance. Am J Cancer Res. 2017;7:1188–97.
Wojtowicz K, Januchowski R, Nowicki M, Zabel M. Inhibition of protein glycosylation reverses the MDR phenotype of cancer cell lines. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2015; 74:49–56.
Fu Y, Li S, Zu Y, et al. Medicinal chemistry of paclitaxel and its analogues. Curr Med Chem. 2009;16:3966–85.
Cao J, Cui S, Li S, et al. Targeted cancer therapy with a 2-deoxyglucose-based adriamycin complex. Cancer Res. 2013;73:1362–73.
Cheng H, Cao X, Xian M, et al. Synthesis and enzyme-specific activation of carbohydrate-geldanamycin conjugates with potent anticancer activity. J Med Chem. 2005;48:645–52.
Goff RD, Thorson JS. Assessment of chemoselective neoglycosylation methods using chlorambucil as a model. J Med Chem. 2010;53:8129–39.
Kumar P, Shustov G, Liang H, et al. Design, synthesis, and preliminary biological evaluation of 6-O-glucose-azomycin adducts for diagnosis and therapy of hypoxic tumors. J Med Chem. 2012;55:6033–46.
Patra M, Awuah SG, Lippard SJ. Chemical Approach to Positional Isomers of Glucose-Platinum Conjugates Reveals Specific Cancer Targeting through Glucose-Transporter-Mediated Uptake in Vitro and in Vivo. J Am Chem Soc. 2016;138:12541–51.
Gynther M, Ropponen J, Laine K, et al. Glucose promoiety enables glucose transporter mediated brain uptake of ketoprofen and indomethacin prodrugs in rats. J Med Chem. 2009;52:3348–53.
Jiao Q, Bi L, Ren Y, Song S, Wang Q, Wang Y-S. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol Cancer. 2018;17:36.
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–108.
Saitou A, Hasegawa Y, Fujitani N ,et al. N-glycosylation regulates MET processing and signaling. Cancer Sci. 2022.
Lopez Sambrooks C, Baro M, Quijano A, et al. Oligosaccharyltransferase inhibition overcomes therapeutic resistance to EGFR tyrosine kinase inhibitors. Cancer Res. 2018;78:5094–106.
Baro M, Lopez Sambrooks C, Quijano A, Saltzman WM, Contessa J. Oligosaccharyltransferase Inhibition Reduces Receptor Tyrosine Kinase Activation and Enhances Glioma Radiosensitivity. Clinical Cancer Res. 2019;25:784–95.
Wei KC, Lin YC, Chen CH, et al. Fucosyltransferase 8 modulates receptor tyrosine kinase activation and temozolomide resistance in glioblastoma cells. Am J Cancer Res. 2021;11:5472–84.
Xu Y, Gao Z, Hu R ,et al. PD-L2 glycosylation promotes immune evasion and predicts anti-EGFR efficacy. J Immunother Cancer. 2021; 9.
Li M, Zhao Y, Zhang W, Zhang S, Zhang S. Multiple-therapy strategies via polysaccharides-based nano-systems in fighting cancer. Carbohyd Polym. 2021;269: 118323.
Sugano M, Egilmez NK, Yokota SJ, et al. Antibody targeting of doxorubicin-loaded liposomes suppresses the growth and metastatic spread of established human lung tumor xenografts in severe combined immunodeficient mice. Can Res. 2000;60:6942–9.
Cao Y, Chen D, Zhao P, et al. Intracellular delivery of mitomycin C with targeted polysaccharide conjugates against multidrug resistance. Ann Biomed Eng. 2011;39:2456–65.
Du Y-Z, Cai L-L, Liu P, You J, Yuan H, Hu F-Q. Tumor cells-specific targeting delivery achieved by A54 peptide functionalized polymeric micelles. Biomaterials. 2012;33:8858–67.
Fernandes E, Ferreira JA, Andreia P, et al. New trends in guided nanotherapies for digestive cancers: A systematic review. J Control Release. 2015;209:288–307.
Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods In Molecular Biology (Clifton, NJ). 2010;624:25–37.
Soni KS, Thomas D, Caffrey T, et al. A polymeric Nanogel-based treatment regimen for enhanced efficacy and sequential administration of synergistic drug combination in pancreatic cancer. J Pharmacol Exp Ther. 2019;370:894–901.
Schjoldager KT, Narimatsu Y, Joshi HJ, Clausen H. Global view of human protein glycosylation pathways and functions. Nat Rev Mol Cell Biol. 2020;21:729–49.
Wu J, Chen S, Liu H, et al. Tunicamycin specifically aggravates ER stress and overcomes chemoresistance in multidrug-resistant gastric cancer cells by inhibiting N-glycosylation. J ExpClin Cancer Res. 2018;37:272.
Li F, Ding J. Sialylation is involved in cell fate decision during development, reprogramming and cancer progression. Protein Cell. 2019;10:550–65.
Sawhney H, Kumar CA. Correlation of serum biomarkers (TSA & LSA) and epithelial dysplasia in early diagnosis of oral precancer and oral cancer. Cancer Biomark. 2011;10:43–9.
Badr HA, Alsadek DMM, Darwish AA, et al. Lectin approaches for glycoproteomics in FDA-approved cancer biomarkers. Expert Rev Proteomics. 2014;11:227–36.
Dao TL, Ip C, Patel J. Serum sialyltransferase and 5’-nucleotidase as reliable biomarkers in women with breast cancer. J Natl Cancer Inst. 1980;65:529–34.
Pietrobono S, Stecca B. Aberrant Sialylation in Cancer: Biomarker and Potential Target for Therapeutic Intervention? Cancers. 2021; 13.
Cadena AP, Cushman TR, Welsh JW. Glycosylation and Antitumor Immunity. Int Rev Cell Mol Biol. 2019;343:111–27.
Steentoft C, Migliorini D, King TR, Mandel U, June CH, Posey AD Jr. Glycan-directed CAR-T cells. Glycobiology. 2018;28:656–69.
Alter G, Ottenhoff THM, Joosten SA. Antibody glycosylation in inflammation, disease and vaccination. Semin Immunol. 2018;39:102–10.
Dalziel M, Crispin M, Scanlan CN, Zitzmann N, Dwek RA. Emerging principles for the therapeutic exploitation of glycosylation. Science. 2014;343:1235681.
Julien S, Picco G, Sewell R, et al. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br J Cancer. 2009;100:1746–54.
Slovin SF, Ragupathi G, Adluri S, et al. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc Natl Acad Sci U S A. 1999;96:5710–5.
Thomas D, Rathinavel AK, Radhakrishnan P. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim Biophys Acta Rev Cancer. 2021;1875: 188464.
Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23:6674–81.
Becker T, Dziadek S, Wittrock S, Kunz H. Synthetic glycopeptides from the mucin family as potential tools in cancer immunotherapy. Curr Cancer Drug Targets. 2006;6:491–517.
Loveland BE, Zhao A, White S, et al. Mannan-MUC1-pulsed dendritic cell immunotherapy: a phase I trial in patients with adenocarcinoma. Clin Cancer Res. 2006;12:869–77.
Bohnenkamp HR, Coleman J, Burchell JM, Taylor-Papadimitriou J, Noll T. Breast carcinoma cell lysate-pulsed dendritic cells cross-prime MUC1-specific CD8+ T cells identified by peptide-MHC-class-I tetramers. Cell Immunol. 2004;231:112–25.
Avigan D, Vasir B, Gong J, et al. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res. 2004;10:4699–708.
Zhang Y, Xie X, Zhao X, et al. Systems analysis of singly and multiply O-glycosylated peptides in the human serum glycoproteome via EThcD and HCD mass spectrometry. J Proteomics. 2018;170:14–27.
Bilan V, Leutert M, Nanni P, Panse C, Hottiger MO. Combining higher-energy collision dissociation and electron-transfer/higher-energy collision dissociation fragmentation in a product-dependent manner confidently assigns proteomewide adp-ribose acceptor sites. Anal Chem. 2017;89:1523–30.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 81974463).
Author information
Authors and Affiliations
Contributions
Writing—Original Draft, CP, BYX; Writing—Review and Editing, CP, BY, KX, YZ; Visualization, CP; Supervision and Revision, KX, YZ. CP and BX contributed equally to this study.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pu, C., Biyuan, Xu, K. et al. Glycosylation and its research progress in endometrial cancer. Clin Transl Oncol 24, 1865–1880 (2022). https://doi.org/10.1007/s12094-022-02858-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02858-z