Abstract
Background
Breast ultrasound and mammography were used in the detection of residual tumor after neoadjuvant chemotherapy. The aim of this study was to evaluate the correlation between clinical and pathological responses with breast density and IHC marker conversion to understand how this information might be used in the future to direct treatment.
Methods
We included 119 patients who underwent CNB and followed NACT. The breast density assessment was based on the mammography examination performed at the time of diagnosis. We evaluated the clinical and pathological responses to NACT by the UICC and Miller–Payne grading systems, respectively.
Results
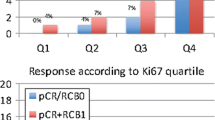
Of 119 patients who met the inclusion criteria, patients with high pre-treatment IHC markers levels showed higher expression of IHC markers regardless of the post-treatment IHC marker level at baseline. However, breast and node tumor sizes before and after NACT were negatively correlated with hormone receptor conversion and positively correlated with Ki-67 conversion (P < 0.05). Patients with low BD were more likely to have a cCR, pCR, TNBC, and postmenopausal status than those with a high BD (P < 0.05). BD was significantly associated with PR and Ki67 conversion but not ER conversion.
Conclusion
Our prospective observational study demonstrated that IHC marker conversion could be used to identify lesion size changes and BD. We also found that a high BD was linked to clinical and pathological responses, molecular subtype, and menopausal status. In the future, additional studies are required to validate the predictive value identified by this research.









Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Colleoni M, Goldhirsch A. Neoadjuvant chemotherapy for breast cancer: any progress? Lancet Oncol. 2014;15:131–2.
Read RL, Flitcroft K, Snook KL, Boyle FM, Spillane AJ. Utility of neoadjuvant chemotherapy in the treatment of operable breast cancer. ANZ J Surg. 2015;85:315–20.
Zhao Y, Dong XQ, Li RG, Ma X, Song J, Li YJ, Zhang DW. Evaluation of the pathological response and prognosis following neoadjuvant chemotherapy in molecular subtypes of breast cancer. OncoTargets Ther. 2015;8:1511–21.
Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–16.
Bertero L, Massa F, Metovic J, et al. Eighth Edition of the UICC Classification of Malignant Tumours: an overview of the changes in the pathological TNM classification criteria—what has changed and why? Virchows Arch. 2018;472(4):519–31.
McCormack VA, dos Silva Santos I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev. 2006;15:1159–69.
Maskarinec G, Pagano IS, Little MA, et al. Mammographic density as a predictor of breast cancer survival: the Multiethnic Cohort. Breast Cancer Res. 2013;15:R7.
Von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804.
Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Ogston KN, Miller ID, Payne S. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12(5):320–7.
Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–92.
Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol. 2009;27:1323–33.
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, WatsonM Davies S, Bernard PS, Parker JS. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50.
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel M. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–23.
Chen S, Chen CM, Yu KD, Zhou RJ, Shao ZM. Prognostic value of a positive-to-negative change in hormone receptor status after neoadjuvant chemotherapy in patients with hormone receptor-positive breast cancer. Ann Surg Oncol. 2012;19:3002–11.
van de Ven S, Smit VT, Dekker TJ, Nortier JW, Kroep JR. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev. 2011;37:422–30.
Lee SH, Chung MA, Quddus MR, Steinhoff MM, Cady B. The effect of neoadjuvant chemotherapy on estrogen and progesterone receptor expression and hormone receptor status in breast cancer. Am J Surg. 2003;186:348–50.
Burcombe RJ, Makris A, Richman PI, Daley FM, Noble S, Pittam M, Wright D, Allen SA, Dove J, Wilson GD. Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant anthracycline chemotherapy for operable breast cancer. Br J Cancer. 2005;92:147–55.
Shet T, Agrawal A, Chinoy R, Havaldar R, Parmar V, Badwe R. Changes in the tumor grade and biological markers in locally advanced breast cancer after chemotherapy—implications for a pathologist. Breast J. 2007;13:457–64.
Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills M, Ashley S, Francis S, Walsh G, Smith IE. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer-a study from the IMPACT trialists. J Clin Oncol. 2005;23:2477–92.
Kasami M, Uematsu T, Honda M, Yabuzaki T, Sanuki J, Uchida Y, Sugimura H. Comparison of estrogen receptor, progesterone receptor and Her-2 status in breast cancer pre- and post-neoadjuvant chemotherapy. Breast. 2008;17:523–7.
Bertos NR, Park M. Breast cancer—one term, many entities? J Clin Investig. 2011;121:3789–96.
Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density: a hormonally responsive risk factor for breast cancer. J Br Menopause Soc. 2006;12(4):186–93.
Bertrand KA, Tamimi RM, Scott CG, Jensen MR, Pankratz V, Visscher D, et al. Mammographic density and risk of breast cancer by age and tumor characteristics. Breast Cancer Res. 2013;15:R104.
Rojas KI, Flores R, Flores CJ, Pinto JA, Gomez HL, Castañeda C. Mamographic density and disease-free survival in [HR+, HER2−] locally advanced breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol. 2014;32:e11536.
Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–7.
Tordai A, Wang J, Andre F, et al. Evaluation of biological pathways involved in chemotherapy response in breast cancer. Breast Cancer Res. 2008;10(2):R37.
Diorio C, Brisson J, Berube S, Pollak M. Genetic polymorphisms involved in insulin-like growth factor (IGF) pathway in relation to mammographic breast density and IGF levels. Cancer Epidemiol Biomark Prev. 2008;17:880e8.
Cho N, Im SA, Cheon GJ, Park IA, et al. Integrated 18F-FDG PET/MRI in breast cancer: early prediction of response to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2018;45(3):328–39.
Mottaghy FM. Is the whole larger than the sum of the parts? Integrated PET/MRI as a tool for response prediction. Eur J Nucl Med Mol Imaging. 2018;45:325–7.
Acknowledgements
We would like to extend our sincere gratitude to our departmental chair for the extensive support. In addition, we would like to give many thanks to our physicians, engineers, and nurses as well as other staff in the department.
Funding
This work was supported by the Heilongjiang Science and Technology Planning Project (Grants: YS17C22) and the Fundamental Research Funds for the Provincial Universities in 2018.
Author information
Authors and Affiliations
Contributions
ZY, ZXL, and ZDW designed this study. ZY, HYX, and ZDW collected data. WXL and ZXL performed the ultrasound and mammographic procedures. ZY, WXL, and HYX wrote this manuscript. ZDW and ZXL revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflicts of interest related to this work.
Ethics approval
Ethical approval was provided by the Institutional Review Board of Harbin Medical University. Ethical approval was obtained from the Hospital Research Ethics Committee.
Informed consent
Informed consent was obtained from all patients included in the study.
Consent for publication
A copy of the consent form can be requested at any stage.
Availability of data and materials
The data sets analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Y., Wang, X., Huang, Y. et al. Response to immunohistochemical markers’ conversion after neoadjuvant chemotherapy in breast cancer patients: association between imaging and histopathologic analysis. Clin Transl Oncol 22, 91–102 (2020). https://doi.org/10.1007/s12094-019-02112-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02112-z