Abstract
Theory predicts intraguild predation (IGP) to be unstable despite its ubiquity in nature, prompting exploration of stabilizing mechanisms of IGP. One of the many ways IGP manifests is through inducible trophic polymorphisms in the intraguild (IG) predator, where a resource-eating predator morph competes with the intraguild (IG) prey for the shared resource while a top predator morph consumes the IG prey. Cannibalism is common in this type of system due to the top predator morph’s specialization on the trophic level below it, which includes the resource-eating predator morph. Here, we explore the consequences of inducible trophic polymorphisms in cannibal predators for IGP stability using an IGP model with and without cannibalism. We employ linear stability analysis and identify regions of coexistence based on the top predator morph’s preference for conspecifics vs. heterospecifics and the IG prey’s competitive ability relative to the resource-eating morph. Our findings reveal that preferential cannibalism (i.e., the preferential consumption of conspecifics) stabilizes the system when the IG prey and resource-eating morph have similar competitive abilities for the shared resource. Though original IGP theory finds that the IG prey must be a superior resource competitor as a general criterion for coexistence, this is not typically the case when the predator has an inducible trophic polymorphism and the resource-eating morph is specialized in resource acquisition. Preferential cannibalism may therefore be a key stabilizing mechanism in IGP systems with a cannibalistic, trophic polymorphic IG predators, providing further insight into what general mechanisms stabilize the pervasive IGP interaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inducible trophic polymorphisms (ITPs), a form of phenotypic plasticity in which consumers can alter morphology, physiology, or behavior to change the trophic level on which they feed (Banerji and Morin 2009), have profound implications on food web dynamics. ITPs occur in a diverse array of taxa, including bacteria (Berleman and Kirby 2007), rotifers (Gilbert 1973), protists (Williams 1961; Giese 1973), insects (Greene 1989), gastropods (Padilla 2001), amphibians (Collins and Cheek 1983; Pfennig 1992), fish (Meyer 1990; Wainwright et al. 1991), and birds (Hulscher 1984; Matthysen 1989; Afik and Karasov 1995). ITPs are a flexible response to changing environmental conditions, enhancing an individual’s fitness in the present environment with regard to resource type and availability. Morphological traits related to feeding in particular are more flexible than once thought (Kishida et al. 2010). Consumption of a prey type can trigger a morphological shift and catalyze a positive feedback between consumption and morphological change (Padilla 2001). In some cases, morphology can even be flexibly reversed within an individual’s lifetime if conditions change (Olsson and Eklov 2005; Kishida and Nishimura 2006; Hoverman and Relyea 2007; Orizaola et al. 2012).
ITPs notably promote cannibalism by rearranging or expanding feeding apparatuses or increasing body size. This mitigates gape size limitation and facilitates consumption of larger prey, including conspecifics (Fox 1975; Polis 1981). The act of consuming larger prey itself can induce rapid growth and increased body size relative to conspecifics, further promoting cannibalism. Because of this, many taxa with ITPs also exhibit cannibal morphs, including fish (Persson et al. 2003; Ribeiro and Qin 2013; Hardie and Hutchings 2015; Amundsen 2016), reptiles (reviewed in Polis and Myers 1985), amphibians (Pfennig 1999; Takatsu and Kishida 2015), insects (de Block and Stoks 2004; Wissinger et al. 2004; Pervez et al. 2021), rotifers (Gilbert 1973), and protists (Kopp and Tollrian 2003; Banerji and Morin 2009). Though specialized morphology is required for some species to engage in cannibalism, for others, it is simply behavioral, even among closely related species (Jefferson et al. 2014).
Cannibalism is common in systems with intraguild predation (IGP) (Polis et al. 1989), where the intraguild (IG) predator competes with the intraguild (IG) prey for a shared resource (Holt and Polis 1997). Despite its ubiquity in nature (Arim and Marquet 2004), original IGP theory predicts considerable regions of instability. A general criterion for coexistence in classic IGP theory is that the IG prey must be a superior competitor for the shared resource (Holt and Polis 1997). At low resource levels, the IG prey outcompetes the IG predator, and at high resource levels, the IG predator reaches densities high enough to overexploit the IG prey. Regions of stable coexistence are therefore limited to intermediate resource levels, in which the strengths of predation and competition are balanced. IGP studies building off the original theory have generally assumed IG prey superiority (Křivan 2000; Faria and Costa 2009; Pal et al. 2014). However, this is unlikely in systems where IG predators exhibit ITPs. A smaller, resource-eating IG predator morph may be as competitive for the shared resource as the IG prey due to morphological resource specialization (Smith and Skúlason 1996) and size-dependent scaling of foraging and metabolic demands (Claessen et al. 2000). Because trophic polymorphic predators challenge the criterion of IG prey competitive superiority, other mechanisms must be at play to enable coexistence.
Cannibalism in the IG predator has been shown to stabilize IGP systems by regulating the predator population when the predator is efficient at exploiting both the IG prey and the shared resource (Rudolf 2007; Toscano et al. 2017; Bassar et al. 2023). However, in nature, the effect of cannibalism depends on the foraging behavior of the cannibal. Cannibalism can divert predation pressure away from IG prey and regulate the predator population, promoting coexistence, or it can strengthen predation pressure on the IG prey due to the increased energetic demands of large cannibal morphs. For instance, Takatsu and Kishida (2015) show that cannibalistic salamander morphs intensify negative impacts on heterospecific prey (tadpole larvae) as a result of the accelerated growth of cannibals. Conversely, others have shown that consumption of heterospecific prey can induce cannibalism (Hoffman and Pfennig 1999; Michimae and Wakahara 2001), relieving the IG prey of consumptive pressure. Stabilizing effects of cannibalism are thus heavily dependent upon the cannibalistic predator’s preference for conspecifics vs. heterospecific IG prey. Studies often assume, however, that the predator has equal or indifferent preference. Evidence of preference in IGP systems is mixed, even among closely related species and sometimes among different populations of the same species. Some studies find preference for conspecifics (Lannoo and Bachmann 1984; Leonardsson 1991; Whiteman et al. 2003; Burgio et al. 2005; Rudolf 2008; Byström et al. 2013; Pervez et al. 2021), others find preference for heterospecifics (Loeb et al. 1994; Gerber and Echternacht 2000; Schausberger and Croft 2000; Montserrat et al. 2006), and still others find a lack of preference either way (Schausberger and Croft 2000; Yasuda et al. 2001; Rudolf 2008). Because predator preference directly alters the strengths of predation and competition experienced by the IG prey, preference is a key determinant of IGP coexistence. The consequences of predator preference, and how it interacts with other determining factors of IGP coexistence such as competitive ability, are nonetheless still unclear.
The present work is motivated by two factors. First, ITPs have uniquely strong effects on the balance of predation and competition in IGP systems by (1) promoting cannibalism and (2) challenging the IG prey competitive superiority criterion. Second, not only is there evidence that IG prey are not always competitively superior to IG predators (Diehl 1995; Navarrete et al. 2000; Vance-Chalcraft et al. 2007; Crumrine 2010), multiple literature reviews find a high propensity for preferential cannibalism, i.e., preference for conspecifics (in piscivorous fish, Byström et al. (2013); across a wide range of taxa, Toscano et al. (2017)). These two aspects of the IGP system–competitive superiority and preferential cannibalism–may therefore be inextricably linked in driving coexistence, particularly in IGP systems with trophic polymorphic predators. However, efforts to incorporate ITPs into IGP models have been limited (see Orlando et al. (2011) as the only exception to our knowledge). This is surprising given their disproportionately large potential for altering system stability through the nature of the two morphs and their consequences on coexistence.
In the present paper, we ask: How does the presence of cannibalism and preferential feeding in a trophic polymorphic IG predator interact with competitive ability of the IG prey to influence the long-term stability of an IGP system? We model an IGP system with a cannibalistic, trophic polymorphic IG predator. We include a preference term to explore the effects of the top predator morph’s preference for conspecifics (resource-eating predator morph) vs. heterospecifics (IG prey) and alter the assumption of IG prey superiority. We find that preferential cannibalism (i.e., preference for conspecifics) expands the region of stable coexistence when the IG predator and IG prey are equal competitors for the resource. This prediction, which can be tested in natural systems, is compelling given that both cannibalism in the top predator morph and similar competitive ability between the resource-eating predator morph and IG prey are likely in IGP systems with trophic polymorphic IG predators. Since this is one of the many ways in which the intraguild predation interaction manifests in nature, preferential cannibalism may be a key stabilizing mechanism in systems with trophic polymorphic IG predators.
Methods
To examine the effect of preferential cannibalism in a trophic polymorphic predator on IGP system stability, we compared two models of varying complexity (Fig. 1) under two scenarios pertaining to IG prey competitive ability. The first model (referred to herein as the “base” model) is an extension of the original Lotka-Volterra IGP model first proposed by Holt and Polis (1997) with the separation of the IG predator into two states: a resource-eating morph that competes with the IG prey for the shared resource and a top predator morph that consumes the IG prey. Biomass moves from one state to the other as a function of resource density, which is intentionally general to encompass changes in frequency of morphs across generations or individuals switching between morphs in a lifetime. In the second model (referred to herein as the “full” model), we build upon the first model to include cannibalism in the IG predator, where the top predator morph consumes both the IG prey and the resource-eating predator morph. We further include a preference parameter, s, that controls the top predator morph’s preference for conspecifics (resource-eating predator morph) or heterospecifics (IG prey). We explore preference over a range of s values, specifically three values of s which represent preference for conspecifics (\(s=0.7\)), preference for heterospecifics (\(s=0.3\)), and no preference (\(s=0.5\)).
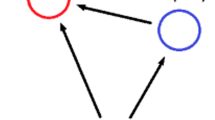
Visual depictions of the two intraguild predation models used in this study. a The base model (Eq. (1)) includes a trophic polymorphic IG predator that switches between two states as a function of prey density: a top predator morph, Z, that feeds on the IG prey, N, and a resource-eating morph, P, that feeds on the shared resource, R. b The full model (Eq. (2)), which builds on the base model to include cannibalism and a preference parameter. Preference for conspecifics occurs when \(s>0.5\); preference for heterospecifics occurs when \(s<0.5\); and no preference occurs when \(s=0.5\). Solid arrows indicate feeding relationships, and broken arrows indicate switching between the two IG predator morphs
Our base IGP model consists of a basal resource R, the IG prey N, and an IG predator, with two distinct states representative of the discrete morphotypes due to the ITP: morphotype P, which eats the resource (R), and morphotype Z, which eats the IG prey (N). The predator switches between the two states at a per capita rate dependent on resource density. This base model consists of four ordinary differential equations:
Model parameters are defined in Table 1. We assume the resource is self-regulated by logistic growth, and consumers forage with type I linear functional responses and suffer constant, density-independent mortality. We also explored versions of these models where the consumers have a type II saturating functional response, the results of which are qualitatively similar and presented in SI 3. We assume that individuals in the P state solely consume the resource and that individuals in the Z state solely consume the IG prey. We further assume density-dependent switching such that foraging effort switches from one state to the other as a function of the biomass density in their own state and availability of prey in the new state. This is to say that biomass of one morph is directed to that of the other morph when the former is crowded and when the resource of the latter is abundant. Without the discrete morphological states and switching between them, this base model collapses to the original IGP model.
Our full model includes cannibalism in the top IG predator morph and a preference parameter, s, to explore the effect of top predator preference for conspecifics (resource-eating predator morph, P) vs. heterospecifics (IG prey, N). Now, morphotype Z eats both the IG prey (N) and conspecifics (P). System dynamics are given by the following:
Here, we assume that cannibalism occurs unidirectionally from predator morph Z onto predator morph P. This makes sense in the context of ITPs, in which one morph is either larger or has morphological, physiological, or behavioral specializations that equips it to consume larger prey (i.e., the IG prey and smaller conspecifics), while the other morph is typically smaller and specialized to feed on the basal resource. Such trophic heterogeneity promotes the occurrence of cannibalism from the larger morph onto the smaller morph and not vice versa.
The switching function in the full model is slightly more complex compared to the base model due to the cannibalism link. Switching into the Z state (and out of the P state) is now a function of both N and P, because Z consumes both the IG prey and the resource-eating morph. Thus, there are two switching parameters dictating switching rate into the Z state: one dependent on IG prey density (\({u}_{NP}\)) and the other dependent on P density (\({u}_{PP}\)). As with the base model, biomass more readily switches from one state to the other when the former state is high in density and the prey species of the latter state is abundant. Following Orlando et al. (2011), we define fitness on the morphological level as the per capita growth rate of a morph’s population excluding the addition or subtraction of individuals from the other morph. The purpose of defining fitness on the morphological level is that the dynamic fitness differences between the two morphs are a key aspect of our model and an important driver of system dynamics.
Model parameterization
Model parameterization follows that used in the original IGP framework (Holt and Polis 1997) for comparability to earlier work (Table 1). By assuming equal attack rates and conversion efficiencies of the top IG predator morph on its prey, we explore preference by altering the preference parameter, s. We therefore interpret \(s=0.5\) as being an equal preference for IG prey and resource-eating predator morph. We use the phrase “preferential cannibalism” to denote that s is greater than 0.5, and therefore the top predator morph Z preferentially consumes conspecifics, P. Similarly, “heterospecific preference” denotes situations when s is less than 0.5, and the top predator morph Z preferentially consumes heterospecific IG prey, N. We focus on three scenarios using the following parameterizations: heterospecific preference (\(s=0.3\)), preferential cannibalism (\(s=0.7\)), and no preference (\(s=0.5\)).
We further explore two scenarios pertaining to the superiority of the IG prey, N, over the resource-eating predator morph, P, for the shared resource. Original IGP theory found a general criterion for coexistence to be that the IG prey is a superior resource competitor (Holt and Polis 1997), and subsequent IGP models have generally assumed IG prey superiority (Křivan 2000; Faria and Costa 2009; Pal et al. 2014). However, when the predator has an ITP that results in two separate states, each specialized on their given resource, it is safe to assume that the resource-eating morph is equipped with a similarly strong competitive ability for the shared resource as the IG prey. To explore the effects of IG prey competitive superiority, or lack thereof, we present two scenarios: one in which N and P are equal competitors and one in which N is superior to P. We do not consider a situation in which the resource-eating morph is superior to the IG prey, as this would largely lead to IG prey extinction due to the predator’s inherent advantage over the prey (consuming its competitor). Under the case of “equal competitors,” the resource-eating predator morph and IG prey are equal competitors for the shared resource: \({c}_{NR}={c}_{PR}=0.5\). Under the case of “prey superiority,” the IG prey is a superior resource competitor, such that \({c}_{NR}=1\) and \({c}_{PR}=0.5\).
We keep the predator morph switching parameters equal to 0.5 and assume that switching happens on a time scale similar to other demographic processes. Though studies quantifying switching rates between morphs are limited, this would reflect morphological switching in organisms such as protists (Banerji and Morin 2009; Orlando et al. 2011). However, lags in switching rates can destabilize population dynamics (Padilla and Adolph 1996; Abrams 2010), and switching that is too rapid can be maladaptive (Kath et al. 2022). These cases typically involve behavioral lags, such as lags in decision making on the part of the predator. Our implementation of switching, however, can be thought of as the sensitivity of biomass transfer rates from one state to the other in response to density changes. Nevertheless, we explore the effects of switching parameters in SI 2.
Lastly, we vary the resource carrying capacity between \(K=1\) and \(K=50\) to examine a wide range of productivity levels for the purpose of relating our results to previous work. Productivity is of interest for two reasons. First, high productivity has been shown to destabilize food webs in general (e.g., paradox of enrichment, Rosenzweig 1971). Second, productivity alters the relative strengths of competition and predation in IGP systems, heavily affecting coexistence outcomes (Holt and Polis 1997; Diehl and Feißel 2000). Original theory found the IG predator overexploits the IG prey at high resource carrying capacities, but work since then has found coexistence to be possible at high productivity levels with the incorporation of various stabilizing mechanisms (e.g., adaptive foraging, Křivan 2000; alternative resources, Daugherty et al. 2007; cannibalism, Rudolf 2007; prey preference, Faria and Costa 2009). We therefore explore the consequences of preferential cannibalism across a gradient of productivity.
Analysis
Following numerical analyses performed in existing cannibalism studies (Rudolf 2006; Orlando et al. 2011; Bassar et al. 2023), we performed numerical simulations and explored stability in our models using linear stability analysis (Gurney and Nisbet 1998; Murdoch et al. 2013). In short, we linearized Eqs. (1) and (2) around their interior equilibrium and then examined the stability of these systems to small perturbations. We first solved for the equilibrium in which all species have positive, non-zero abundances using the “Solve” function in Mathematica (Wolfram Research, Inc., Mathematica, Version 12.0, Champaign, IL, 2019). We then evaluated the Jacobian matrix at this solution and numerically computed the eigenvalues using the “Eigenvalues” function, selecting the eigenvalue(s) in which the real part is negative and therefore stable. We further performed numerical simulations to show the effects of preference and competitive superiority on dynamics and equilibrium densities in SI 1. Dynamics were simulated in the R programming language (v4.2.3; R Core Team 2023) using the R package “deSolve deSolve” (v1.34; Soetaert et al. 2010).
Results
Within the region of four-species coexistence, the preference parameter, s, is negatively related to the IG prey’s superiority over the resource-eating morph, P, for the shared resource (Fig. 2). When the IG prey, N, is a similar competitor as P for the resource (\({c}_{NR}\) is close to 0.5 and \({c}_{PR}=0.5\)), the region of coexistence exists in the parameter space in which preference is skewed towards conspecifics (\(s>0.5\), between ~0.6 and ~0.8). As N becomes increasingly superior to P for the resource (\({c}_{NR}\) approaches 1 as \({c}_{PR}\) remains 0.5), coexistence is maintained when preference switches from conspecific preference (\(s>0.5\)), to no preference (\(s=0.5\)), and eventually to heterospecific preference (\(s<0.5\)). Outside of the region of coexistence, if preference for conspecifics is too high, the IG predator drives itself extinct through heavy preferential cannibalism, resulting in a stable equilibrium of just the IG prey and the resource (NR). If the preference parameter is too low (preference heavily skewed towards heterospecifics), the IG predator drives the IG prey extinct through strong intraguild predation, resulting in a stable equilibrium of the IG predator morphs with the resource (ZPR). See Section 2 of the Supplementary Information for numerical simulations of the dynamics in each of these three regions (Fig. S1).
Regions of stable coexistence across the ratio of resource consumption rate by the IG prey to that of the resource-eating predator morph, \({c}_{NR}/{c}_{PR}\), and preference parameter, s. Orange regions denote stable coexistence between all species and blue regions denote stable equilibria between certain species. R is the resource, N is the IG prey, P is the resource-eating IG predator morph, and Z is the top IG predator morph. When \({c}_{NR}/{c}_{PR}=1\), the IG prey and resource-eating morph are equal competitors for the shared resource. As \({c}_{NR}/{c}_{PR}\) increases, the IG prey becomes increasingly more competitively superior to the resource-eating morph
When the IG prey, N, is a superior competitor for the shared resource, R, the model without cannibalism is stable across a wide range of top predator consumption rates on the IG prey and resource carrying capacities (Fig. 3a). In the case of IG prey superiority, cannibalism greatly decreases coexistence overall (Fig. 3b–d). The region of stable NR equilibrium increases when cannibalism is added. The IG predator has a disadvantage by cannibalizing the resource-eating morph, P, which suffers strong competitive pressure from the superior IG prey. Strong cannibalism and competition lead to a dwindling P population, reducing the amount of biomass that can switch into the top predator morph, Z, resulting in extinction. Note the large region of stable ZPR equilibrium that is introduced under the heterospecific preference scenario (\(s=0.3\), Fig. 3b). Here, Z excludes N through a combination of preferential consumption of N and benefit of having an alternative resource, P. Conversely, when Z preferentially consumes conspecifics (\(s=0.7\), Fig. 3d), N confers an advantage from not being consumed as heavily as its competitor. The IG predator largely drives itself extinct through the preferential consumption of conspecifics, P, which are competitively inferior to the IG prey, resulting in a large region of NR equilibrium. The region of coexistence is maximized when preference is not skewed either way (\(s=0.5\), Fig. 3c), wherein the IG prey’s competitive advantage is balanced out by the top predator morph’s equal consumption of N and P.
Regions of stability across carrying capacity, K, and the top predator morph’s consumption rate on the IG prey, \({c}_{ZN}\), when the prey is superior (a–d) and when the prey and predator are equal resource competitors (e–h). Examined across four scenarios: base model without cannibalism (Eq. (1), a, e), and full model (Eq. (2)) with heterospecific preference (b, f), no preference (c, g), and conspecific preference (d, h). Variables are defined in Fig. 2. Orange regions denote stable coexistence between all species, blue regions denote stable equilibria between certain species, and gray regions denote neutral equilibria
When the IG prey and resource-eating morph are equal competitors for the resource, the region of coexistence is nonexistent without cannibalism (Fig. 3e). Cannibalism is not present here to regulate the predator population and prevent overexploitation of the IG prey, which no longer benefits from being the superior resource competitor. Including cannibalism and a preference for heterospecifics (\(s=0.3\), Fig. 3f) introduces a negligible region of coexistence. Without preference either way (\(s=0.5\), Fig. 3g), there is a slight increase in the space of coexistence. Coexistence greatly increases when cannibals preferentially consume conspecifics (\(s=0.7\), Fig. 3h) due to greater regulation of the IG predator population, reducing overexploitation of the IG prey. When the IG prey is not competitively superior, preferential cannibalism simultaneously reduces predatory pressure from Z and competitive pressure from P on the IG prey, facilitating its persistence over a larger range of IG predation rates.
Increasing the resource carrying capacity, K, does not lead to the extinction of the IG prey in any of the model formulations (Fig. 3a–h) due to the stabilizing nature of density-dependent switching. We find that all species’ equilibrium densities increase and saturate with increasing values of resource carrying capacity (Fig. S2), as has been shown before. We further find that the results presented in this paper are not sensitive to switching rates (Fig. S3). The results do not change drastically when consumers have a type II functional response (Fig. S4). Though neither of the present models with type I functional responses yield limit cycles, we find that these models with type II functional responses cause limit cycles only under high values of the preference parameter (Fig. S5), and the cycles increase in amplitude as carrying capacity increases (Fig. S6).
The main result that conspecific preference maximizes coexistence when the prey and predator are equal competitors is robust to multiple parameter combinations (Figs. 3, 4 and 5). Comparing the top predator morph’s consumption rate on the IG prey, \({c}_{ZN}\), against the top predator morph’s conversion efficiency on the IG prey, \({e}_{ZN}\), produces similar results that preferential cannibalism maximizes the region of coexistence when the IG prey and resource-eating morph are similar resource competitors (Fig. 4). When the IG prey is a superior competitor, coexistence is maximized without cannibalism (Fig. 4a), and adding cannibalism to the model greatly reduces stability regardless of preference (Fig. 4b–d). A lack of preference leads to the largest region of coexistence when cannibalism is present in the model (\(s=0.5\), Fig. 4c). When the IG prey and resource-eating predator morph are equal competitors, preferential cannibalism maximizes coexistence (\(s=0.7\); Fig. 4h). Here, we begin to see regions of bistability appear, in which there exists a stable equilibrium of the IG predator morphs with the resource (ZPR) or just the IG prey with the resource (NR).
Regions of stability across the top predator morph’s consumption rate on the IG prey, \({c}_{ZN}\), and the top predator morph’s conversion efficiency on the IG prey, \({e}_{ZN}\), when the prey is superior (a–d) and when the prey and predator are equal resource competitors (e–h). Examined across four scenarios: base model without cannibalism (Eq. (1), a, e), and full model (Eq. (2)) with heterospecific preference (b, f), no preference (c, g), and conspecific preference (d, h). Variables are defined in Fig. 2. Orange regions denote stable coexistence between all species, blue regions denote stable equilibria between certain species, gray regions denote neutral equilibria, and yellow regions denote bistability
Regions of stability across the top predator morph’s consumption rate on the IG prey, \({c}_{ZN}\), and the top predator morph’s consumption rate on conspecifics, \({c}_{ZP}\), when the prey is superior (a–c) and when the prey and predator are equal resource competitors (d–f). Examined across three scenarios: the full model (Eq. (2)) with heterospecific preference (a, d), no preference (b, e), and conspecific preference (c, f). Variables are defined in Fig. 2. Orange regions denote stable coexistence between all species, blue regions denote stable equilibria between certain species, gray regions denote neutral equilibria, and yellow regions denote bistability
Finally, we look at the top predator’s consumption rate on the IG prey, \({c}_{ZN}\), against the top predator’s cannibalism consumption rate on conspecifics, \({c}_{ZP}\) (Fig. 5). Only the three preference scenarios are compared since the base model does not include cannibalism and therefore does not have the \({c}_{ZP}\) term. Again, we find the same pattern, further supporting the main result that preferential cannibalism stabilizes the IGP system by maximizing the region of coexistence when the prey and predator are equal competitors (Fig. 5g). Similar to Fig. 4, bistability occurs in a small region where either the IG prey persists with the resource (NR) or the IG predator morphs persist with the resource (ZPR).
Discussion
Holt and Polis’ (1997) seminal paper on intraguild predation (IGP) predicted unstable dynamics under most conditions. Studies have since found IGP to be ubiquitous in natural food webs (Arim and Marquet 2004), prompting an exploration of stabilizing mechanisms. IGP manifests in many ways, one being through inducible trophic polymorphisms in the IG predator, where the top predator morph consumes the IG prey and the resource-eating morph competes with the IG prey for the shared resource (Banerji and Morin 2009). ITPs promote cannibalism in the predator by causing a divergence in the trophic levels on which each morph feeds along with facilitating size heterogeneity (Kopp and Tollrian 2003). Our study produces predictions of IGP stability in systems with cannibalistic, trophic polymorphic IG predators: preferential cannibalism in the top predator morph relaxes the requirement for IG prey competitive superiority, promoting coexistence when the prey and predator are equal competitors for the resource. This result is robust across a wide range of parameter values (Figs. 3, 4, 5 and S3). Preferential cannibalism may therefore be a strong stabilizing mechanism in natural IGP systems when the IG predator has an inducible trophic polymorphism and is a strong competitor, providing further understanding as to the conditions in which IGP exists stably in nature. To our knowledge, this is the first theoretical study to jointly explore inducible trophic polymorphisms, cannibalism, and preference on IGP stability.
The present results are especially compelling given that (1) IG prey are not likely to be competitively superior when the IG predator exhibits an inducible trophic polymorphism due to morphological resource specializations (Smith and Skúlason 1996) and size-dependent scaling of foraging and metabolic demands (Claessen et al. 2000) and (2) preferential cannibalism is widespread (Byström et al. 2013; Toscano et al. 2017). Our work combines these two phenomena to show that the interaction between them promotes IGP stability when the IG predator has an inducible trophic polymorphism. The underlying mechanism is preferential cannibalism as a strong form of intraspecific density dependence in the IG predator population (Polis 1981). By preferentially consuming conspecifics, the top predator morph regulates its own population and promotes the persistence of the IG prey by both diverting predatory pressure away from the IG prey and limiting the population size of its equally strong competitor, the resource-eating morph. This allows the IG prey to coexist with the IG predator without being the superior resource competitor, a general criterion of original IGP theory (Polis and Holt 1992; Holt and Polis 1997). Our results therefore indicate that preferential cannibalism in trophic polymorphic IG predators may be a key reason why we often see in nature that the IG prey is not competitively superior (Diehl 1995; Vance-Chalcraft et al. 2007; Crumrine 2010). Though we did not examine the case of the IG predator as a superior resource competitor, others have shown that coexistence is similarly enhanced when stabilizing mechanisms are at play, such as a high cannibalism consumption rate (Rudolf 2006; Toscano et al. 2017), alternative prey (Daugherty et al. 2007), or prey switching (Wei 2019).
Prey switching is a stabilizing mechanism that enhances coexistence over a broad range of conditions in omnivory models (Abrams and Matsuda 2004; Faria and Costa 2009; Pal et al. 2014; Wang et al. 2018; Wei 2019), preventing IG prey overexploitation at high carrying capacities (Křivan 2000; Křivan and Diehl 2005; Faria and da Silveira Costa 2010). Though the switching mechanism we consider here is not explicitly behavioral, we see a similar stabilizing effect from the density-dependent switching between morphs. This stabilizing effect is most clearly illustrated in the large regions of coexistence in Figs. 3a, 4a and 5a. This scenario is closest to the original Lotka-Volterra IGP model, which predicts limited coexistence even when the prey is a superior competitor, resulting in IG prey overexploitation at high carrying capacities (Holt and Polis 1997). The only difference in our base model is the inclusion of switching between morphs. Without switching, we recover the original IGP prediction of reduced coexistence as carrying capacity increases. Instead, we see coexistence maintained at high carrying capacities (Fig. 3a). When the IG prey is not competitively superior, switching alone is not enough to stabilize the system, as has been found in similar IGP models, such as those that employ adaptive foraging (Křivan 2000).
Studies show that switching is stabilizing unless there is a significant lag in switching between states (Padilla and Adolph 1996; Abrams 2010) or switching occurs at a high rate (Kath et al. 2022). Although we do not incorporate explicit time lags in shifts between morphological states, our results are robust to a broad range of switching parameter values (SI 2). Nonetheless, many species, notably protists with inducible trophic polymorphisms, have quick response times. For example, Tetrahymena vorax takes about 4.5 h to switch states, which is about half of their generation time of 8 h (Orlando et al. 2011).
The present study is intentionally general to encompass the many forms that inducible trophic polymorphisms can take. Our separate IG predator state variables and switching functions represent changes in biomass as a function of prey availability, which can occur within or across generations. In some species, morphotypes are induced during development, and individuals are more or less fixed in that morphology throughout their lifetime, such as distinct cannibal fish morphotypes (e.g., perch, Persson et al. 2003; barramundi, Ribeiro and Qin 2013; cod, Hardie and Hutchings 2015; charr, Amundsen 2016). Others can flexibly reverse morphology in their lifetime, typically up until a point in development (e.g., jaw morphology in cichlids, Meyer 1990; broad-headed morphology in salamanders, Michimae and Wakahara 2002; snout and body shape in perch, Olsson and Eklöv 2005; body and tail depth in tadpoles, Kishida and Nishimura 2006, Orizaola et al. 2012). Still others, like protists and rotifers, can respond to changing conditions at almost any point in their life cycle, rearranging buccal cavities when larger prey becomes available and dividing to typical morphs when that prey is depleted (Williams 1961; Giese 1973; Kopp and Tollrian 2003; Banerji and Morin 2009). Because we assume biomass is shifting from one predator state to another, our model provides a general framework that can be modified in the future to explore more specific forms of ITPs.
Our study is motivated by a well-known protist system: IG predator Blepharisma, IG prey Tetrahymena, and shared resource, bacteria (Diehl and Feißel 2000; Price and Morin 2004). Blepharisma is an omnivorous protist that has an inducible trophic polymorphism and also engages in cannibalism (Giese 1973; Lennartz and Bovee 1980). The microstome state is small and specialized on bacteria, while the macrostome state is large with gape structures specialized for feeding on smaller protists, such as bacterivorous Tetrahymena and conspecifics. When small protists are present, Blepharisma individuals that are large enough to capture smaller protists experience a positive feedback of enlargement and rearrangement of the buccal cavity and oral ciliature, facilitating growth into macrostomes. Macrostomes can then either divide into microstomes, if protist prey is depleted, or macrostomes, if protist prey is still available (Woodie, personal observation). This inducible expression of morphology is similar to that seen in an amphibian or fish, where the large cannibal morphs can be induced during development if conditions call for it, but their future offspring can flexibly take on a different morphology if conditions change.
Although this is the first study to examine preferential cannibalism in a trophic polymorphic IG predator, the results can be compared to the only other study to our knowledge of cannibalism and ITPs in IG predators (Orlando et al. 2011). Orlando et al. (2011) modeled cannibalism and different switching strategies between morphs in the protist, Tetrahymena vorax. They found constant switching rates between cannibals and typical morphs stabilize population dynamics, whereas variable switching (switching rates that increase fitness) leads to exclusion of the IG prey. This makes sense in light of the model parameterization; the cannibalistic top predator morph has a higher attack rate and conversion efficiency on the IG prey than conspecifics. The predator thus confers too much of an advantage, switching between morphs to maximize their fitness along with consuming IG prey more heavily than conspecifics. There is no mechanism at play to prevent the exclusion of the IG prey (e.g., preferential cannibalism). Our dynamic switching function is similar to their variable switching function in that predators more readily switch out of morphological states when intraspecific competition is high or their given resource is in low abundance, but the mechanism preventing the predator from having too much of a benefit is the preferential consumption of conspecifics when the predator and prey are similar resource competitors (s = 0.7; Figs. 3h, 4h and 5f). When the prey has a bit more of an advantage through superior resource competition, conspecific preference is no longer necessary, and cannibalism alone—without preference either way—is enough to facilitate coexistence (s = 0.5; Figs. 3c, 4c and 5b).
To date, cannibalism in IGP systems has primarily been studied in the context of life history IGP, in which adult predators prey on juvenile conspecifics and heterospecific IG prey (Rudolf 2006; Toscano et al. 2017; Hin and de Roos 2019; Bassar et al. 2023). These studies find cannibalism in LHIGP systems can promote coexistence, even when the IG prey is not competitively superior, suggesting that cannibalism plays a similar stabilizing role regardless of the type of stage structure (LHIGP or ITPs). For instance, Toscano et al. (2017) found in a study of cannibalism in LHIGP systems that only if juvenile IG predators are superior competitors for the resource can cannibalism promote coexistence. The mechanism is the same as in the present study: When the IG prey is competitively superior, strong cannibalism reduces the juvenile (or resource-eating predator morph) population, leading to competitive exclusion. This is why we see large regions of NR equilibrium when the IG prey is superior and the top predator morph preferentially consumes conspecifics (Figs. 3d, 4d and 5c). Furthermore, Hin and de Roos (2019) find that a high cannibalistic preference, in combination with juvenile predator resource specialization, leads to coexistence in LHIGP systems. It is compelling that the conditions leading to the results in the present study of a particular type of stage structure (ITPs) align well with studies that employ a different type of stage structure (LHIGP).
Since the consumption of conspecifics necessarily reduces overall population size, cannibalism generally increases the fitness of individuals but not populations (Polis 1981). Because of this, cannibalism studies have consistently considered fitness at levels lower than the whole population, typically the individual level (see reviews and citations within: Fox 1975; Polis 1981; Rosenheim and Schreiber 2022) or the morphological level (the present study, and in Orlando et al. 2011). This is because cannibalism, by nature of removing individuals from the population, naturally has a negative effect on population growth rate. The negative population-level effect is a crucial population regulation mechanism that has been shown to stabilize many models with cannibalism (see reviews and citations within: Claessen et al. 2004; Rosenheim and Schreiber 2022), especially those involving IGP interactions (Rudolf 2006; Toscano et al. 2017; Bassar et al. 2023). However, previous IGP models define fitness on the population level (Křivan 2000). Under this definition of fitness, cannibalism is often considered maladaptive especially when parameterization allows for the population regulation effect. Considering fitness at the population level is interesting for future cannibalism studies. The potential for cannibalism to be stabilizing while simultaneously increasing total population growth rate will likely depend on the type of cannibalism, mentioned briefly in a recent review (Rosenheim and Schreiber 2022). For instance, cannibalism in the form of cannibal morphotypes almost always decreases overall population growth rates by nature of cannibal morphs increasing in density quickly and inflicting strong conspecific mortality (Persson et al. 2003; Amundsen 2016). Conversely, filial cannibalism can induce positive population growth rate as it is a unique form of parental care (Rosenheim and Schreiber 2022). Future studies should explore the potential for population regulation in IG predators with cannibal morphotypes using a population-level definition of fitness, i.e., can cannibalism have a regulatory effect while also increasing the growth rate of the population?
Conclusion
Despite decades of focused attention, there are still major gaps in our understanding of what mechanisms stabilize intraguild predation interactions, which are widespread in nature and diverse in the ways they manifest. Inducible trophic polymorphisms are one way that IGP manifests in nature, yet efforts to incorporate them into the theoretical framework of IGP are extremely limited (but see Orlando et al. 2011). The present study finds that preferential cannibalism is the mechanism responsible for enhancing coexistence between cannibalistic trophic polymorphic IG predators and IG prey that lack competitive superiority. These results are compelling given that both cannibalism and similar competitive ability between the resource-eating IG predator morph and IG prey are particularly likely in IGP systems with trophic polymorphic IG predators. This is because ITPs challenge the assumption of IG prey superiority and promote cannibalism. The results of the present study may help explain why evidence suggests two common occurrences in IGP systems are preferential cannibalism (reviewed by Byström et al. 2013 and Toscano et al. 2017) and lack of IG prey competitive superiority (Diehl 1995; Navarrete et al. 2000; Vance-Chalcraft et al. 2007; Crumrine 2010). Preferential cannibalism may therefore be a key stabilizing mechanism in systems with trophic polymorphic IG predators that compete strongly with their IG prey. The present work contributes to broader efforts to understand what mechanisms drive coexistence of the ubiquitous IGP interaction in order to bridge the gap between theory and nature.
Data availability
The code used to generate the data in this study is available through a Dryad Digital Repository: https://doi.org/10.5061/dryad.t4b8gtj76.
References
Abrams PA (2010) Implications of flexible foraging for interspecific interactions: lessons from simple models. Funct Ecol 24(1):7–17. https://doi.org/10.1111/j.1365-2435.2009.01621.x
Abrams PA, Matsuda H (2004) Consequences of behavioral dynamics for the population dynamics of predator-prey systems with switching. Popul Ecol 46(1):13–25. https://doi.org/10.1007/s10144-003-0168-2
Afik D, Karasov WH (1995) The trade-offs between digestion rate and efficiency in warblers and their ecological implications. Ecology 76(7):2247–2257. https://doi.org/10.2307/1941699
Amundsen PA (2016) Contrasting life-history strategies facilitated by cannibalism in a stunted Arctic charr population. Hydrobiologia 783(1):11–19. https://doi.org/10.1007/s10750-015-2600-y
Arim M, Marquet PA (2004) Intraguild predation: a widespread interaction related to species biology: Intraguild predation. Ecol Lett 7(7):557–564. https://doi.org/10.1111/j.1461-0248.2004.00613.x
Banerji A, Morin PJ (2009) Phenotypic plasticity, intraguild predation and anti-cannibal defences in an enigmatic polymorphic ciliate. Funct Ecol 23(2):427–434. https://doi.org/10.1111/j.1365-2435.2008.01499.x
Bassar RD, Coulson T, Travis J (2023) Size-dependent intraguild predation, cannibalism, and resource allocation determine the outcome of species coexistence. Am Nat 201(5):712–724. https://doi.org/10.1086/723600
Berleman JE, Kirby JR (2007) Multicellular development in myxococcus xanthus is stimulated by predator-prey interactions. J Bacteriol 189(15):5675–5682. https://doi.org/10.1128/JB.00544-07
Burgio G, Santi F, Maini S (2005) Intra-guild predation and cannibalism between Harmonia axyridis and Adalia bipunctata adults and larvae: laboratory experiments. Bull Insectology 58(2):135
Byström P, Ask P, Andersson J, Persson L (2013) Preference for cannibalism and ontogenetic constraints in competitive ability of piscivorous top predators. PLoS ONE 8(7):e70404. https://doi.org/10.1371/journal.pone.0070404
Claessen D, de Roos AM, Persson L (2000) Dwarfs and giants: cannibalism and competition in size-structured populations. Am Nat 155(2):219–237. https://doi.org/10.1086/303315
Claessen D, de Roos AM, Persson L (2004) Population dynamic theory of size–dependent cannibalism. Proc Royal Soc B 271(1537):333–340. https://doi.org/10.1098/rspb.2003.2555
Collins JP, Cheek JE (1983) Effect of food and density on development of typical and cannibalistic salamander larvae in ambystoma tigrinum nebulosum. Am Zool 23(1):77–84. https://doi.org/10.1093/icb/23.1.77
Crumrine PW (2010) Size-structured cannibalism between top predators promotes the survival of intermediate predators in an intraguild predation system. J North Am Benthol Soc 29(2):636–646. https://doi.org/10.1899/09-006.1
Daugherty MP, Harmon JP, Briggs CJ (2007) Trophic supplements to intraguild predation. Oikos 116(4):662–677. https://doi.org/10.1111/j.0030-1299.2007.15378.x
De Block M, Stoks R (2004) Life history responses depend on timing of cannibalism in a damselfly. Freshw Biol 49(6):775–786. https://doi.org/10.1111/j.1365-2427.2004.01224.x
Diehl S (1995) Direct and indirect effects of omnivory in a littoral lake community. Ecology 76(6):1727–1740. https://doi.org/10.2307/1940706
Diehl S, Feißel M (2000) Effects of enrichment on three-level food chains with omnivory. Am Nat 155(2):200–218. https://doi.org/10.1086/303319
Faria LD, da Silveira Costa MI (2010) Omnivorous food web, prey preference and allochthonous nutrient input. Ecol Complex 7(1):107–114. https://doi.org/10.1016/j.ecocom.2009.08.003
Faria LD, Costa MIS (2009) The interplay among prey preference, nutrient enrichment and stability in an omnivory system. Braz J Biol 69(4):1027–1035. https://doi.org/10.1590/S1519-69842009000500006
Fox LR (1975) Cannibalism in natural populations. Annu Rev Ecol Syst 6(1):87–106
Gerber GP, Echternacht AC (2000) Evidence for asymmetrical intraguild predation between native and introduced Anolis lizards. Oecologia 124(4):599–607. https://doi.org/10.1007/s004420000414
Giese AC (1973) Blepharisma: the biology of a light sensitive protozoan. Stanford Univ. Press, pp 266–303
Gilbert JJ (1973) Induction and ecological significance of gigantism in the rotifer Asplanchna sieboldi. Science 181(4094):63–66. https://doi.org/10.1126/science.181.4094.63
Greene E (1989) A diet-induced developmental polymorphism in a caterpillar. Science 243(4891):643–646. https://doi.org/10.1126/science.243.4891.643
Gurney W, Nisbet RM (1998) Ecological dynamics. Oxford University Press
Hardie DC, Hutchings JA (2015) Cannibalistic growth polyphenism in Atlantic cod. Evol Ecol Res 16(7):569–580
Hin V, de Roos AM (2019) Cannibalism prevents evolutionary suicide of ontogenetic omnivores in life-history intraguild predation systems. Ecol Evol 9(7):3807–3822. https://doi.org/10.1002/ece3.5004
Hoffman EA, Pfennig DW (1999) Proximate causes of cannibalistic polyphenism in larval tiger salamanders. Ecology 80(3):1076–1080. https://doi.org/10.1890/0012-9658(1999)080[1076:PCOCPI]2.0.CO;2
Holt RD, Polis GA (1997) A theoretical framework for intraguild predation. Am Nat 149(4):745–764. https://doi.org/10.1086/286018
Hoverman JT, Relyea RA (2007) How flexible is phenotypic plasticity? Developmental windows for trait induction and reversal. Ecology 88(3):693–705. https://doi.org/10.1890/05-1697
Hulscher JB (1984) Growth and abrasion of the oystercatcher bill in relation to dietary switches. Neth J Zool 35(1–2):124–154. https://doi.org/10.1163/002829685X00109
Jefferson DM, Ferrari MC, Mathis A, Hobson KA, Britzke ER, Crane AL, Blaustein AR, Chivers DP (2014) Shifty salamanders: Transient trophic polymorphism and cannibalism within natural populations of larval ambystomatid salamanders. Front Zool 11(1):76. https://doi.org/10.1186/s12983-014-0076-7
Kath NJ, Gaedke U, Van Velzen E (2022) The double-edged sword of inducible defences: costs and benefits of maladaptive switching from the individual to the community level. Sci Rep 12(1):10344. https://doi.org/10.1038/s41598-022-13895-7
Kishida O, Nishimura K (2006) Flexible architecture of inducible morphological plasticity. J Anim Ecol 75(3):705–712. https://doi.org/10.1111/j.1365-2656.2006.01091.x
Kishida O, Trussell GC, Mougi A, Nishimura K (2010) Evolutionary ecology of inducible morphological plasticity in predator–prey interaction: toward the practical links with population ecology. Popul Ecol 52(1):37–46. https://doi.org/10.1007/s10144-009-0182-0
Kopp M, Tollrian R (2003) Trophic size polyphenism in Lembadion bullinum: costs and benefits of an inducible offense. Ecology 84(3):641–651. https://doi.org/10.1890/0012-9658(2003)084[0641:TSPILB]2.0.CO;2
Křivan V (2000) Optimal intraguild foraging and population stability. Theor Popul Biol 58(2):79–94. https://doi.org/10.1006/tpbi.2000.1480
Křivan V, Diehl S (2005) Adaptive omnivory and species coexistence in tri-trophic food webs. Theor Popul Biol 67(2):85–99. https://doi.org/10.1016/j.tpb.2004.09.003
Lannoo MJ, Bachmann MD (1984) Aspects of cannibalistic morphs in a population of Ambystoma t. tigrinum larvae. Am Midl Nat 112(1):103. https://doi.org/10.2307/2425463
Lennartz DC, Bovee EC (1980) Induction of macrostome formation in Blepharisma americanum (Suzuki, 1954) by alpha-tocopheryl succinate. Trans Am Microsc Soc 99(3):310. https://doi.org/10.2307/3226006
Leonardsson K (1991) Effects of cannibalism and alternative prey on population dynamics of Saduria Entomon (Isopoda). Ecology 72(4):1273–1285. https://doi.org/10.2307/1941101
Loeb MLG, Collins JP, Maret TJ (1994) The role of prey in controlling expression of a trophic polymorphism in Ambystoma tigrinum nebulosum. Funct Ecol 8(2):151. https://doi.org/10.2307/2389898
Matthysen E (1989) Seasonal variation in bill morphology of nuthatches Sitta europaea: dietary adaptations or consequences. Ardea 77(1):117–125
Meyer A (1990) Ecological and evolutionary consequences of the trophic polymorphism in Cichlasoma citrinellum (Pisces: Cichlidae). Biol J Linn 39(3):279–299. https://doi.org/10.1111/j.1095-8312.1990.tb00517.x
Michimae H, Wakahara M (2001) Factors which affect the occurrence of cannibalism and the broad-headed “cannibal” morph in larvae of the salamander Hynobius retardatus. Behav Ecol Sociobiol 50(4):339–345. https://doi.org/10.1007/s002650100368
Michimae H, Wakahara M (2002) A tadpole-induced polyphenism in the salamander Hynobius retardatus. Evolution 56(10):2029–2038. https://doi.org/10.1111/j.0014-3820.2002.tb00129.x
Montserrat M, Janssen A, Magalhães S, Sabelis MW (2006) To be an intra-guild predator or a cannibal: Is prey quality decisive? Ecol Entomol 31(5):430–436. https://doi.org/10.1111/j.1365-2311.2006.00804.x
Murdoch WW, Briggs CJ, Nisbet RM (2013) Consumer-resource dynamics (MPB-36). Princeton University Press
Navarrete SA, Menge BA, Daley BA (2000) Species interactions in intertidal food webs: prey or predation regulation of intermediate predators? Ecology 81(8):2264–2277. https://doi.org/10.1890/0012-9658(2000)081[2264:SIIIFW]2.0.CO;2
Olsson J, Eklöv P (2005) Habitat structure, feeding mode and morphological reversibility: factors influencing phenotypic plasticity in perch. Evol Ecol Res 7(8):1109–1123
Orizaola G, Dahl E, Laurila A (2012) Reversibility of predator-induced plasticity and its effect at a life-history switch point. Oikos 121(1):44–52. https://doi.org/10.1111/j.1600-0706.2011.19050.x
Orlando PA, Brown JS, Buhse HE, Whelan CJ (2011) Switching strategies, population dynamics, and mechanisms of co-existence in food webs with Jekyll-and-Hyde species. Evol Ecol Res 13(5):495–511
Padilla DK, Adolph SC (1996) Plastic inducible morphologies are not always adaptive: the importance of time delays in a stochastic environment. Evol Ecol 10:105–117
Padilla DK (2001) Food and environmental cues trigger an inducible offence. Evol Ecol Res 3(1):1–13
Pal N, Samanta S, Chattopadhyay J (2014) Revisited Hastings and Powell model with omnivory and predator switching. Chaos Solit Fractals 66:58–73. https://doi.org/10.1016/j.chaos.2014.05.003
Persson L, de Roos AM, Claessen D et al (2003) Gigantic cannibals driving a whole-lake trophic cascade. PNAS 100(7):4035–4039. https://doi.org/10.1073/pnas.0636404100
Pervez A, Kumar R, Chandra S (2021) Food preference, growth and development of three aphidophagous ladybirds preying on conspecific and heterospecific eggs. Int J Trop Insect Sci 42(1):603–613. https://doi.org/10.1007/s42690-021-00579-4
Pfennig DW (1992) Proximate and functional causes of polyphenism in an anuran tadpole. Funct Ecol 6(2):167. https://doi.org/10.2307/2389751
Pfennig DW (1999) Cannibalistic tadpoles that pose the greatest threat to kin are most likely to discriminate kin. Proc R Soc B Biol Sci 266(1414):57–61. https://doi.org/10.1098/rspb.1999.0604
Polis GA (1981) The evolution and dynamics of intraspecific predation. Annu Rev Ecol Syst 12(1):225–251. https://doi.org/10.1146/annurev.es.12.110181.001301
Polis GA, Holt RD (1992) Intraguild predation: the dynamics of complex trophic interactions. Trends Ecol Evol 7(5):151–154. https://doi.org/10.1016/0169-5347(92)90208-S
Polis GA, Myers CA (1985) A survey of intraspecific predation among reptiles and amphibians. J Herpetol 19(1):99. https://doi.org/10.2307/1564425
Polis GA, Myers CA, Holt RD (1989) The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu Rev Ecol Syst 20(1):297–330
Price JE, Morin PJ (2004) Colonization history determines alternate community states in a food web of intraguild predators. Ecology 85(4):1017–1028. https://doi.org/10.1890/03-0157
R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ribeiro FF, Qin JG (2013) Modelling size-dependent cannibalism in barramundi Lates calcarifer: cannibalistic polyphenism and its implication to aquaculture. PLoS ONE 8(12):e82488. https://doi.org/10.1371/journal.pone.0082488
Rosenheim JA, Schreiber SJ (2022) Pathways to the density-dependent expression of cannibalism, and consequences for regulated population dynamics. Ecology 103(10):e3785. https://doi.org/10.1002/ecy.3785
Rosenzweig ML (1971) Paradox of enrichment: destabilization of exploitation ecosystems in ecological time. Science 171(3969):385–387. https://doi.org/10.1126/science.171.3969.385
Rudolf VH (2007) The interaction of cannibalism and omnivory: consequences for community dynamics. Ecology 88(11):2697–2705. https://doi.org/10.1890/06-1266.1
Rudolf VH (2008) Impact of cannibalism on predator–prey dynamics: size-structured interactions and apparent mutualism. Ecology 89(6):1650–1660. https://doi.org/10.1890/07-0709.1
Schausberger P, Croft BA (2000) Cannibalism and intraguild predation among phytoseiid mites: are aggressiveness and prey preference related to diet specialization? Exp Appl Acarol 24:709–725. https://doi.org/10.1023/A:1010747208519
Smith TB, Skúlason S (1996) Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu Rev Ecol Syst 27(1):111–133. https://doi.org/10.1146/annurev.ecolsys.27.1.111
Soetaert K, Petzoldt T, Setzer RW (2010) Solving Differential Equations in R: Package deSolve. J Stat Softw 33(9):1–25. https://doi.org/10.18637/jss.v033.i09
Takatsu K, Kishida O (2015) Predator cannibalism can intensify negative impacts on heterospecific prey. Ecology 96(7):1887–1898. https://doi.org/10.1890/14-1616.1
Toscano BJ, Hin V, Rudolf VH (2017) Cannibalism and intraguild predation community dynamics: coexistence, competitive exclusion, and the loss of alternative stable states. Am Nat 190(5):617–630. https://doi.org/10.1086/693997
Vance-Chalcraft HD, Rosenheim JA, Vonesh JR, Osenberg CW, Sih A (2007) The influence of intraguild predation on prey suppression and prey release: a meta-analysis. Ecology 88(11):2689–2696. https://doi.org/10.1890/06-1869.1
Wainwright PC, Osenberg CW, Mittelbach GG (1991) Trophic polymorphism in the pumpkinseed sunfish (lepomis gibbosus linnaeus): effects of environment on ontogeny. Funct Ecol 5(1):40. https://doi.org/10.2307/2389554
Wang X, Zhang G, Lai J (2018) Dynamics of an intraguild predation model with an adaptive IGpredator. Math Biosci 302:19–26. https://doi.org/10.1016/j.mbs.2018.05.014
Wei HC (2019) A mathematical model of intraguild predation with prey switching. Math Comput Simul 165:107–118. https://doi.org/10.1016/j.matcom.2019.03.004
Whiteman HH, Sheen JP, Johnson EB, VanDeusen A, Cargille R, Sacco TW (2003) Heterospecific prey and trophic polyphenism in larval tiger salamanders. Copeia 1:56–67. https://doi.org/10.1643/0045-8511(2003)003[0056:HPATPI]2.0.CO;2
Williams NE (1961) Polymorphism in Tetrahymena vorax. J Protozool 8(4):403–410. https://doi.org/10.1111/j.1550-7408.1961.tb01235.x
Wissinger S, Steinmetz J, Alexander JS, Brown W (2004) Larval cannibalism, time constraints, and adult fitness in caddisflies that inhabit temporary wetlands. Oecologia 138(1):39–47. https://doi.org/10.1007/s00442-003-1397-y
Yasuda H, Kikuchi T, Kindlmann P, Sato S (2001) Relationships between attack and escape rates, cannibalism, and intraguild predation in larvae of two predatory ladybirds. J Insect Behav 14:373–384. https://doi.org/10.1023/A:1011175430247
Acknowledgements
We thank Helen Regan, Matthew Daugherty, Nicole Rafferty, and an anonymous reviewer for helpful comments and feedback on the manuscript.
Funding
K.E.A. and C.A.W. were supported by a grant from the National Science Foundation #DEB-1553718. C.A.W. was supported by The Spieth Award and The Lance and Maureen Loomer Endowed Award in Biology from the University of California, Riverside.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. C.A.W. conducted data collection and analysis, while K.E.A. advised data analysis and aided in interpretation of results. The first draft was written by C.A.W., and K.E.A. provided feedback and revisions on subsequent drafts. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The authors declare that they have complied with the ethical standards of the journal and their institution.
Consent to participate
No human subjects were used for this research.
Competing interests
The authors declare no competing interests.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Woodie, C.A., Anderson, K.E. Preferential cannibalism as a key stabilizing mechanism of intraguild predation systems with trophic polymorphic predators. Theor Ecol 17, 59–72 (2024). https://doi.org/10.1007/s12080-024-00575-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-024-00575-7