Abstract
CCN2, also known as connective tissue growth factor (CTGF) is a transcriptional target of TGF-β signaling. Unlike its original name (“CTGF”) suggested, CCN2 is not an actual growth factor but a matricellular protein that plays an important role in fibrosis, inflammation and connective tissue remodeling in a variety of diseases, including cancer. In pancreatic ductal adenocarcinoma, CCN2 signaling induces stromal infiltration and facilitates a strong tumor-stromal interaction. In many types of cancer, CCN2 overexpression has been associated with poor outcome. CMS4 (Consensus Molecular Subtype 4) is a recently identified aggressive colorectal cancer subtype, that is characterized by up-regulation of genes involved in epithelial-to-mesenchymal transition, TGF-β signaling, angiogenesis, complement activation, and extracellular matrix remodeling. In addition, a high influx of stromal fibroblasts contributes to the mesenchymal-like gene expression profile of this subtype. Furthermore, compared with the other three CMS groups, CMS4 tumors have the worst prognosis. Based on these observations, we postulated that CCN2 might contribute to colorectal cancer progression, especially in the CMS4 subtype. This review discusses the available literature on the role of CCN2 in colorectal cancer, with a focus on the ‘fibrotic subtype’ CMS4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is one of the most common causes of cancer-related mortality in the Western world (Siegel et al. 2016). CRC is a highly heterogeneous disease. Traditional CRC staging takes into account the depth of invasive growth, the histological differentiation grade and the presence of metastases in lymph nodes and in distant organs. More recently, the CRC Subtyping Consortium has identified four Consensus Molecular Subtypes (CMS) based on integrative analysis of molecular data linked to tumor phenotype and clinical features (Guinney et al. 2015). CMS1 tumors are often microsatellite instable and are characterized by strong activation of immune pathways. This subtype responds best to immune checkpoint inhibition, presumably due to a deficient mismatch repair pathway, which leads to a high mutational load and the generation of tumor-specific neo-antigens (Le et al. 2015; de Weger et al. 2012). Tumors of CMS2 are characterized by a high level of copy number alterations, epithelial differentiation, up-regulation of WNT and MYC targets, and chromosomal instability. CMS3 tumors are likely to harbor activating mutations in KRAS, and show a marked deregulation of metabolic pathways; CMS3 tumors also display high-level chromosomal instability (CIN). CMS4 tumors are characterized by high expression of genes involved in epithelial-to-mesenchymal transition (EMT), Transforming Growth Factor β (TGF-β) signaling, angiogenesis and extracellular matrix remodeling. CMS4 tumors also exhibit a high density of tumor-associated fibroblasts. Importantly, CMS4 tumors have a poorer prognosis compared with the other subtypes, due to an increased likelihood of distant relapse (Guinney et al. 2015; Becht et al. 2016).
CCN2, is well-known for its involvement in connective tissue remodeling in various diseases, including cancer. CCN2 is a transcriptional target of TGF- β, that binds growth factors, extracellular matrix protein, and cell surface molecules, including integrins, thereby modulating signaling activity in multiple pathways (reviewed by (Lau 2016; Cicha and Goppelt-Struebe 2009)). In preclinical studies of pancreatic ductal adenocarcinoma (PDAC), a cancer type with particularly strong tumor-stroma interaction and stromal infiltration, CCN2 has been identified as a major determinant of tumor growth and metastasis, and as a valid therapeutic target (Dornhofer et al. 2006; Bennewith et al. 2009). A phase 1/2 trial with neo-adjuvant xanti-CCN2 (“anti-CTGF”) antibody treatment (FG3019) in locally advanced PDAC is underway (NCT02210559). Furthermore, CTGF overexpression has been associated with poor prognosis in several tumor types, including B-cell acute lymphoblastic leukemia (Boag et al. 2007; Sala-Torra et al. 2007), breast cancer (Chien et al. 2011), gastric cancer (Liu et al. 2008), glioma (Xie et al. 2004), melanoma (Hutchenreuther et al. 2015), pancreatic cancer (Bennewith et al. 2009) and prostate cancer (Yang et al. 2005). Based on the findings in other cancer types, and on the notion that CMS4 tumors are stroma-rich and have a poor prognosis, we suggest that CCN2 could also play a role in the fibrotic response that characterizes CMS4 CRC.
CCN2 and colorectal cancer progression
TGF-β acts as a tumor suppressor in colorectal cancer initiation, by inhibiting excessive cell proliferation. However, in later stages of tumor development, TGF-β plays a crucial pro-metastatic role and its expression is associated with worse outcome (Massague 2008; Calon et al. 2012). mRNA and protein levels of CCN2, a well-known transcriptional target of TGF-β, were found to be increased in more advanced Duke’s and TNM stages of CRC (Ladwa et al. 2011).
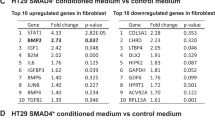
CRC can metastasize via blood or lymphatic vessels. TGF-β signaling has been implicated in formation of metastases and facilitation of secondary organ colonization (Calon et al. 2012). By recruiting myofibroblasts to the invasive tumor front via TGF-β signaling, tumor cells facilitate their own invasion and metastatic spread (Massague 2008). A similar role has been proposed for CCN2 based on the observed correlation of CCN2 expression with α-smooth muscle actin expression in ileal carcinoids, an endocrine carcinoma that is extremely rich in fibrosis (Cunningham et al. 2010). Furthermore, conditioned medium from colon cancer cells can stimulate CCN2 expression in peritoneal fibroblasts (Yokota et al. 2016).
Although the contribution of lymphangiogenesis to CRC progression is currently unknown, it does correlate with poor prognosis (Sundlisaeter et al. 2007). We recently identified involvement of CCN2 not only in fibrotic matrix remodeling but also in lymphangiogenesis downstream of TGF-β in experimental kidney fibrosis (Kinashi et al., unpublished data). TGF-β activates CCN2 expression through canonical pSmad2/3 signaling, but depending on cell type and context also through RhoA, Ras, and MEKK 1/2 (reviewed by (Cicha and Goppelt-Struebe 2009)). The TGF-β pathway regulates lymphangiogenesis and stromal infiltration and activation, which may in part be mediated by induction of its transcriptional target CCN2.
CCN2 and prognosis of colorectal cancer
Several research groups have studied the relationship between CCN2 expression and CRC prognosis, with conflicting results. Lin and others (2005; 2011) performed immunohistochemical analyses of CCN2 expression in primary CRC, and found that high CCN2 levels were associated with a decreased risk of peritoneal metastasis and with significantly higher disease-free and overall survival. In other studies however, CCN2 levels were higher in more advanced CRC stages (Ladwa et al. 2011; Guo et al. 2015), and activation of the TAZ-AXL-CCN2 axis in CRC was associated with higher stage, higher grade and poorer survival in CRC patients (Yuen et al. 2013; Zhang et al. 2016). Using gene expression data from 800 primary CRC samples (from publically available data sets used by Guinney et al. (2015)), we found that relapse-free survival as well as overall survival of CRC patients with high CCN2 expression (N = 200; top-25 %) was significantly shorter than in CRC patients with low CCN2 expression (N = 600; lower 75 %), p < 0.001 and p = 0.011, respectively (Fig. 1a, b). Taken together, the data on CCN2 expression in relation to prognosis in CRC remains inconclusive. To resolve this issue, new studies are needed that couple the use of well-validated anti-CCN2 antibodies on immunohistochemistry with RNA analyses in thoroughly annotated large cohorts of CRC patients.
High CCN2(/CTGF) expression is associated with poor prognosis Kaplan Meier curves showing the disease-free (a) and overall (b) survival of the CCN2(/CTGF)-high (upper 25 %) and CCN2(/CTGF)-low (lower 75 %) expression subgroups. The inset shows the expression of CCN2(/CTGF) in the high and low subgroups
CCN2 expression has been linked to drug resistance in several types of cancer (Tsai et al. 2014; Lai et al. 2011; Yin et al. 2010). It has also been suggested that CCN2 expression contributes to therapy resistance in CRC, since overexpression of CCN2 induced resistance to radiotherapy (combined with simvastatin) in colorectal cancer cell lines, whereas CCN2 knockdown increased the radiosensitivity (Lim et al. 2015). Data on this subject are very limited however, and more research is needed to elucidate the role of CCN2 in the response to standard chemotherapy and targeted therapy in CRC.
CCN2 and CMS4
In a collection of 2476 primary CRC samples (Guinney et al. 2015), we compared the expression levels of CCN2(/CTGF) mRNA between the four CMS of CRC. This revealed that expression is particularly high in CMS4 (Fig. 2a). Furthermore, CMS4 tumors were strongly enriched in the CCN2(/CTGF)-high group, while the canonical subtypes (CMS2, CMS3) were enriched in the CCN2(/CTGF)-low subgroup (Fig. 2b). Interestingly, CCN2 was identified as an upstream activating regulator of a 19 gene-based risk profile that predicts poor CRC prognosis and benefit from adjuvant chemotherapy (Kim et al. 2014). Considering the strong association between CCN2 expression and the CMS4 colorectal cancer subtype, CCN2 could be an interesting target for therapy.
CCN2(/CTGF) and CMS (a) CCN2(/CTGF) mRNA expression in the consensus molecular CRC subtypes. (b) Analysis of the percentage CMS1–4 contributing to the CCN2(/CTGF)-high and CCN2(/CTGF)-low subgroups. The genomics analysis and visualization application R2 (http://r2.amc.nl) was used to analyze the expression of CCN-2(/CTGF) in the four consensus molecular subtypes (CMS1–4)
Conclusion
The available data on the role of CCN2(/CTGF) in colorectal cancer progression and prognosis is limited, and in part contradictory. The high expression of CCN2(/CTGF)x in CMS4 colorectal cancer and its established role in tissue fibrosis warrant further investigation to establish the possible value of xCCN2(/CTGF) as a marker and potential therapeutic target in CRC, especially in the aggressive mesenchymal subtype.
Abbreviations
- CMS:
-
Consensus Molecular Subtype
- CRC:
-
Colorectal cancer
- CCN2:
-
CCN-family protein 2
- CTGF:
-
Connective tissue growth factor
- EMT:
-
Epithelial-to-mesenchymal transition
- PDAC:
-
Pancreatic ductal adenocarcinoma
- TGF-β:
-
Transforming Growth Factor β
References
Becht E, de Reynies A, Giraldo NA, Pilati C, Buttard B, Lacroix L, Selves J, Sautes-Fridman C, Laurent-Puig P, Fridman WH (2016) Immune and Stromal Classification of Colorectal Cancer Is Associated with Molecular Subtypes and Relevant for Precision Immunotherapy. Clin Cancer Res. doi:10.1158/1078-0432.CCR-15-2879
Bennewith KL, Huang X, Ham CM, Graves EE, Erler JT, Kambham N, Feazell J, Yang GP, Koong A, Giaccia AJ (2009) The role of tumor cell-derived connective tissue growth factor (CTGF/CCN2) in pancreatic tumor growth. Cancer Res 69(3):775–784. doi:10.1158/0008-5472.CAN-08-0987
Boag JM, Beesley AH, Firth MJ, Freitas JR, Ford J, Brigstock DR, de Klerk NH, Kees UR (2007) High expression of connective tissue growth factor in pre-B acute lymphoblastic leukaemia. Br J Haematol 138(6):740–748. doi:10.1111/j.1365-2141.2007.06739.x
Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, Byrom D, Riera A, Rossell D, Mangues R, Massague J, Sancho E, Batlle E (2012) Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell 22(5):571–584. doi:10.1016/j.ccr.2012.08.013
Chien W, O'Kelly J, Lu D, Leiter A, Sohn J, Yin D, Karlan B, Vadgama J, Lyons KM, Koeffler HP (2011) Expression of connective tissue growth factor (CTGF/CCN2) in breast cancer cells is associated with increased migration and angiogenesis. Int J Oncol 38(6):1741–1747. doi:10.3892/ijo.2011.985
Cicha I, Goppelt-Struebe M (2009) Connective tissue growth factor: context-dependent functions and mechanisms of regulation. Biofactors 35(2):200–208. doi:10.1002/biof.30
Cunningham JL, Tsolakis AV, Jacobson A, Janson ET (2010) Connective tissue growth factor expression in endocrine tumors is associated with high stromal expression of alpha-smooth muscle actin. Eur J Endocrinol 163(4):691–697. doi:10.1530/EJE-10-0420
de Weger VA, Turksma AW, Voorham QJ, Euler Z, Bril H, van den Eertwegh AJ, Bloemena E, Pinedo HM, Vermorken JB, van Tinteren H, Meijer GA, Hooijberg E (2012) Clinical effects of adjuvant active specific immunotherapy differ between patients with microsatellite-stable and microsatellite-instable colon cancer. Clin Cancer Res 18(3):882–889. doi:10.1158/1078-0432.CCR-11-1716
Dornhofer N, Spong S, Bennewith K, Salim A, Klaus S, Kambham N, Wong C, Kaper F, Sutphin P, Nacamuli R, Hockel M, Le Q, Longaker M, Yang G, Koong A, Giaccia A (2006) Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res 66(11):5816–5827. doi:10.1158/0008-5472.CAN-06-0081
Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa EMF, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S (2015) The consensus molecular subtypes of colorectal cancer. Nat Med 21(11):1350–1356. doi:10.1038/nm.3967
Guo Y, Li X, Lin C, Zhang Y, Hu G, Zhou J, Du J, Gao K, Gan Y, Deng H (2015) MicroRNA133b inhibits connective tissue growth factor in colorectal cancer and correlates with the clinical stage of the disease. Mol Med Rep 11(4):2805–2812. doi:10.3892/mmr.2014.3075
Hutchenreuther J, Vincent KM, Carter DE, Postovit LM, Leask A (2015) CCN2 expression by tumor stroma is required for melanoma metastasis. J Invest Dermatol 135(11):2805–2813. doi:10.1038/jid.2015.279
Kim SK, Kim SY, Kim JH, Roh SA, Cho DH, Kim YS, Kim JC (2014) A nineteen gene-based risk score classifier predicts prognosis of colorectal cancer patients. Mol Oncol 8(8):1653–1666. doi:10.1016/j.molonc.2014.06.016
Ladwa R, Pringle H, Kumar R, West K (2011) Expression of CTGF and Cyr61 in colorectal cancer. J Clin Pathol 64(1):58–64. doi:10.1136/jcp.2010.082768
Lai D, Ho KC, Hao Y, Yang X (2011) Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res 71(7):2728–2738. doi:10.1158/0008-5472.CAN-10-2711
Lau LF (2016) Cell surface receptors for CCN proteins. J Cell Commun Signal 10(2):121–127. doi:10.1007/s12079-016-0324-z
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, LA D Jr (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372(26):2509–2520. doi:10.1056/NEJMoa1500596
Lim T, Lee I, Kim J, Kang WK (2015) Synergistic effect of simvastatin plus radiation in gastric cancer and colorectal cancer: implications of BIRC5 and connective tissue growth factor. Int J Radiat Oncol Biol Phys 93(2):316–325. doi:10.1016/j.ijrobp.2015.05.023
Lin BR, Chang CC, Che TF, Chen ST, Chen RJ, Yang CY, Jeng YM, Liang JT, Lee PH, Chang KJ, Chau YP, Kuo ML (2005) Connective tissue growth factor inhibits metastasis and acts as an independent prognostic marker in colorectal cancer. Gastroenterology 128(1):9–23
Lin BR, Chang CC, Chen RJ, Jeng YM, Liang JT, Lee PH, Chang KJ, Kuo ML (2011) Connective tissue growth factor acts as a therapeutic agent and predictor for peritoneal carcinomatosis of colorectal cancer. Clin Cancer Res 17(10):3077–3088. doi:10.1158/1078-0432.CCR-09-3256
Liu LY, Han YC, SH W, Lv ZH (2008) Expression of connective tissue growth factor in tumor tissues is an independent predictor of poor prognosis in patients with gastric cancer. World J Gastroenterol 14(13):2110–2114
Massague J (2008) TGFbeta in Cancer. Cell 134(2):215–230. doi:10.1016/j.cell.2008.07.001
Sala-Torra O, Gundacker HM, Stirewalt DL, Ladne PA, Pogosova-Agadjanyan EL, Slovak ML, Willman CL, Heimfeld S, Boldt DH, Radich JP (2007) Connective tissue growth factor (CTGF) expression and outcome in adult patients with acute lymphoblastic leukemia. Blood 109(7):3080–3083. doi:10.1182/blood-2006-06-031096
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30. doi:10.3322/caac.21332
Sundlisaeter E, Dicko A, Sakariassen PO, Sondenaa K, Enger PO, Bjerkvig R (2007) Lymphangiogenesis in colorectal cancer--prognostic and therapeutic aspects. Int J Cancer 121(7):1401–1409. doi:10.1002/ijc.22996
Tsai HC, Huang CY, HL S, Tang CH (2014) CCN2 enhances resistance to cisplatin-mediating cell apoptosis in human osteosarcoma. PLoS One 9(3):–e90159. doi:10.1371/journal.pone.0090159
Xie D, Yin D, Wang HJ, Liu GT, Elashoff R, Black K, Koeffler HP (2004) Levels of expression of CYR61 and CTGF are prognostic for tumor progression and survival of individuals with gliomas. Clin Cancer Res 10(6):2072–2081
Yang F, Tuxhorn JA, Ressler SJ, McAlhany SJ, Dang TD, Rowley DR (2005) Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res 65(19):8887–8895. doi:10.1158/0008-5472.CAN-05-1702
Yin D, Chen W, O'Kelly J, Lu D, Ham M, Doan NB, Xie D, Wang C, Vadgama J, Said JW, Black KL, Koeffler HP (2010) Connective tissue growth factor associated with oncogenic activities and drug resistance in glioblastoma multiforme. Int J Cancer 127(10):2257–2267. doi:10.1002/ijc.25257
Yokota M, Kojima M, Higuchi Y, Nishizawa Y, Kobayashi A, Ito M, Saito N, Ochiai A (2016) Gene expression profile in the activation of subperitoneal fibroblasts reflects prognosis of patients with colon cancer. Int J Cancer 138(6):1422–1431. doi:10.1002/ijc.29851
Yuen HF, McCrudden CM, Huang YH, Tham JM, Zhang X, Zeng Q, Zhang SD, Hong W (2013) TAZ expression as a prognostic indicator in colorectal cancer. PLoS One 8(1):–e54211. doi:10.1371/journal.pone.0054211
Zhang SD, McCrudden CM, Yuen HF, Leung KL, Hong WJ, Kwok HF (2016) Association between the expression levels of TAZ, AXL and CTGF and clinicopathological parameters in patients with colon cancer. Oncol Lett 11(2):1223–1229. doi:10.3892/ol.2015.3999
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ubink, I., Verhaar, E.R., Kranenburg, O. et al. A potential role for CCN2/CTGF in aggressive colorectal cancer. J. Cell Commun. Signal. 10, 223–227 (2016). https://doi.org/10.1007/s12079-016-0347-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-016-0347-5