Abstract
The Ca2+ transport ATPase (SERCA) of sarcoplasmic reticulum (SR) plays an important role in muscle cytosolic signaling, as it stores Ca2+ in intracellular membrane bound compartments, thereby lowering cytosolic Ca2+ to induce relaxation. The stored Ca2+ is in turn released upon membrane excitation to trigger muscle contraction. SERCA is activated by high affinity binding of cytosolic Ca2+, whereupon ATP is utilized by formation of a phosphoenzyme intermediate, which undergoes protein conformational transitions yielding reduced affinity and vectorial translocation of bound Ca2+. We review here biochemical and biophysical evidence demonstrating that release of bound Ca2+ into the lumen of SR requires Ca2+/H+ exchange at the low affinity Ca2+ sites. Rise of lumenal Ca2+ above its dissociation constant from low affinity sites, or reduction of the H+ concentration by high pH, prevent Ca2+/H+ exchange. Under these conditions Ca2+ release into the lumen of SR is bypassed, and hydrolytic cleavage of phosphoenzyme may yield uncoupled ATPase cycles. We clarify how such Ca2+pump slippage does not occur within the time length of muscle twitches, but under special conditions and in special cells may contribute to thermogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcoplasmic reticulum (SR) membrane vesicles, originally referred to as “relaxing factor”, were first isolated from skeletal muscle by Ebashi and Lipmann (1962), and Hasselbach and Makinose (1962), and were shown to contain a P–type ATPase (SERCA1 isoform) sustaining Ca2+ transport. In muscle cells, this transport activity plays an important role in lowering cytosolic Ca2+ as required for relaxation of contractile elements, and storing transported Ca2+ in the lumen of SR for subsequent release and contractile activation (Carafoli 2002; Clapham 2007). General information on SERCA1 catalytic function and molecular structure is given in several reviews (de Meis and Vianna 1979; Inesi et al. 1990; Andersen and Vilsen 1995; Toyoshima 2008; Møller et al. 2010).
SERCA1 is a 996 amino acid membrane bound protein (MacLennan et al. 1985) comprising ten transmembrane helical segments, and a globular headpiece that protrudes from the cytosolic side of the membrane and includes three distinct domains (A, N and P). Catalytic activation follows high affinity binding of cytosolic Ca2+ within the transmembrane region of the enzyme (Fig. 1). Activation is followed by utilization of ATP bound to the N domain, and formation of phosphorylated enzyme intermediate by transfer of the ATP γ-phosphate to an aspartyl residue (Asp-351) in the P domain. Conformational transition of the phosphoenzyme then promotes vectorial translocation of bound Ca2+ and release of Ca2+ into the lumen of SR. Finally, the phosphoenzyme undergoes hydrolytic cleavage with catalytic assistance by an A domain critical sequence (Thr-Gly-Glu), leading to a new cycle.
Two-dimensional folding model of the SERCA1 sequence. The diagram shows ten transmembrane segments (M1 to M10) including six residues (Glu-309, Glu-771, Asn-796, Thr-799, Asp-800 and Glu-908) contributing oxygen atoms for calcium binding, enzyme activation, and transport. The extramembranous headpiece comprises: a nucleotide binding domain (N); the P domain, with several residues conserved in P-type ATPases, including Asp-351 (in red) that undergoes phosphorylation to form the catalytic phosphoenzyme intermediate (EP); and the A domain with the Thr-Gly-Glu conserved sequence involved in catalytic assistance of EP hydrolytic cleavage
Ca2+/ATP coupling ratios
Cooperative binding of 2 Ca2+ per ATPase (Inesi et al. 1980) implies transport of 2 Ca2+ per catalytic cycle, if both bound Ca2+ are translocated with maximal efficiency. Ratios of 2 Ca2+ per ATP were in fact observed under conditions permitting free Ca2+ to remain low in the lumen of the vesicles: (a) steady state experiments in which oxalate is used for complexation of lumenal Ca2+ (Martonosi and Feretos 1964) and (b) pre-steady state experiments in which lumenal Ca2+ has yet to rise (Fig. 2a; Inesi et al. 1988). On the other hand, Ca2+/ATP ratios lower than 2 have been observed with native SR vesicles as well as reconstituted systems (Yu and Inesi 1995), under conditions permitting lumenal Ca2+ to rise (mM) while Ca2+ in the outer medium remains sufficiently high (μM) for ATPase activation (Fig. 2b). Under these conditions, the lumenal Ca2+ concentration is higher than the dissociation constant of Ca2+ from the lumenal sites, and therefore the phosphoenyme bypasses the Ca2+ release step and proceeds to hydrolytic cleavage of Pi, with consequent reduction of the Ca2+/ATP transport ratio. Uncoupled ATPase subsides if EGTA is added to the outer medium to reduce free Ca2+ below the ATPase activating level (Fig. 2b).
Pre-steady state measurements of ATPase activity and Ca2± transport by native SR vesicles obtained from skeletal muscle. a Initial phosphoenzyme formation and Ca2+ occlusion (2Ca2+/1EP) are observed within the first cycle following addition of ATP. Ca2+ uptake and Pi production rates follow with molar ratios of 2:1. Time resolution in the millisecond time scale was obtained with rapid mixing instruments. b Pre-steady state experiments extended to the second time scale, show that the initial rates of Ca2+ uptake and Pi production begin with a ratio of 2:1, but the Ca2+ uptake rate undergoes saturation, while uncoupled ATPase activity continues as long as the medium Ca2+ is maintained above the ATPase activation level. Uncoupled ATPase ceases if EGTA is added to chelate medium Ca2+. Reaction mixtures contained 20–50 μg SR protein/ml, 10 mM PIPES, pH 7.0, 100 mM KCl, 5 mM MgSO4, 0.2 mM CaCl2 and 0.2 mM EGTA. Radioactive tracers added according to the experimental schedule. Reaction started with 100 mM ATP and stopped by acid quenching. 1 mM EGTA added when indicated. Temperature 25 °C. Derived from Inesi et al. (1988) and Yu and Inesi (1995)
A variable stoichiometric ratio (i.e., Ca2+/ATP) of active transport may be considered to be an intrinsic feature of the pump, if the ATPase reaction sequence allows an alternate pathway leading to hydrolytic cleavage of Pi without vectorial displacement of Ca2+ (Johnson et al. 1985; Inesi and de Meis 1989). The importance of this phenomenon, referred to as slippage of the pump, is related to heat production and thermogenesis, when the free energy derived from ATP hydrolysis is not utilized for active transport (de Meis et al. 1997; de Meis 2001; de Meis et al. 2005).
Ca2+/H+ exchange at the lumenal gate
Exchange of Ca2+ with H+ upon vectorial translocation is a specific feature of the Ca2+ ATPase (Lewis et al. 2012), facilitating lumenal Ca2+ release (Yu et al. 1994; Bublitz et al. 2013). Evidence of Ca2+/H+ exchange, H+ counter transport (Chiesi and Inesi 1980; Yamaguchi and Kanazawa 1985; Ueno and Sekine 1981) and electrogenicity (Morimoto and Kasai 1986; Cornelius and Møller 1991; Obara et al. 2005) in the operation of the Ca2+ ATPase was obtained with vesicular fragments of SR membrane and with ATPase reconstituted in phospholipids vesicles lacking non specific H+ or Ca2+ channels. It is shown in Fig. 3a that the molar ratio of Ca2+/H+ counter transport is 1 when the lumenal and medium pH is near neutrality. However, a higher number of acidic residues involved in Ca2+ binding (Glu-771, Asp-800, Glu-309, Glu-908) is likely to participate in Ca2+/H+ exchange (Bublitz et al. 2013; Obara et al. 2005), even though only one H+ per Ca2+ may actually be counter transported. In this case, the remaining H+ undergo lumenal dissociation. The Ca2+/H+ exchange is facilitated by acidic residues pK changes, as the phosphoenzyme undergoes its catalytic transition (Yu et al. 1994).
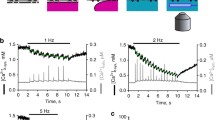
a ATP-dependent Ca2± uptake, H± countertransport, and development of transmembrane electrical potential in reconstituted SERCA1 proteoliposomes. Proteoliposomes prepared at pH 7.0 were diluted (5.0 μg protein/ml) in a medium (pH 7.0) containing 10 mM PIPES, 100 mM K2SO4, 5 mM MgSO4, 50 μM CaCl2, and 50 μM arsenazo III, or 200 μM lumenal pyranine, or 1 μM oxonol VI. The reaction was started at 11 °C by the addition of 0.2 mM ATP and followed by differential absorption spectrometry. b Charge measurements on native SR Ca2±ATPase (SERCA1) adsorbed on a solid supported membrane (SSM). The current transients were obtained after rapid delivery of 100 μM ATP to ATPase preincubated with 10 μM free Ca2+ and 100 mM KCl, at pH 7 (solid line) or pH 7.8 (dotted line). Derived from Yu et al. (1994) and Lewis et al. (2012)
Further evidence for Ca2+/H+ exchange is provided by measurements of charge transfer upon addition of Ca2+ or ATP to microsomal vesicles adsorbed on a solid supported membrane (SSM) (Tadini-Buoninsegni et al. 2004, 2006, 2010). Related electrogenic events are recorded as current transients due to flow of electrons along the external circuit toward the electrode surface, as required to compensate for the potential difference across the vesicular membrane produced by displacement of positive charge upon vectorial translocation in the direction of the SSM electrode. In fact, when ATP is added to the membrane bound ATPase adsorbed on the SSM in the presence of saturating Ca2+, a current transient is observed due to vectorial translocation and dissociation of bound Ca2+ in the direction of the SSM electrode after phosphoenzyme formation by utilization of ATP (Tadini-Buoninsegni et al. 2004, 2006). The electrical current recorded by the SSM method is a measure of the rate of change of the transmembrane potential and is not sensitive to stationary currents. Therefore, only the electrogenic signal generated within the first cycle is observed, whereas steady state events after the first cycle are not detected. It is shown in Fig. 3b that the net charge produced by ATP addition at neutral pH decreases significantly if ATP addition is performed at alkaline pH. This indicates that when lack of H+ limits H+/Ca2+ exchange (i.e. alkaline pH), vectorial translocation of bound Ca2+ in the direction of the SSM electrode is prevented, even though K+ is present in high concentration and may neutralize acidic residues at alkaline pH. This indicates a requirement for specific H+ binding at the Ca2+ sites, in order to obtain Ca2+ release.
The specific relevance of ATP dependent charge transfer is demonstrated by interference of mutations (Asp-351 to Asn) preventing phosphoenzyme formation (Tadini-Buoninsegni et al. 2006). Furthermore, cation/H+ exchange at the transport sites following phosphoenzyme formation occurs in Ca2+ ATPases, but does not occur in copper ATPases (Lewis et al. 2012).
The importance of Ca2+/H+ exchange in determining release of bound Ca2+ from the phosphoenzyme can be also demonstrated in steady state experiments. It is shown in Fig. 4 that the maximal levels of accumulated Ca2+ are significantly reduced if the pH is raised above 7 (consider that the physiological intracellular pH is 6.8, while the extracellular pH is 7.4)). This indicates that if exchange is limited due to low H+ concentration, Ca2+ is less likely to dissociate from the phosphoenzyme. On the other hand, while Ca2+ translocation is reduced, steady state ATPase activity is increased as the pH is raised, and continues after maximal levels of Ca2+ uptake are reached. It is apparent that alkaline pH reduces Ca2+/H+ exchange and dissociation of bound Ca2+, whereby the phosphoenyme bypasses the Ca2+ release step and proceeds to hydrolytic cleavage of Pi. Therefore, reduction of the Ca2+/ATP transport ratio can be produced either by a high Ca2+ concentration or a low H+ concentration in the lumen of the vesicles.
Maximal levels of Ca2± uptake and rates of ATPase activity in the absence of oxalate as a function of pH. Experiments performed as in Fig. 2B, except for pH regulation with 50 mM MES or HEPES buffer. The reaction was started by addition of 1 mM ATP, at 25 °C. Original data
A diagram of the sequential steps in the ATPase mechanism, derived from the original reaction diagram of de Meis and Vianna (1979) and modified to show Ca2+/H+ exchange and a pathway for slippage of the Ca2+ pump, is given in Scheme 1.
In the diagram on Scheme 1, solid lines indicate the optimal pathway, beginning with enzyme activation by high affinity and cooperative binding of two Ca2+, yielding E1 · 2Ca2+. Utilization of ATP yields ADP · E1 ~ P · 2Ca2+, followed by release of ADP and utilization of the phosphorylation potential to change vectorial orientation and affinity of the Ca2+ sites. Bound Ca2+ is then released into the lumenal medium in exchange for H+. Hydrolytic cleavage of nH+ · E2-P and transition of nH+ · E2 to E1 finally yields closure of the lumenal gate and exposure of the Ca2+ sites to the cytosolic medium. Formation of E1 · 2Ca2+ then starts a new cycle.
The dotted lines in Scheme 1 indicate that if lumenal Ca2+ is higher than its dissociation constant, or lumenal H+ is too low to sustain exchange, Ca2+ release and formation of nH+ · E2 are prevented. Interference with completion of the ATPase cycle would then cause reversal to E1 ~ P · 2Ca2+ (see below Fig. 5b), whereby phosphorylation potential leads directly to hydrolytic cleavage, rather than utilization for active transport. This is rendered possible as low concentration of ADP prevents its re-binding, and the remaining proximity of the A domain to the phosphorylation site allows catalytic assistance by the critical Thr-Gly-Glu sequence.
a Equilibrium levels of phosphoenzyme obtained through utilization of Pi by SERCA1 at acid or alkaline pH, in the absence or presence of Ca2±. Reaction medium: 50 mM MES (pH 6.0) or HEPES (pH 7.5), 20 % Me2SO4, 10 mM MgCl2, 100 mM KCl, 2 mM EGTA or 1 mM CaCl2 (in the absence of EGTA), and 50 μg protein/ml. The reaction was started by the addition of 50 μM [32P]Pi. The samples were acid quenched after 2 min incubation at 30 °C, and processed by electrophoresis for determination of radioactive ATPase protein. b Ca2± concentration and pH dependence of phosphoryl transfer from phosphoenzyme to ADP, to yield ATP. Phosphoenzyme was obtained by incubating 3.0 mg of protein/ml in 60 mM Tris-maleate, pH 6.3, 4 mM [32P]Pi, 20 mM MgC12, 0.5 mM EGTA. Following a 2 min incubation at 30 °C, the reaction mixture was diluted 10-fold with 60 mM Tris-maleate (pH 8.0), 1 mM ADP, CaC12 and EGTA to yield free Ca2+ as indicated. The samples were acid quenched after 5 min incubation and processed for determination of ATP. Derived from Lewis et al. (2012) and de Meis and Inesi (1982)
Strong evidence for the role of protons and the nH+ · E2 state is provided with experiments of enzyme phosphorylation by utilization of Pi (Masuda and de Meis 1973). It is shown in Fig. 5a that this reaction (i.e., reverse reaction of phosphoenzyme hydrolytic cleavage) is enhanced by acid pH, and is inhibited by alkaline pH and Ca2+. This indicates a requirement for proton occupancy of acidic residues involved in Ca2+ binding (i.e., nH+ · E2). On the other hand (Fig. 5b), further reversal of the cycle upon addition of ADP to form ATP, requires a switch to alkaline pH and addition of mM Ca2+, in order to replace protons with Ca2+ on the low affinity binding sites (i.e. transition of nH+ · E2-P to E2-P · 2Ca2+ and ADP · E1 ~ P · 2Ca2+).
High resolution crystal structures of most states (or their analogs) comprising the ATPase reaction sequence have been obtained, and are described in detailed reviews (Toyoshima 2008; Møller et al. 2010; Toyoshima and Inesi 2004). Comparison of these structures reveals rearrangements of transmembrane helices upon Ca2+ binding, phosphoenzyme formation, occlusion and then dissociation of bound Ca2+, which are mechanically linked to specific bending and rotation patterns of each headpiece domain. These movements provide an explanation for the long range linkage of phosphorylation and Ca2+ binding domains, including the roles of critical amino acids in substrate binding, catalytic reactions, and Ca2+ transport. They also demonstrate that the states included in the reaction diagram are structurally distinct, and possess specific features that are functionally relevant. It is of interest that movement of M1-M2 causes displacement of membrane helix M4L from M5 and M6, thereby opening the lumenal gate and allowing outflow of Ca2+, following Ca2+/H+ exchange. The lumenal gate is then closed upon cleavage of phosphate and dissociation of H+, when reverse rotation of the A domain is accompanied by upward displacement of M4L and reduction of the space between the M4 and M6 helices.
Effects of accessory polypeptides
Several studies have demonstrated that sarcolipin (SLN), a 31 amino acid polypeptide (Odermatt et al. 1997; Odermatt et al. 1998) is constitutively bound to the Ca2+ ATPase (SERCA1) of (at least human and rabbit) fast twitch skeletal muscle, and produces uncoupling of ATP utilization and Ca2+ transport, with a consequent thermogenic effect (Mall et al. 2006; Bal et al. 2012). However, these studies were performed by genetic manipulations and reconstitution procedures, which may not apply to the physiologic signaling mechanism of a fast twitch in native muscles, but rather reflect phenomena occurring under special circumstance and/or in other tissues (see below). In fact, other studies have shown that incorporation of SLN into proteoliposomes with SERCA simply results in a lower apparent affinity for calcium and a lower turnover rate (Gorski et al. 2013).
It is of interest that the Ca2+ ATPase SERCA2 isoform, prevalent in cardiac muscle (Lytton et al. 1992), is associated with phospholamban (PLN), a 52 amino acid polypeptide, to some extent similar to SLN. The definite effect of PLB on SERCA2 is a lower Ca2+ binding affinity (Koss and Kranias 1996; MacLennan and Kranias 2003; Toyoshima et al. 2003) and/or a slower E1 to 2Ca2+ · E1 transition (Cantilina et al. 1993). This results in a higher Ca2+ concentration requirement for Ca2+ transport activation (Fig. 6).
a Ca2± signaling in cultured cardiac myocytes subjected to field stimulation. Following stimulation the cytosolic Ca2+ concentration rises from 0.04 μM to 0.6 μM, and then returns to the 0.04 μM resting level within 0.6 s. b Rates of Ca2± uptake by cardiac sarcoplasmic reticulum vesicles as a function of free Ca2± concentration. Before the measurements, the vesicles were pre-incubated with either a control buffer (filled circle), or with a monoclonal antibody neutralizing phospholamban (filled triangele). Note how neutralization of phospholamban decreases the Ca2+ concentration required for activation of the transport ATPase. Note also how the cytosolic Ca2+ concentrations observed at the low and high ends of the Ca2+ signal, correspond to Ca2+ levels indufficient or suitable to yield SERCA activation. Derived from Prasad and Inesi (2012) and Cantilina et al. (1993)
Diagram outlining the sequential reactions on a SERCA catalytic and transport cycle as explained in the text above. The solid lines indicate the optimal pathway of a well coupled ATP utilization and net Ca2+. The dotted lines indicate a short cut of the enyme cycle, whereby ATP utilization is uncoupled from net Ca2+ transport, as explained in the text above
Both SLN and PLN reside within a groove surrounded by transmembrane helices M2, M4, M6 and M9, as shown by crystallographic studies (Toyoshima et al. 2013; Winther et al. 2013; Akin et al. 2013), and also indicated by NMR (Buffy et al. 2006) and cross-linking experiments (Sahoo et al. 2013). This is a critical position, since structural studies demonstrate that the helices delimiting this groove undergo displacements affecting Ca2+ binding, Ca2+ dissociation, as well as opening and closing of the lumenal gate. This explains how the presence of SLN and PLN may affect rates of movements and related partial reactions of the ATPase cycle. Some difference in the effects of the two polypeptides could be related to specific sequences and points of interactions (Sahoo et al. 2013), as well as to the cytosolic segment of PLN (absent in SLN) which may interact with SERCA headpiece domains and delay their movements to yield the E1 .2Ca2+ state. It is worth considering that a slight shift of the Ca2+ concentration required for ATP activation would affect the rates of Ca2+ transport at low levels of cytoplasmic Ca2+, and therefore the efficiency of twitch relaxation. However, it is not likely that the Km (equilibrium constant) of the Ca2+ sites on lumenal orientation would be significantly affected. The physiological interest of PLB and SLN is related to the reversibility of their effects upon phosphorylation catalyzed by signaling kinases (Koss and Kranias 1996; MacLennan and Kranias 2003; Toyoshima et al. 2003).
Contractile relaxation and uncoupling of the Ca2+ pump
Considering the possibility of Ca2+ pump uncoupling, an important question is whether uncoupling of SERCA1 interferes with reduction of cytosolic Ca2+ below the level allowing contractile relaxation of muscle fibers. The evidence presented above indicates that the Ca2+ pump is perfectly coupled when the SR lumen Ca2+ in is low, even if cytosolic Ca2+ out is relatively high. In the light of this information, we consider that relaxation of a muscle twitch occurs in less than 1 s and, within this time, lumenal Ca2+ does not reach a concentration higher than its dissociation from E2-P · 2Ca2+, as shown in experiments performed with rabbit native SR vesicles (Fig. 2). On the other hand, when cytosolic Ca2+ is reduced to a level producing contractile relaxation, such a Ca2+ level will be also low with regard to SERCA activation, and the ATPase would then proceed at very low rates or remain inactive. Therefore during relaxation, the pump remains quiescent, mostly in the Mg2+ bound E1 state (Toyoshima et al. 2013), with no significant slippage. Slippage of the pump would occur if lumenal Ca2+ were to become higher than its dissociation constant from E2-P · 2Ca2+, and cytosolic Ca2+ were to remain above the level required for full ATPase activation. This may occur upon prolonged muscle activity, if cytosolic Ca2+ is maintained relatively high by multiple action potentials and Ca2+ flux through plasma membrane voltage sensitive channels, as expected in shivering thermogenesis. Alternatively, a rise of intracellular pH above 7.0, may affect intracellular Ca2+ signaling, as recently reported for G protein signaling (Isom et al. 2013). In this case, some degree of SERCA uncoupling would be produced, contributing to the muscle twitching observed in alkalosis. Furthermore, thermogenic uncoupling may occur in tissues where SERCA is inserted in membrane compartments allowing lumenal Ca2+ rise to high levels, while cytosolic Ca2+ remains sufficiently high. Most importantly, it was reported that in brown fat, in addition to uncoupling of the mitochondrial respiratory chain, uncoupled SERCA contributes to non shivering thermogenesis (de Meis et al. 2006).
Abbreviations
- SERCA:
-
Sarcoplasmic reticulum Ca2+ ATPase
- SSM:
-
Solid supported membrane
- SR:
-
Sarcoplasmic reticulum
- SLN:
-
Sarcolipin
- PLN:
-
Phospholamban
References
Akin BL, Hurley TD, Chen Z, Jones LR (2013) The structural basis for phospholamban inhibition of the calcium pump in sarcoplasmic reticulum. J Biol Chem (in press) DOI 10.1074/jbc.M113.501585
Andersen JP, Vilsen B (1995) Structure-function relationships of cation translocation by Ca2+ and Na+, K+-ATPases studied by site-directed mutagenesis. FEBS Lett 359:101–106
Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, Rowland LA, Bombardier E, Goonasekera SA, Tupling AR, Molkentin JD, Periasamy M (2012) Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med 18:1575–1579
Bublitz M, Musgaard M, Poulsen H, Thøgersen L, Olesen C, Schiøtt B, Morth JP, Møller JV, Nissen P (2013) Ion pathways in the sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem 288:10759–10765
Buffy JJ, Buck-Koehntop BA, Porcelli F, Traaseth NJ, Thomas DD, Veglia G (2006) Defining the intramembrane binding mechanism of sarcolipin to calcium ATPase using solution NMR spectroscopy. J Mol Biol 358:420–429
Cantilina T, Sagara Y, Inesi G, Jones LR (1993) Comparative studies of cardiac and skeletal sarcoplasmic reticulum ATPases. Effect of a phospholamban antibody on enzyme activation by Ca2+. J Biol Chem 268:17018–17025
Carafoli E (2002) Calcium signaling: a tale for all seasons. Proc Natl Acad Sci U S A 99:1115–1122
Chiesi M, Inesi G (1980) Adenosine 5′-triphosphate dependent fluxes of manganese and hydrogen ions in sarcoplasmic reticulum vesicles. Biochemistry 19:2912–2918
Clapham DE (2007) Calcium signaling. Cell 131:1047–1122
Cornelius F, Møller JV (1991) Electrogenic pump current of sarcoplasmic reticulum Ca2+-ATPase reconstituted at high lipid/protein ratio. FEBS Lett 284:46–50
de Meis L (2001) Role of the sarcoplasmic reticulum Ca2+-ATPase on heat production and thermogenesis. Biosci Rep 21:113–137
de Meis L, Inesi G (1982) ATP synthesis by sarcoplasmic reticulum ATPase following Ca2+, pH, temperature, and water activity jumps. J Biol Chem 257:1289–1294
de Meis L, Vianna AL (1979) Energy interconversion by the Ca2+ dependent ATPase of the sarcoplasmic reticulum. Annu Rev Biochem 48:275–292
de Meis L, Bianconi ML, Suzano VA (1997) Control of energy fluxes by the sarcoplasmic reticulum Ca2+-ATPase: ATP hydrolysis. ATP synthesis and heat production. FEBS Lett 406:201–204
de Meis L, Arruda AP, Carvalho DP (2005) Role of sarco/endoplasmic reticulum Ca2+-ATPase in thermogenesis. Biosci Rep 25:181–190
de Meis L, Arruda AP, da Costa RM, Benchimol M (2006) Identification of a Ca2+ −ATPase in brown adipose tissue mitochondria: regulation of thermogenesis by ATP and Ca2+. J Biol Chem 281:16384–16390
Ebashi S, Lipmann F (1962) Adenosine-triphosphate linked concentration of calcium ions in a particulate fraction of rabbit muscle. J Cell Biol 14:389–400
Gorski PA, Glaves JP, Vangheluwe P, Young HS (2013) Sarco(endo)plasmic reticulum calcium ATPase (SERCA) inhibition by sarcolipin is encoded in its luminal tail. J Biol Chem 288:8456–8467
Hasselbach W, Makinose M (1962) ATP and active transport. Biochem Biophys Res Commun 7:132–136
Inesi G, de Meis L (1989) Regulation of steady state filling in sarcoplasmic reticulum. Roles of back-inhibition, leakage, and slippage of the calcium pump. J Biol Chem 264:5929–5936
Inesi G, Kurzmack M, Coan C, Lewis DA (1980) Cooperative calcium binding and ATPase activation in sarcoplasmic reticulum vesicles. J Biol Chem 255:3025–3031
Inesi G, Kurzmack M, Lewis D (1988) Kinetic and equilibrium characterization of an energy-transducing enzyme and its partial reactions. Methods Enzymol 157:154–190
Inesi G, Sumbilla C, Kirtley ME (1990) Relationships of molecular structure and function in Ca2+ transport ATPase. Physiol Rev 70:749–760
Isom DG, Sridharan V, Baker R, Clement ST, Smalley DM, Dohlman HG (2013) Protons as second messenger regulators of G protein signaling. Mol Cell 51:4531–4538
Johnson EA, Tanford C, Reynolds JA (1985) Variable stoichiometry in active ion transport: theoretical analysis of physiological consequences. Proc Natl Acad Sci U S A 82:5352–5356
Koss KL, Kranias EG (1996) Phospholamban: a prominent regulator of myocardial contractility. Circ Res 79:1059–1063
Lewis D, Pilankatta R, Inesi G, Bartolommei G, Moncelli MR, Tadini-Buoninsegni F (2012) Distinctive features of catalytic and transport mechanisms in mammalian sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) and Cu+ (ATP7A/B) ATPases. J Biol Chem 287:32717–32727
Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH (1992) Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem 267:14483–14489
MacLennan DH, Kranias EG (2003) Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4:566–577
MacLennan DH, Brandl CJ, Korczak B, Green NM (1985) Amino-acid sequence of a Ca2+- Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature 316:696–700
Mall S, Broadbridge R, Harrison SL, Gore MG, Lee AG, East JM (2006) The presence of sarcolipin results in increased heat production by Ca2+-ATPase. J Biol Chem 281:36597–36602
Martonosi A, Feretos R (1964) Sarcoplasmic reticulum II. Correlation between Adenodine Triphosphatase activity and Ca2+ uptake. J Biol Chem 239:659–668
Masuda H, de Meis L (1973) Phosphorylation of the sarcoplasmic reticulum membrane by orthophosphate. Inhibition by calcium ions. Biochemistry 12:4581–4585
Møller JV, Olesen C, Winther AM, Nissen P (2010) The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Q Rev Biophys 43:501–566
Morimoto T, Kasai M (1986) Reconstitution of sarcoplasmic reticulum Ca2+-ATPase vesicles lacking ion channels and demonstration of electrogenicity of Ca2+-pump. J Biochem (Tokyo) 99:1071–1080
Obara K, Miyashita N, Xu C, Toyoshima I, Sugita Y, Inesi G, Toyoshima C (2005) Structural role of countertransport revealed in Ca2+ pump crystal structure in the absence of Ca2+. Proc Natl Acad Sci U S A 102:14489–14496
Odermatt A, Taschner PE, Scherer SW, Beatty B, Khanna VK, Cornblath DR, Chaudhry V, Yee WC, Schrank B, Karpati G, Breuning MH, Knoers N, MacLennan DH (1997) Characterization of the gene encoding human sarcolipin (SLN), a proteolipid associated with SERCA1: absence of structural mutations in five patients with Brody disease. Genomics 45:541–553
Odermatt A, Becker S, Khanna VK, Kurzydlowski K, Leisner E, Pette D, MacLennan DH (1998) Sarcolipin regulates the activity of SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem 273:12360–12369
Prasad AM, Inesi G (2012) Regulation and rate limiting mechanisms of Ca2+ ATPase (SERCA2) expression in cardiac myocytes. Mol Cell Biochem 361:85–96
Sahoo SK, Shaikh SA, Sopariwala DH, Bal NC, Periasamy M (2013) Sarcolipin protein interaction with sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) is distinct from phospholamban protein, and only sarcolipin can promote uncoupling of the SERCA pump. J Biol Chem 288:6881–6889
Tadini-Buoninsegni F, Bartolommei G, Moncelli MR, Inesi G, Guidelli R (2004) Time-resolved charge translocation by sarcoplasmic reticulum Ca-ATPase measured on a solid supported membrane. Biophys J 86:3671–3686
Tadini-Buoninsegni F, Bartolommei G, Moncelli MR, Guidelli R, Inesi G (2006) Pre-steady state electrogenic events of Ca2+/H+ exchange and transport by the Ca2+-ATPase. J Biol Chem 281:37720–3772735
Tadini-Buoninsegni F, Bartolommei G, Moncelli MR, Pilankatta R, Lewis D, Inesi G (2010) ATP-dependent charge movement in ATP7B Cu+-ATPase is demonstrated by pre-steady state electrical measurements. FEBS Lett 584:4619–4622
Toyoshima C (2008) Structural aspects of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. Arch Biochem Biophys 476:3–11
Toyoshima C, Inesi G (2004) Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu Rev Biochem 73:269–292
Toyoshima C, Asahi M, Sugita Y, Khanna R, Tsuda T, MacLennan DH (2003) Modeling of the inhibitory interaction of phospholamban with the Ca2+ ATPase. Proc Natl Acad Sci U S A 100:467–472
Toyoshima C, Iwasawa S, Ogawa H, Hirata A, Tsueda J, Inesi G (2013) Crystal structures of the calcium pump and sarcolipin in the Mg2+-bound E1 state. Nature 495:260–264
Ueno T, Sekine T (1981) A role of H+ flux in active Ca2+ transport into sarcoplasmic reticulum vesicles. II. H+ ejection during Ca2+ uptake. J Biochem (Tokyo) 89:1247–1252
Winther AM, Bublitz M, Karlsen JL, Møller JV, Hansen JB, Nissen P, Buch-Pedersen MJ (2013) The sarcolipin-bound calcium pump stabilizes calcium sites exposed to the cytoplasm. Nature 495:265–269
Yamaguchi M, Kanazawa T (1985) Coincidence of H+ binding and Ca2+ dissociation in the sarcoplasmic reticulum Ca-ATPase during ATP hydrolysis. J Biol Chem 260:4896–4900
Yu X, Inesi G (1995) Variable stoichiometric efficiency of Ca2+ and Sr2+ transport by the sarcoplasmic reticulum ATPase. J Biol Chem 270:4361–4367
Yu X, Hao L, Inesi G (1994) pK change of acidic residues contributes to cation countertransport in the Ca-ATPase of sarcoplasmic reticulum. Role of H+ in Ca2+ ATPase countertransport. J Biol Chem 269:16656–16661
Acknowledgments
The authors were partially supported by a National Institutes of Health RO1 Grant (G.I.), and Ente Cassa di Risparmio di Firenze and Italian Ministry of Education, University and Research (F.T.-B.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Inesi, G., Tadini-Buoninsegni, F. Ca2+/H+ exchange, lumenal Ca2+ release and Ca2+/ATP coupling ratios in the sarcoplasmic reticulum ATPase. J. Cell Commun. Signal. 8, 5–11 (2014). https://doi.org/10.1007/s12079-013-0213-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-013-0213-7