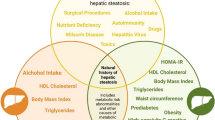
Abstract
Introduction
For the first time in nearly half a century, fatty liver disease has undergone a change in name and definition, from the exclusive term, non-alcoholic fatty liver disease (NAFLD), to the inclusion-based, metabolic-associated fatty liver disease (MAFLD). This has led investigators across the globe to evaluate the impact the nomenclature change has had on the epidemiology and natural history of the disease.
Methods
This systematic review provides a comprehensive overview on how the shift in name and diagnostic criteria has influenced point prevalence in different geographic regions, as well as morbidity and mortality risk, whilst highlighting gaps in the literature that need to be addressed.
Conclusions
MAFLD prevalence is higher than NAFLD prevalence, carries a higher risk of overall mortality, with greater granularity in risk-stratification amongst MAFLD subtypes.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic steatohepatitis (NASH) first entered the hepatology vernacular in 1980, when Ludwig and colleagues described the histologic finding of fatty change with lobular inflammation resembling alcoholic hepatitis in 20 patients who did not consume alcohol and in whom there was no alternate cause of liver disease [1]. Understanding of the condition has expanded exponentially over the past 4 decades as worldwide non-alcoholic fatty liver disease (NAFLD) has become the most prevalent condition affecting the liver, mirroring the burgeoning obesity pandemic [2] and rapidly emerging as one of the foremost indications for liver transplantation [3, 4].
In 2020, an international consensus panel—comprising select experts in the field from 22 countries across the globe—revisited the nomenclature and posited the term metabolic dysfunction-associated fatty liver disease, or simply metabolic-associated fatty liver disease (MAFLD) [5, 6]. This newly proposed term endeavors to better encapsulate the pathophysiological basis for the condition, remove stigmatizing terminology, and acknowledge the heterogeneity encountered in clinical practice, with reference to the co-existence of multiple etiologies of liver disease in a single patient. Moreover, a more inclusive diagnostic criteria may positively influence enrollment into clinical trials and highlight with greater precision the synergistic impact on clinical outcomes.
For both NAFLD and MAFLD, evidence of ≥ 5% hepatosteatosis is a sine qua non for diagnosis irrespective of detection modality. Whereas NAFLD is reliant on excluding alternate causes of liver disease (i.e., alcohol-related fatty liver, viral hepatitis, and drug-induced steatosis) [7, 8], MAFLD requires at least one of the following to be present: (1) overweight according to body mass index (specific threshold for those of Asian ethnicity versus other ethnicities); (2) type 2 diabetes mellitus (T2DM) as per standard diagnostic criteria; and/or (3) metabolic ‘dysfunction’ defined by presence of at least two of seven clinical and biochemical criteria [5] (Table 1).
However, a debate has since ensued on the world stage with several leading commentators voicing concerns over the timing of the name change and proposed criteria for diagnosis. Criticisms include reference to the operational definition for ‘metabolic health’, the risk of confusing colleagues outside the discipline whereby disease awareness remains substandard, as well as the potential for unintended negative consequences on the clinical development and regulatory approval pathways of novel therapeutics [9,10,11]. As such, of the three major international hepatology societies, only the Asian Pacific Association for the Study of the Liver (APASL) has officially endorsed the paradigm shift [12], with observers eagerly awaiting consensus statements from European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD). EASL and AASLD have undertaken a joint formal Delphi process to address the merits of adopting the name change in fatty liver disease [13, 14], with the consensus yet to be published at the time of this review. A particular focus has been on ensuring the shift from NAFLD to MAFLD does not inadvertently impact stakeholder enthusiasm around drug and biomarker development.
Controversies in nosology aside, the dawn of MAFLD has brought with it fertile ground for research, with further opportunities for investigation remaining on the horizon. Herein, we look to summarize in the form of a narrative review, the literature on the influence the name change from NAFLD to MAFLD has had on epidemiology and clinical outcomes in adults and provide key areas for future research efforts.
Methodology
A search of OVID Medline, EMBASE and Web of Science databases from inception to May 2023 was carried out to identify studies reporting on the differences in prevalence and clinically relevant outcomes for NAFLD and MAFLD in the same cohort (Supplementary Fig. 1). The search terms included NAFLD and MAFLD and their associated terms. Studies reporting prevalence as a secondary outcome whereby the primary outcome was to determine an association between fatty liver disease and one more clinicobiochemical parameter(s) were excluded if the study exclusion criteria led to sampling and/or ascertainment bias.
Prevalence
Given the geographic variation in acceptance of the new term MAFLD, it is not surprising that prevalence studies have been most represented by those conducted in Asia compared to Europe, North America and other regions as detailed below (Table 2).
North America: Lin et al. provide one of the earliest insights into NAFLD vs MAFLD prevalence, through post-hoc analysis of the third National Health and Nutrition Examination Survey from 1988 to 1994 (NHANES III) in the United States (USA) [15]. Hepatosteatosis was determined through ultrasonography (US) with MAFLD prevalence reported to be 31.2%, while NAFLD prevalence 33.2%. A further two studies utilizing NHANES III report the prevalence of both NAFLD and MAFLD to be similar, between 30 and 33% [16, 17]. Concerns arise with the methodology of these studies given a near 1.5-fold higher NAFLD prevalence from NHANES III than existing literature in the pre-MAFLD era (~ 18–20%) [18,19,20,21]. Nguyen et al. conducted an equivalent but seemingly more accurate analysis from NHANES III, with MAFLD prevalence 20.1% and NAFLD prevalence 18.3%, with 74.7% concordance of NAFLD-MAFLD and a greater proportion with non-NAFLD-MAFLD than non-MAFLD-NAFLD (16.8% vs 8.5%) [22]. Despite adoption of the same operational definitions for NAFLD and MAFLD among studies, the differences in prevalence estimates from NHANES III demonstrate the inter-reporter variability in epidemiologic studies in fatty liver disease has not been remedied by the nomenclature change. Contemporary iterations of NHANES validate that MAFLD marginally increases fatty liver disease prevalence in the USA, whether case ascertainment is through the United States Fatty Liver Index (US-FLI) or accepted elastographic parameters on vibration-controlled transient elastography (VCTE) [23,24,25]. A study by Wong et al. from NHANES 2011–2018 reported that MAFLD prevalence increased from 34.4% to 38.1% (p < 0.01) between 2011 to 2018, with 7.6% of MAFLD patients having concomitant liver disease (5.5% alcohol-related, 1.6% hepatitis C, 0.5% chronic hepatitis B [CHB]) [26]. This rise in fatty liver disease is supported by another study by Zhang et al. from NHANES 1999–2016, with both NAFLD and MAFLD prevalence rising in parallel [27] (Table 2).
Asia: In mainland China, nine cross-sectional, US-based studies have reported the MAFLD prevalence to be between 20.4 and 48.4%, and in all but one study [28] higher than NAFLD prevalence (18.4–45.3%) [28,29,30,31,32,33,34,35,36]. The difference is accounted for by the region in which they occurred, age of participants (all adults vs ≥ 40 years old vs 45–70 years old only), epoch of study (ranging from 2006 to 2020) and varying prevalence of central and general obesity and T2DM. Seven of these studies reported the NAFLD-MAFLD concordance to be between 78 and 90% [28, 29, 31, 32, 34,35,36], with all but one [28] finding non-NAFLD-MAFLD cohort significantly higher than the non-MAFLD-NAFLD proportion. While studies report similar rate of co-existent CHB (between 2 and 5% [29, 30, 35]), rates for simultaneous alcohol-related liver disease (ARLD) is vastly different (from 0.4 to 17.6% [28,29,30, 35]). Notably, in the study by Wang et al., there was a marked difference in ARLD between females and males with MAFLD (0.5 vs 15.8%, respectively) [29].
In one cross-sectional population-based study of adults from Hong Kong conducted between 2008 and 2010 and utilizing proton magnetic resonance spectroscopy to detect hepatosteatosis, MAFLD prevalence was 25.9% and NAFLD prevalence 25.7%, with an overall concordance of 89.2% (5.8% with MAFLD without NAFLD and 5.1% NAFLD without MAFLD) [37]. One study from the Taiwan Biobank cohort of 22,909 adults who underwent US, MAFLD prevalence was higher than NAFLD (38.9% vs 36.9%) with 79.7% concordance between fatty liver disease definitions and the majority of MAFLD-only cohort due to concomitant viral hepatitis, particularly CHB [38].
A FLI-based study from South Korea among close to 10 million participants aged between 40 and 64 years recruited in 2009–2010, the NAFLD-MAFLD concordance was 72.4% (26.1% MAFLD without NAFLD, 1.5% NAFLD without MAFLD), with point prevalence 37.3% and 28.0% for MAFLD and NAFLD, respectively [39]. In all but one of six smaller studies originating in Korea, the finding that MAFLD prevalence is higher than NAFLD prevalence is once more replicated (MAFLD 25–46% vs NAFLD 22–41%) [40,41,42,43,44,45]. Akin to the studies reporting from China, the differences in fatty liver prevalence between studies can be accounted for by varying geographic region of sampled cohort, gender and age profile of population studied, and prevalence of metabolic risk factors and co-existent liver disease in each cohort. The Hong Kong and FLI-based Korean studies report similar co-prevalence of viral hepatitis as those from China, 4.9% and 4.6% respectively; however, concomitant ARLD differed substantially, 1.1% in Hong Kong and 22.2% in Korea, despite the same definition for excessive consumption (> 30 g/day in males, > 20 g/day in females). Co-existent ARLD in > 10% of MAFLD-only participants was seen among all Korean studies reporting this outcome and mirrors the prevalence in China (Table 2).
In the earliest report establishing prevalence differences with the shift in fatty liver nomenclature from Japan, Fujii et al. report MAFLD prevalence to be 35.0%, higher than NAFLD prevalence 27.4% and with 69.9% concordance from a health examination registry of over 2000 adults undertaking US between 2014 and 2019 [46]. Although concurrent liver disease in the MAFLD-only cohort was not specified, presence of hepatitis C antibody and alcohol consumption ≥ 60 g/day were exclusion criteria, suggesting it was related to lower degrees of alcohol excess, CHB and/or alternate liver disease. Furthermore, 90% of isolated MAFLD participants were male, markedly disparate compared with the gender distribution among those with overlap NAFLD-MAFLD (69% male) and isolated NAFLD (64% male). In other studies from Japan, the concordance between fatty liver disease definitions is seemingly lower than other parts of the globe, with a higher MAFLD-only cohort than reported elsewhere in Asia or beyond [47,48,49], suggesting this difference may be driven by a higher prevalence of simultaneous liver disease or greater degree of metabolic dysfunction among Japanese fatty liver disease patients. Unfortunately, alternate etiology of liver disease has not been reported in any of these studies. Once more, all but one study reporting the difference in NAFLD and MAFLD prevalence in Japan revealed a higher prevalence of the latter [46,47,48,49,50,51] (Table 2). However, the methodology of this study comes into question given other authors determined the prevalence of NAFLD to be much lower in the same cohort studied [51], and as per the NHANES III reports highlights the fraught nature of reporting on fatty liver disease epidemiology.
A single study from Sri Lanka utilizing the well-conducted Ragama Health Study which enrolled those between 35 and 64 years old and utilizing US, the concordance was high at 87.7% (8.6% MAFLD without NAFLD, 3.7% NAFLD without MAFLD) with a MAFLD prevalence of 33.2% higher than NAFLD prevalence 31.5% [52] (Table 2).
Oceania: One FLI-based cross-sectional study from a regional center in Australia revealed concordance in the two diagnoses was 82.5% (17.5% MAFLD without NAFLD, 0% NAFLD without MAFLD), with a MAFLD prevalence of 47.2% again higher than NAFLD prevalence 38.7% [53]. Co-existent alcohol-related fatty liver was 16.9% in the Australian MAFLD cohort compared to 8.9% in the Sri Lankan cohort, despite lower threshold used to determine excessive alcohol consumption in the Sri Lankan study. Neither study reported on prevalence of viral hepatitis (Table 2).
Europe: To date, only one study has been published from Europe, specifically from the Rotterdam study in the Netherlands, recruiting adults older than 45 years between 2009 and 2014 and undertaking US [54]. NAFLD and MAFLD prevalence were 29.5% and 34.3%, respectively, with 80.4% concordance (Table 2). However, there is some uncertainty in the results from this study given participants consuming greater than 60 g of alcohol per day and those with viral hepatitis were excluded, such that concomitant liver disease in the MAFLD participants was related to moderate alcohol excess and use of steatogenic medications.
Epidemiology
A uniform finding among studies was that in comparison to those meeting a diagnosis for NAFLD, those with MAFLD were more likely to be males (paralleling difference in excessive alcohol consumption between genders in these studies), have more participants with one or more components of metabolic syndrome, and a greater proportion with indeterminate or high-risk for fibrosis on basis of non-invasive tests (Fig. 1.). This is likely related to the inherently inclusive diagnostic criteria for MAFLD, requiring one or more clinical features of metabolic dysfunction to be present, as well as for allowing for co-existence of alternate etiology of liver disease, which may result in an additive or synergistic impact on fibrogenesis. This requires further evaluation in prospective studies. These differences are even more stark when comparing participants meeting the diagnosis for MAFLD without NAFLD compared to NAFLD without MAFLD, raising the prospect of greater granularity in risk stratifying patients with fatty liver disease, with a small proportion with potentially otherwise ‘metabolically healthy’ fatty liver. Apart from a minority (n = 5/35, 14%) [15, 16, 28, 44, 50], the epidemiologic studies consistently demonstrate that point prevalence is higher for MAFLD when redefined from NAFLD, which fits in with its inherently inclusive diagnostic criteria.
Another point to highlight is that the difference in prevalence of MAFLD and NAFLD is linked to the background prevalence of other causes of liver disease, with a greater difference in point prevalence in those populations in which alternate causes of liver disease are more prevalent. Once more this is related to the polarizing diagnostic criterion of co-factor for liver disease, which allows for existence of alternate etiologies of liver disease for MAFLD but not NAFLD (Table 1) [5, 7, 8].
Natural history
Beyond epidemiology, a highly relevant aspect of the name change for clinicians is in determining any major differences in clinical outcomes. This allows a physician to appropriately counsel the patient on prognostication and focus therapeutic efforts in ameliorating this risk. Many researchers have made concerted efforts in establishing if the shift in nomenclature has resulted in a disease with heightened risk, particularly given the allowance for co-occurrence of alternate etiology of liver disease in the definition.
Mortality
Overall: Nguyen and colleagues trichotomized NHANES III participants into non-NAFLD-MAFLD, overlap NAFLD-MAFLD and non-MAFLD-NAFLD groups, and demonstrated a significant difference in 15-year all-cause mortality between the groups, 26.2% vs 21.1% vs 10.6% (p < 0.0001), respectively [22]. Those with MAFLD without NAFLD had a 2.4-fold increased risk for mortality compared to NAFLD without MAFLD on a model adjusted for demographic features, smoking status, viral hepatitis, fibrosis stage and weight (Table 3 and Fig. 2a). Older age, current or former smoking status, being African American and viral hepatitis were all independently associated with all-cause mortality on a multivariable regression model. Similarly, Kim et al. utilized the NHANES III database and stratified participants according to the three fatty liver groups per Nguyen et al. and reported on long-term outcome, with a median follow-up time of 23.2 years [55]. Once more, the authors demonstrated that those with MAFLD are at higher risk for all-cause mortality than those with NAFLD. First, participants with MAFLD had a 17% increased risk of all-cause mortality compared with those without MAFLD on a comprehensive multivariable Cox proportional model, while there was no difference between those with NAFLD compared to non-NAFLD (p = 0.35). Second, adjusting for the same demographic, lifestyle, clinical and laboratory covariates, only participants with NAFLD-MAFLD and non-NAFLD-MAFLD were determined to have a significantly increased risk for all-cause mortality compared with those without any steatosis (Table 3 and Fig. 2a); no difference was observed for those with non-MAFLD-NAFLD.
It is noteworthy that a single study also reporting from NHANES III did not demonstrate a difference in all-cause mortality in those with MAFLD or NAFLD, when compared to non-MAFLD/NAFLD participants [56]. Although alcohol-related liver disease was accounted for in this model, the definition was based on a lower threshold for alcohol consumption (≥ 20 g/day in males; ≥ 10 g/day in females) than the other two studies reporting from NHANES III (≥ 30 g/day in males; ≥ 20 g/day in females), which might have influenced the findings. There were other differences between the covariates included in respective models, which might also have led to a disparity in findings. Finally, in Huang et al. study [17] also utilizing NHANES, MAFLD but not NAFLD was determined to increase risk of overall mortality compared with non-fatty liver disease participants, except when the model was adjusted for metabolic risk factors, suggesting it is these risk factors which associate with heightened mortality risk.
In the report by Wang et al. from the Kailuan Study in China following over 150,000 participants for a median of 12.7 years, the annual all-cause mortality rate of MAFLD was higher than NAFLD for all age groups and between genders [29], consistent with NHANES III literature. Furthermore, on subgroup analysis, meeting the MAFLD criteria according to T2DM or metabolic dysfunction had a more profound negative impact on mortality than overweight/obesity criterion (HR 1.41 [95% CI 1.18–1.67] up to HR 4.26 [95% CI 1.74–10.43] vs HR 0.32 [95% CI 0.15–0.57] up to HR 1.06 [0.64–1.74] in various age-/gender-stratified groups); the cumulative number of criteria met led to higher risk of mortality (HR up to 4.26 [95% CI 1.74–10.43] for one criteria met vs HR up to 11.40 [95% CI 2.69–48.35] for two or three criteria met); and presence of an additional cause of liver disease (viral and/or alcohol-related) compounded the risk of mortality (HR up to 1.77 [95% CI 1.27–2.48] without additional liver disease vs up to 9.86 [95% CI 2.44–39.98] with co-existent liver disease). This concept of a difference in outcome according to MAFLD criteria met was also reported from NHANES III, again with T2DM and metabolic dysfunction presenting a greater risk than MAFLD with overweight/obesity alone [57].
Two studies originating from different cohorts in Korea have reported on the comparison in mortality between NAFLD and MAFLD. One study including adults aged 40–70 years once more demonstrated the impact of a name change has on mortality, with MAFLD conferring a 36% higher risk of all-cause mortality after adjustment for relevant covariates, while there was no difference in mortality in NAFLD participants with the same regression model (Table 3) [58]. The adverse impact of MAFLD on mortality remained even after adjusting for viral hepatitis and excess alcohol consumption (HR 1.33, 95% CI 1.05–1.69). In another study reporting from the large cross-sectional Kangbuk Samsung Health Study, enrolling all adults 18 years and older between 2002 and 2012 and followed for a median of 5.7 years, all-cause mortality was higher in MAFLD participants than those without MAFLD (log-rank p-value < 0.001 from Kaplan–Meier curve) [43], with insignificant difference between NAFLD vs non-NAFLD (p = 0.20). There was delineation in survival curves for groups stratified as non-MAFLD-NAFLD, NAFLD-MAFLD and non-NAFLD-MAFLD (log-rank p < 0.001), with the MAFLD-only group at highest risk of mortality (HR 1.67, 95% CI 1.40–1.99) and the NAFLD-only group at no higher risk of mortality than non-steatotic participants (HR 0.84, 95% CI 0.57–1.23) on unadjusted models, with the statistical significance dissipating in the MAFLD-only group on multivariable models (Table 3 and Fig. 1a).
Cardiovascular disease and cancer-related: A strength of the study by Younossi et al. was in reporting on cause-specific mortality data, with CVD-related death most common cause of mortality in MAFLD participants (34.5%) followed by extra-hepatic malignancy (20.4%), both outnumbering liver-related death (6.7%) during the 20-year follow-up period [56]. All the authors reporting from NHANES III were consistent in reporting CVD as the foremost cause of death, closely followed by cancer [22, 55, 57]. Nguyen and colleagues demonstrated that the mortality difference between MAFLD-only, NAFLD-MAFLD and NAFLD-only groups persisted for cardiovascular disease (CVD)-related mortality (p = 0.009) and non-cancer/non-CVD-related mortality (p = 0.002) but not cancer-related mortality (p = 0.2) [22].
On sensitivity analysis of cause-specific mortality, Kim et al. established there was no difference in CVD-related mortality and cancer-related mortality in the fully-adjusted model between MAFLD vs non-MAFLD (p = 0.69 and p = 0.41, respectively) and NAFLD vs non-NAFLD (p = 0.48 and p = 0.89, respectively) [55]. However, non-NAFLD-MAFLD conferred a heightened risk for cancer-related mortality on the complete model, with non-steatotic participants as the reference (Table 3 and Fig. 2c). This may suggest that co-factor for liver disease may be a determinant for increased risk of carcinogenesis, although there was no granular data on specific malignancies to draw conclusions about where this risk may lie (i.e., hepatic vs extra-hepatic).
Similarly, Moon et al. found no difference in CVD-related mortality between those with and without MAFLD (p-value 0.66–0.98 on all adjusted models), but once more MAFLD portended a higher cancer-related mortality (HR 1.52 [95% CI 1.01–2.30] up to HR 1.63 [95% CI 1.13–2.36] on adjusted models), except for when the model was adjusted for viral hepatitis and excess alcohol consumption (Table 3) [58]. This adds further weight to the hypothesis that cancer-related mortality in MAFLD may account for by the synergistic carcinogenesis occurring with additional liver disease. Contrary to Kim et al. the authors of this study were able to reveal cause-specific cancer deaths, with ‘other’ unspecified cancer (51.0%) far outweighing liver (18.4%) and lung (16.3%) as the most common cause of cancer-related death [58].
In another Korean study by Kim and colleagues with shorter duration of follow-up, CVD-related mortality was higher in MAFLD participants than those without MAFLD (log-rank p-value < 0.001 from Kaplan–Meier curve) as well as NAFLD vs non-NAFLD (p = 0.002) [43], while those by Lee et al. [39] and Yoo et al. [45] demonstrate an apparent stepwise hierarchy in risk stratification for CVD mortality in fatty liver disease with the advent of the nomenclature change; highest for isolated MAFLD, followed by concordant fatty liver disease, and the least risk—with near equipoise to the general non-fatty liver disease controls—in those with NAFLD-alone (Table 3).
Liver-related: Death from liver disease was not well reported among studies, with authors utilizing NHANES III raising difficulties with accessing this linked data due to the few numbers of liver-related deaths [22, 55, 57]. However, Younossi et al. were able to access this specific data and reported liver-related mortality was higher for MAFLD than NAFLD (3.01%, 95% CI 1.99–4.03 vs 1.81%, 95% CI 0.95–2.66), and although the influence of various covariates on CVD-related and extra-hepatic malignancy-related mortality was similar between the two groups, it was markedly different for liver-related mortality [56]. Whereas high-risk for fibrosis was the greatest influence over liver-related mortality in both MAFLD and NAFLD (HR 17.15, 95% CI 4.55–64.65 and HR 9.26, 95% CI 1.84–46.33, respectively), the other covariates with most influence were alcohol-related liver disease (HR 4.50, 95% CI 1.89–10.75) and chronic kidney disease (HR 2.92, 95% CI 1.21–7.01) for MAFLD, while they were high C-reactive protein (CRP) (HR 4.47, 95% CI 1.35–14.77) and insulin resistance (HR 3.57, 95% CI 1.35–9.42) for NAFLD. It is vital to point out the differences in definitions between MAFLD and NAFLD once again, with the latter not allowing for inclusion of excessive alcohol consumption. As such, by definition, excessive alcohol consumption cannot be a predictor for liver-related outcome in NAFLD and by extension, this would impact how other covariates interact between the disease and outcome.
In the Korean study by Moon and colleagues, MAFLD was predictive for liver-related mortality even after comprehensive multivariable analysis accounting for demographic variables, comorbidities and high-sensitivity CRP (Table 3) [58].
To surmise, these studies suggest that meeting the diagnostic criteria for MAFLD is more hazardous than a diagnosis of NAFLD, with a higher risk of mortality (in particular, all-cause and liver-related), MAFLD without NAFLD (i.e., fatty liver in the presence of co-factor for liver disease) leads to a compounded risk of death, fulfilling different criterion for MAFLD may impact mortality risk (with highest risk for those meeting T2DM and metabolic dysfunction criteria) and that there is a small cohort of fatty liver patients in the community who are metabolically ‘healthier’ that do not appear to have adverse outcomes compared to non-steatotic ‘healthy’ participants (Figs. 2 and 3.). The reported differential mortality risk between NAFLD and MAFLD may be in part resultant from the multivariate models utilized to adjust for risk (e.g., not being able to adjust for relevant metabolic covariates given these are contained within the MAFLD diagnostic criteria) or due to the inclusive nature of the MAFLD diagnostic criteria, allowing for co-existence of alternate etiologies of liver disease, which as discussed earlier may have a deleterious and compounding impact on mortality. This has implications for public health and research efforts as it stratifies the ballooning problem of fatty liver into at-risk groups for which targeted interventions are most needed.
Morbidity
The literature describing the differential morbidity between NAFLD and MAFLD is less certain and mature than mortality data. While some authors report a higher incidence of general and central obesity [52], T2DM [52] and CVD [39, 52] in MAFLD compared with NAFLD, others have found an increased morbidity from extra-hepatic disease in MAFLD and NAFLD compared to non-fatty liver participants, but no difference between the two conditions [28, 30, 42, 59]. However, this area of research is still in its infancy and the volume of studies is limited in comparison to those conducted in the NAFLD-alone arena, which have allowed for numerous meta-analyses in individual outcomes [60,61,62,63,64,65,66,67].
Scarce literature has examined the impact of the change in nomenclature on malignancy risk, whether primary hepatic or extra-hepatic. A single Korean study examining over 10 million adults aged between 40 and 64 years demonstrated that the risk for incident colorectal cancer was highest among those with isolated MAFLD (HR 1.32, 95% CI 1.28–1.35), followed by those with NAFLD-MAFLD (HR 1.18, 95% CI 1.16–1.20), and last in those with non-MAFLD-NAFLD (HR 1.16, 95% CI 1.06–1.28) [68]. A study by Yuan and colleagues [35] examining a large cohort from China followed for a median duration of over 12 years reported contrary results regarding extra-hepatic malignancy with NAFLD-MAFLD carrying an increased risk for colorectal cancer (HR 1.19, 95% CI 1.00–1.41), thyroid cancer (HR 1.62, 95% CI 1.11–2.35), renal cancer (HR 1.58, 95% CI 1.19–2.09), prostate cancer (HR 1.48, 95% CI 1.04–2.11) and breast cancer (HR 1.29, 95% CI 1.02–1.64), but MAFLD alone not increasing the risk for any of the twelve malignancies investigated. However, these models adjusted for excessive alcohol consumption, with the authors also demonstrating that those with MAFLD and excessive alcohol consumption had a higher risk of developing extra-hepatic malignancy (HR 1.14, 95% CI 1.01–1.29), which was not seen in those with MAFLD and viral hepatitis (HR 1.17, 95% CI 0.83–1.65) or MAFLD without co-factor for liver disease (HR 1.03, 95% CI 0.97–1.10).
Only a single study has reported on incidence of hepatocellular carcinoma (HCC) in those with NAFLD compared to MAFLD. In this retrospective study from Geneva examining HCC incidence between 1999 and 2014, the MAFLD-HCC age-standardized incidence rose from 1.30 (95% CI 0.75–2.10) to 5.03 (95% CI 4.01–6.23) per 100,000, with a fivefold higher age-standardized incidence than NAFLD-HCC in males and twofold higher age-standardized incidence in females in 2014 [69].
Future directions
Although the debate surrounding the nomenclature shift may rage on, researchers continue to examine how the diagnostic criteria have impacted epidemiology and natural history of fatty liver disease. Yet more is to be learnt over the coming years and decades. We recommend the following priorities in studies reporting on the epidemiology and clinical outcomes of fatty liver disease:
-
1.
Prevalence studies originating beyond the US and Asia, to better establish if there is geographic variation for prevalence between these two conditions and the factors contributing to these differences (environmental vs genetic vs other)
-
2.
Exploring differences in liver-related outcome, CVD-related outcome and incidence of hepatic and extra-hepatic malignancy between NAFLD and MAFLD
-
3.
Evaluating the morbidity and mortality of MAFLD across gender and different age groups, including older persons, to determine whether there are age- or gender-specific differences in clinical outcomes over time
-
4.
Investigating differences in outcome according to the specific MAFLD criteria being met (overweight/obesity vs T2DM vs metabolic dysfunction, as well as general vs central obesity), number of MAFLD criteria met (1 vs 2 vs 3), and in relation to presence or absence of co-factor for liver disease (particularly the impact co-occurrence of viral and alcohol-related liver disease have on hepatic and extra-hepatic malignancy)
-
5.
Determining how diet and lifestyle, including participation in physical activity, influence outcome in MAFLD compared to NAFLD
Conclusions
The dawn of MAFLD has led to an increased prevalence of fatty liver disease, with a heightened risk for overall mortality. However, much is still to be established about the impact of the name change, particularly on non-fatal clinical outcomes including CVD, liver decompensation and malignancy.
Data availability
This is a review article utilising data from other authors/published papers and, as such, data availability statement not required.
References
Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 1980;55(7):434–438
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64(1):73–84
Burra P, Becchetti C, Germani G. NAFLD and liver transplantation: disease burden, current management and future challenges. JHEP Rep 2020;2(6): 100192
Wong RJ, Singal AK. Trends in liver disease etiology among adults awaiting liver transplantation in the United States, 2014–2019. JAMA Netw Open 2020;3(2): e1920294
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73(1):202–209
Eslam M, Sanyal AJ, George J, International Consensus P. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020;158(7):1999-2014.e1
European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64(6):1388–402
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67(1):328–357
Younossi ZM, Rinella ME, Sanyal AJ, Harrison SA, Brunt EM, Goodman Z, et al. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology 2021;73(3):1194–1198
Singh SP, Anirvan P, Reddy KR, Conjeevaram HS, Marchesini G, Rinella ME, et al. Non-alcoholic fatty liver disease: Not time for an obituary just yet! J Hepatol 2021;74(4):972–974
Ratziu V, Rinella M, Beuers U, Loomba R, Anstee QM, Harrison S, et al. The times they are a-changin’ (for NAFLD as well). J Hepatol 2020;73(6):1307–1309
Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int 2020;14(6):889–919
AASLD. Reaching Consensus on NAFLD Nomenclature 2022 [Available from: https://www.aasld.org/news/reaching-consensus-nafld-nomenclature.
EASL. NAFLD Nomenclature Consensus Meeting High-Level Output 2022 [Available from: https://easl.eu/news/nafld-nomenclature-consensus-meeting-high-level-output/.
Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int 2020;40(9):2082–2089
Zhang P, Dong X, Zhang W, Wang S, Chen C, Tang J, et al. Metabolic-associated fatty liver disease and the risk of cardiovascular disease. Clin Res Hepatol Gastroenterol 2023;47(1): 102063
Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: which has closer association with all-cause and cause-specific mortality?-Results from NHANES III. Front Med (Lausanne) 2021;8: 693507
Paik J, Golabi P, Younoszai Z, Mishra A, Trimble G, Younossi ZM. Chronic kidney disease is independently associated with increased mortality in patients with nonalcoholic fatty liver disease. Liver Int 2019;39(2):342–352
Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol 2012;10(6):646–650
Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ 2011;343: d6891
Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2013;178(1):38–45
Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential clinical characteristics and mortality outcomes in persons With NAFLD and/or MAFLD. Clin Gastroenterol Hepatol 2021;19(10):2172–81 e6
Aimuzi R, Xie Z, Qu Y, Jiang Y, Luo K. Associations of urinary organophosphate esters metabolites and diet quality with nonalcoholic/metabolic dysfunction-associated fatty liver diseases in adults. Ecotoxicol Environ Saf 2023;254: 114720
Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int 2021;41(6):1290–1293
Xie ZQ, Li HX, Tan WL, Yang L, Ma XW, Li WX, et al. Association of serum vitamin C With NAFLD and MAFLD among adults in the United States. Front Nutr 2021;8: 795391
Wong RJ, Cheung R. Trends in the prevalence of metabolic dysfunction-associated fatty liver disease in the United States, 2011–2018. Clin Gastroenterol Hepatol 2022;20(3):e610–e613
Zhang HJ, Wang YY, Chen C, Lu YL, Wang NJ. Cardiovascular and renal burdens of metabolic associated fatty liver disease from serial US national surveys, 1999–2016. Chin Med J (Engl) 2021;134(13):1593–1601
Xu X, Zhou X, Tian T, Ding Y, Yu C, Zhao W, et al. Comparison of clinical characteristics and outcomes of MAFLD and NAFLD in Chinese Health examination populations. J Clin Transl Hepatol 2023;11(4):777–786
Wang X, Wu S, Yuan X, Chen S, Fu Q, Sun Y, et al. Metabolic dysfunction-associated fatty liver disease and mortality among chinese adults: a prospective cohort study. J Clin Endocrinol Metab 2022;107(2):e745–e755
Liang Y, Chen H, Liu Y, Hou X, Wei L, Bao Y, et al. Association of MAFLD with diabetes, chronic kidney disease, and cardiovascular disease: a 4.6-year cohort study in China. J Clin Endocrinol Metab 2022;107(1):88–97
Yu C, Wang M, Zheng S, Xia M, Yang H, Zhang D, et al. Comparing the diagnostic criteria of MAFLD and NAFLD in the Chinese population: a population-based prospective cohort study. J Clin Transl Hepatol 2022;10(1):6–16
Liu Q, Zhao G, Li Q, Wu W, Zhang Y, Bian H. A comparison of NAFLD and MAFLD diagnostic criteria in contemporary urban healthy adults in China: a cross-sectional study. BMC Gastroenterol 2022;22(1):471
Miao L, Yang L, Guo LS, Shi QQ, Zhou TF, Chen Y, et al. Metabolic dysfunction-associated fatty liver disease is associated with greater impairment of lung function than nonalcoholic fatty liver disease. J Clin Transl Hepatol 2022;10(2):230–237
Wang Y, Yu Y, Zhang H, Chen C, Wan H, Chen Y, et al. Cardiovascular and renal burdens among patients with MAFLD and NAFLD in China. Front Endocrinol (Lausanne) 2022;13: 968766
Yuan X, Wang X, Wu S, Chen S, Wang Y, Wang J, Lu Y, Sun Y, Fu Q, Wang L. Associations between metabolic dysfunction-associated fatty liver disease and extrahepatic cancers: a cohort in China. HepatoBiliary Surg Nutr 2022. https://hbsn.amegroups.org/article/view/96588/pdf
Zeng J, Qin L, Jin Q, Yang RX, Ning G, Su Q, et al. Prevalence and characteristics of MAFLD in Chinese adults aged 40 years or older: a community-based study. Hepatobiliary Pancreat Dis Int 2022;21(2):154–161
Wong VW, Wong GL, Woo J, Abrigo JM, Chan CK, Shu SS, et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease. Clin Gastroenterol Hepatol 2021;19(10):2161–71.e5
Cheng YM, Wang CC, Kao JH. Metabolic associated fatty liver disease better identifying patients at risk of liver and cardiovascular complications. Hepatol Int 2023;17(2):350–356
Lee H, Lee YH, Kim SU, Kim HC. Metabolic Dysfunction-Associated Fatty Liver Disease and Incident Cardiovascular Disease Risk: A Nationwide Cohort Study. Clin Gastroenterol Hepatol 2021;19(10):2138–47.e10
Chun HS, Lee M, Lee JS, Lee HW, Kim BK, Park JY, et al. Metabolic dysfunction associated fatty liver disease identifies subjects with cardiovascular risk better than non-alcoholic fatty liver disease. Liver Int 2022. https://pubmed.ncbi.nlm.nih.gov/36585250/
Choi JM, Park HE, Han YM, Lee J, Lee H, Chung SJ, et al. Non-alcoholic/metabolic-associated fatty liver disease and helicobacter pylori additively increase the risk of arterial stiffness. Front Med (Lausanne) 2022;9: 844954
Kim H, Lee CJ, Ahn SH, Lee KS, Lee BK, Baik SJ, et al. MAFLD predicts the risk of cardiovascular disease better than NAFLD in asymptomatic subjects with health check-ups. Dig Dis Sci 2022;67(10):4919–4928
Kim KS, Hong S, Ahn HY, Park CY. Metabolic dysfunction-associated fatty liver disease and mortality: a population-based cohort study. Diabetes Metab J 2023. https://doi.org/10.4093/dmj.2021.0327
Seo JY, Bae JH, Kwak MS, Yang JI, Chung SJ, Yim JY, et al. The risk of colorectal adenoma in nonalcoholic or metabolic-associated fatty liver disease. Biomedicines 2021;9(10):1401
Yoo TK, Lee MY, Kim SH, Zheng MH, Targher G, Byrne CD, et al. Comparison of cardiovascular mortality between MAFLD and NAFLD: a cohort study. Nutr Metab Cardiovasc Dis 2023;33(5):947–955
Fujii H, Fukumoto S, Enomoto M, Uchida-Kobayashi S, Kimura T, Tamori A, et al. The FibroScan-aspartate aminotransferase score can stratify the disease severity in a Japanese cohort with fatty liver diseases. Sci Rep 2021;11(1):13844
Bessho R, Kashiwagi K, Ikura A, Yamataka K, Inaishi J, Takaishi H, et al. A significant risk of metabolic dysfunction-associated fatty liver disease plus diabetes on subclinical atherosclerosis. PLoS One 2022;17(5): e0269265
Tateda T, Iino C, Sasada T, Sato S, Igarashi G, Kawaguchi S, et al. Evaluation of metabolic dysfunction-associated fatty liver disease using FibroScan, diet, and microbiota: a large cross-sectional study. PLoS One 2022;17(11): e0277930
Sogabe M, Okahisa T, Kurihara T, Kagawa M, Ueda H, Kawaguchi T, et al. Comparison of the role of alcohol consumption and qualitative abdominal fat on NAFLD and MAFLD in males and females. Sci Rep 2022;12(1):16048
Tanaka M, Mori K, Takahashi S, Higashiura Y, Ohnishi H, Hanawa N, et al. Metabolic dysfunction-associated fatty liver disease predicts new onset of chronic kidney disease better than fatty liver or nonalcoholic fatty liver disease. Nephrol Dial Transplant 2023;38(3):700–711
Mori K, Tanaka M, Hosaka I, Mikami T, Endo K, Hanawa N, et al. Metabolic dysfunction-associated fatty liver disease is associated with an increase in systolic blood pressure over time: linear mixed-effects model analyses. Hypertens Res 2023;46(5):1110–1121
Niriella MA, Ediriweera DS, Kasturiratne A, De Silva ST, Dassanayaka AS, De Silva AP, et al. Outcomes of NAFLD and MAFLD: results from a community-based, prospective cohort study. PLoS One 2021;16(2): e0245762
Kemp W, Clayton-Chubb D, Majeed A, Glenister KM, Magliano DJ, Lubel J, et al. Impact of renaming NAFLD to MAFLD in an Australian regional cohort: Results from a prospective population-based study. J Gastroenterol Hepatol 2022;37(2):395–403
van Kleef LA, Ayada I, Alferink LJM, Pan Q, de Knegt RJ. Metabolic dysfunction-associated fatty liver disease improves detection of high liver stiffness: the Rotterdam Study. Hepatology 2022;75(2):419–429
Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol 2021;75(6):1284–1291
Younossi ZM, Paik JM, Al Shabeeb R, Golabi P, Younossi I, Henry L. Are there outcome differences between NAFLD and metabolic-associated fatty liver disease? Hepatology 2022;76(5):1423–1437
Zhang YC, Lyu ZY, Ma B, Li LM, Wang W, Sheng C, et al. A new risk stratification strategy for fatty liver disease by incorporating MAFLD and fibrosis score in a large US population. Hepatol Int 2022;16(4):835–845
Moon JH, Kim W, Koo BK, Cho NH, Innovative Target Exploration of Nc. Metabolic dysfunction-associated fatty liver disease predicts long-term mortality and cardiovascular disease. Gut Liver 2022;16(3):433–42
Yoneda M, Yamamoto T, Honda Y, Imajo K, Ogawa Y, Kessoku T, et al. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: a retrospective nationwide claims database study in Japan. J Gastroenterol 2021;56(11):1022–1032
Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care 2018;41(2):372–382
Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Schattenberg JM, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut 2022;71(1):156–162
Mantovani A, Petracca G, Beatrice G, Csermely A, Tilg H, Byrne CD, et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut 2022;71(4):778–788
Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut 2021;70(5):962–969
Mantovani A, Zaza G, Byrne CD, Lonardo A, Zoppini G, Bonora E, et al. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: a systematic review and meta-analysis. Metabolism 2018;79:64–76
Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 2014;11(7): e1001680
Orci LA, Sanduzzi-Zamparelli M, Caballol B, Sapena V, Colucci N, Torres F, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol 2022;20(2):283–92 e10
Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol 2016;65(3):589–600
Lee H, Lee HW, Kim SU, Chang KH. Metabolic dysfunction-associated fatty liver disease increases colon cancer risk: a nationwide cohort study. Clin Transl Gastroenterol 2022;13(1): e00435
Myers S, Neyroud-Caspar I, Spahr L, Gkouvatsos K, Fournier E, Giostra E, et al. NAFLD and MAFLD as emerging causes of HCC: a populational study. JHEP Rep 2021;3(2): 100231
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
All the authors have made substantial contributions to the following: (1) the conception and design of the review (SKR, KV, DCC), or acquisition of data (KV, DCC), or analysis and interpretation of data (KV, DCC, AM, JL, DS, WK, SKR), (2) drafting the article (KV, SKR) or revising it critically for important intellectual content (all the authors), and (3) final approval of the version to be submitted (all authors). KV is the guarantor for this article.
Corresponding author
Ethics declarations
Conflict of interest
Karl Vaz, Daniel Clayton-Chubb, Ammar Majeed, John Lubel, David Simmons, William Kem, Stuart K. Roberts declares that they have no conflict of interest.
Ethical approval
Not required.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vaz, K., Clayton-Chubb, D., Majeed, A. et al. Current understanding and future perspectives on the impact of changing NAFLD to MAFLD on global epidemiology and clinical outcomes. Hepatol Int 17, 1082–1097 (2023). https://doi.org/10.1007/s12072-023-10568-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10568-z