Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) and metabolic-associated fatty liver disease (MAFLD) shares common pathophysiological mechanisms with type 2 diabetes, making them significant risk factors for type 2 diabetes. The present study aimed to assess the epidemiological feature of type 2 diabetes in patients with NAFLD or MAFLD at global levels.
Methods
Published studies were searched for terms that included type 2 diabetes, and NAFLD or MAFLD using PubMed, EMBASE, MEDLINE, and Web of Science databases from their inception to December 2022. The pooled global and regional prevalence and incidence density of type 2 diabetes in patients with NAFLD or MAFLD were evaluated using random-effects meta-analysis. Potential sources of heterogeneity were investigated using stratified meta-analysis and meta-regression.
Results
A total of 395 studies (6,878,568 participants with NAFLD; 1,172,637 participants with MAFLD) from 40 countries or areas were included in the meta-analysis. The pooled prevalence of type 2 diabetes among NAFLD or MAFLD patients was 28.3% (95% confidence interval 25.2–31.6%) and 26.2% (23.9–28.6%) globally. The incidence density of type 2 diabetes in NAFLD or MAFLD patients was 24.6 per 1000-person year (20.7 to 29.2) and 26.9 per 1000-person year (7.3 to 44.4), respectively.
Conclusions
The present study describes the global prevalence and incidence of type 2 diabetes in patients with NAFLD or MAFLD. The study findings serve as a valuable resource to assess the global clinical and economic impact of type 2 diabetes in patients with NAFLD or MAFLD.
Similar content being viewed by others
Background
Type 2 diabetes is a worldwide public health burden, which accounts for about 90–95% of diabetes mellitus [1]. Over the past 40 years, the number of adults with diabetes mellitus has quadrupled from 108 million in 1980 to 463 million in 2019 globally [2, 3]. This figure is predicted to be 579 million in 2030 and 700 million in 2045 [2, 4]. Moreover, it is estimated that the amount of direct health care expenditures attributed to type 2 diabetes in 2007 was $232 billion, whereas in 2019, it soared to $760 billion [5]. Thus, it is essential to curb the rise of type 2 diabetes in order to minimize economic expenses and protect individuals and communities from its enduring negative consequences.
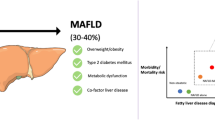
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide, encompassing a spectrum that includes hepatic steatosis, non-alcoholic steatohepatitis (NASH), advanced liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). Currently, one in four people in the world suffers from NAFLD [6]. Eventually, 20 million people will die from NAFLD-related liver disease [7]. In clinical settings, NAFLD has been reported to share similar pathophysiological mechanism (such as insulin resistance, defective hepatic lipidic profile, and abnormal triglyceride metabolism) with type 2 diabetes [8,9,10] and is strongly associated with it [9, 11, 12]. A recent meta-analysis has revealed that NAFLD increases the risk of type 2 diabetes incident by approximately twofold [13]. Therefore, reducing the prevalence of NAFLD could help mitigate the impact of type 2 diabetes to some extent. However, to the best of our knowledge, there are no reports on global prevalence and incidence density of type 2 diabetes among NAFLD patients that can be used to delineate its epidemiological features [13,14,15,16,17].
Furthermore, given that NAFLD is closely related to several metabolic diseases, the term metabolic-associated fatty liver disease (MAFLD) was proposed in 2020 to better describe these coexisting conditions and reflect the etiology of fatty liver diseases [18, 19]. MAFLD is defined based on evidence of hepatic steatosis and is simultaneously accompanied by at least one of the following conditions: obesity, diabetes, or metabolic dysregulation [18]. Of note, these conditions are closely related to type 2 diabetes. However, no research to date has reported on the epidemiological features of type 2 diabetes among MAFLD patients.
Therefore, the present study utilized a comprehensive meta-analysis to report the worldwide incidence and prevalence of type 2 diabetes among individuals with NAFLD or MAFLD.
Methods
Search terms, included and excluded criteria
The present systematic review and meta-analysis were in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines [20]. The study protocol was registered in PROSPERO with an ID number of CRD42022335692 (https://www.crd.york.ac.uk/prospero/).
Two researchers independently conducted a literature search in the PubMed, EMBASE, MEDLINE, and Web of Science databases in the period from their inception to December 2022 using a combination of the following search terms related to type 2 diabetes in NAFLD or MAFLD: "fatty liver" (OR "NAFLD" OR "nonalcoholic fatty liver disease" OR "nonalcoholic steatohepatitis "OR "hepatic steatosis" OR "MAFLD" OR " metabolic associated fatty liver disease") AND "diabetes" NOT "animals"[mesh]. The search included all observational studies conducted in adults (≥ 18 years old) published in peer-reviewed journals until December 2022. In addition to the initial database search, references obtained from the retrieved publications were also searched to identify any additional articles that may have been missed.
The following publications were excluded: reviews or abstracts; studies that were unable to ascertain the NAFLD diagnosis; studies on patients with viral hepatitis B and C (HBV/HCV), or other liver-related disease or excess alcohol consumption; studies with patients under 18 years old or pregnant individuals; studies that did not report screening for excess alcohol use; studies reporting on type 1 diabetes; studies that included only groups with certain metabolic condition, such as morbidly obese patients; and studies not published in the English language. If multiple studies were conducted using the same population, the most recent data were utilized for the present analysis.
Data extraction and analysis
Usable data from eligible studies were independently extracted by two authors (H. L. and J. J.). Any discrepancies in their decisions were resolved through consultation with a third author (L. C.). The following data were extracted from each study: title, author (s), study year, publication year, study location (country and region), study design, NAFLD diagnosis, sample size, average age, percentage of males, and number of participants with NAFLD or MAFLD.
To estimate the pooled prevalence, the prevalence rates were combined in random-effects meta-analyses (normal-normal model) that took into account inter-study heterogeneity. Moreover, meta-analysis was performed using logit transformed proportions to obtain better statistical properties [21]. To address the potential for misestimation due to liver index and hospital records of NAFLD definition, a sensitivity analysis was performed after excluding those studies, based on previous reports [5, 6]. The percentage of males, average age of the sample population, geographic region, diagnostic method, follow-up duration, and publication year were examined both in univariate and multivariate meta-regression models. A funnel plot, Begg-Mazumdar’s rank correlation test, and Egger’s regression test were used to estimate whether there were any publication or related biases. The detailed data analysis was observed in Additional file 1. Supplementary Methods [5, 6, 21,22,23].
In addition, Joinpoint Regression Analysis was used to calculate the average annual percent change (AAPC) and the corresponding 95% CIs in the temporal trend of each part with natural log-transformed rates. All analyses were performed using the Stata version 17.0 software (Stata Corp, College Station, TX, USA), R version 4.0.5, and Joinpoint version 4.7.0.0. Bilateral P < 0.05 was considered as statistically significant.
Furthermore, GRADEpro version 3.6.1 was used to assess evidence quality and grading of recommendation strength in the included studies in the quantitative synthesis—meta-analysis, in accordance to the grading of recommendation, assessment, and evaluation (GRADE) instrument [24]. This assessment was based on the considerations of study design, consistency, directness, heterogeneity, precision, publication bias, and other aspects reported by studies included in this systematic review. The quality of the evidence was rated as high, moderate, low, or very low [25].
Patient and public involvement statement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Results
The electronic search yielded 26,131 articles (Additional file 2: Fig. S1). A total of 20,017 records were retained after removing the duplicates. Furthermore, 18,116 records with ineligible titles and abstracts were excluded based on the aforementioned exclusion criteria, in addition to 1506 records with no available information, not describing observational studies, including teenage subjects, not written in English, without baseline data, and with the same study participants. As a result, 395 reports were included in the final study analysis. Of those, 364 studies focused on the epidemiological research on type 2 diabetes in NAFLD patients, while 42 studies reported on the MAFLD research.
Prevalence of type 2 diabetes among patients with NAFLD or MAFLD
The pooled global prevalence of type 2 diabetes among patients with NAFLD or MAFLD was 28.1% (95% confidence interval [CI] 25.1–31.3%). Specifically, a total of 340 studies including a total 5,563,639 patients with NAFLD were included in the study (Additional file 3: Table S1 [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361,362]). Briefly, the median age of these participants was 50.6 (range 33.1–78.0) years, with 51.2% of NAFLD patients being male (range 9.0–94.3%) (Additional file 3: Table S2). The estimated global prevalence of type 2 diabetes among patients with NAFLD was 28.3% (95% confidence interval [CI] 25.2–31.6%; Fig. 1). The prevalence of type 2 diabetes among patients with NAFLD was dramatically increased from 28.4% (95% CI 24.4–32.7%) in 2002 to 34.1% (95% CI 30.8–37.5%) in 2022 (Additional file 2: Fig. S2), similar to the results of the AAPC, especially for Australia, South America, and Southeast Asia (all P < 0.05) (Additional file 3: Table S3). Across the region, pooled prevalence estimates ranged from 24.1% in East Asia (n = 102; 95% CI 21.3–27.2%; I2 = 100.0%) to 47.1% in Africa (n = 4; 95% CI 20.6–59.7%; I2 = 95.1%). The pooled prevalence of type 2 diabetes in NAFLD patients across countries is listed in Fig. 2. Across diagnosis of NAFLD, the pooled prevalence of type 2 diabetes among patients with NAFLD was the lowest in index (n = 20; 17.4%; 95% CI 11.8–25.1%; I2 = 100.0%) and the highest in hospital records (n = 19; 45.2%; 95% CI 31.2–60.0%; I2 = 100.0%). Furthermore, sensitivity analyses results that excluded studies that using liver index and hospital records as NAFLD definitions, were consistent with the overall study findings (28.2% vs. 28.3%; Additional file 3: Table S4).
For secondary analysis (Additional file 3: Table S5), the pooled prevalence values for type 2 diabetes among patients with NAFLD were 15.7% (n = 19; 95% CI 11.7–20.7%; I2 = 99.8%) and 22.2% (n = 25; 95% CI 17.2–28.0%; I2 = 99.8%) for males and females, respectively. The pooled prevalence of type 2 diabetes among patients with lean-NAFLD was 15.2% (n = 19; 95% CI 10.8–21.0%; I2 = 99.5%), while the prevalence among non-lean NAFLD patients was 25.9% (n = 23; 95% CI 20.3–32.5%; I2 = 99.8%). Moreover, the present study further investigated the prevalence of type 2 diabetes among NAFLD patients with or without Corona Virus Disease 2019 (Covid-19). As shown, the prevalence of type 2 diabetes for those with Covid-19 was 18.7% (n = 2; 95% CI 5.8–46.1%; I2 = 99.2%) and 28.4% (n = 338; 95% CI 25.2–31.7%; I2 = 100.0%) for those without Covid-19.
The univariate meta-regression analysis indicated that study design (P = 0.02), mean age (P < 0.0001), proportion of males (P = 0.03), publication year (P = 0.01), and NAFLD diagnosis (P < 0.0001) were significantly associated with the prevalence of type 2 diabetes (Additional file 3: Table S6). In the multivariate analysis, mean age (P < 0.0001), publication year (P = 0.03), and NAFLD diagnosis (P < 0.0001) remained significantly associated with the prevalence of type 2 diabetes.
In addition, a total of 40 studies, including 1,169,899 patients with MAFLD were evaluated in the analyses (Additional file 3: Table S7 [171, 248, 251, 271, 307,308,309, 313, 335, 349, 363,364,365,366,367,368,369,370,371,372,373,374,375,376,377,378,379,380,381,382,383,384,385,386,387,388,389,390,391]). The median age of the participants was 50.9 (range 35.1–68.4) years, with 53.3% of MAFLD patients being male (range 23.9–85.4%; Additional file 3: Table S8). The pooled global prevalence of type 2 diabetes among patients with MAFLD was 26.2% (95% CI 23.9–28.6%; Additional file 2: Fig. S3). Across the geographic location, the highest pooled prevalence of type 2 diabetes in MAFLD patients was reported in West Asia (n = 1; 81.5%; 95% CI 79.3–83.5%) and the lowest prevalence in East Asia (n = 21; 21.1%; 95% CI 18.6–23.8%; I2 = 99.3%). The pooled prevalence of type 2 diabetes in MAFLD patients across countries is shown in Additional file 2: Fig. S4. Moreover, the results from AAPC analysis indicated that there was an increased trend of prevalence in type 2 diabetes populations with MAFLD (Additional file 3: Table S9). For meta-regression analysis, only geographic region (P < 0.05) was significantly associated with the prevalence rates both in univariate and multivariate meta-regression analyses (Additional file 3: Table S10).
Incidence density of type 2 diabetes among patients with NAFLD or MAFLD
The pooled global incidence density of type 2 diabetes among patients with NAFLD or MAFLD was 24.0 per 1000-person year (95% CI 20.3–28.4). To be specific, a total of 36 studies evaluated in the incidence analysis, included 1,327,087 patients with NAFLD (Additional file 3: Table S11 [43, 82, 174, 203, 221, 233, 262, 274, 285, 349, 392,393,394,395,396,397,398,399,400,401,402,403,404,405,406,407,408,409,410,411,412,413,414,415,416,417]). Briefly, the median age of these participants was 48.3 (range 30.7–69.4) years, and a median follow-up time of 5.05 years. In addition, 61.2% (range 36.0–90.5) of patients with NAFLD were male (Additional file 3: Table S12). The estimated global incidence density of type 2 diabetes among patients with NAFLD was 24.6 per 1000-person year (95% CI 20.7 to 29.2; Fig. 3). There was a substantial decrease in the incidence of type 2 diabetes among patients with NAFLD from 28.3 per 1000-person year in 2003 to 16.1 per 1000-person year in 2022 (Additional file 2: Fig. S5), while no significant trend of AAPC analysis was observed (Additional file 3: Table S13). Across the region, pooled incidence density estimates ranged from 17.0 per 1000-person year (n = 1; 95% CI 16.1 to 18.0) in Australia to 81.6 per 1000-person year (n = 3; 95% CI 57.5 to 114.6; I2 = 99.5%) in North America. The pooled incidence density of type 2 diabetes in NAFLD patients across countries is listed in Fig. 4. Subgroup differences were significant in NAFLD diagnosis, where the pooled incidence density was the highest in liver biopsy (n = 9; 41.9 per 1000-person year; 95% CI 24.5 to 70.6; I2 = 99.9%) and the lowest in liver index (n = 5; 20.8 per 1000-person year; 95% CI 13.0 to 33.2; I2 = 100.0%), except for mixed diagnostic methods (the definition of NAFLD in a study using more than one diagnostic method). In the secondary analysis, the pooled type 2 diabetes incidence density among patients with NAFLD for males was slightly lower than that of females (9.7 vs. 10.5). Additionally, sensitivity analyses results, which excluded studies that used index and hospital records for NAFLD diagnosis, also demonstrated results similar to those observed in the whole population (Additional file 3: Table S14). Only the region (P = 0.04) demonstrated a significant association with the incidence density of type 2 diabetes in the univariate and multivariate analysis (Additional file 3: Table S15).
Moreover, only three studies from East Asia with a total of 5222 patients with MAFLD were included in the incidence analysis (Additional file 3: Table S16 [349, 418, 419]). The median age of these participants was 45.2 (range 40.0–54.9) years, with a median follow-up time of 10 years (Additional file 3: Table S17). Studies on the incidence density of type 2 diabetes among patients with MAFLD were all from China and had a pooled incidence density of 26.9 per 1000-person year (95% CI 7.3 to 44.4; Additional file 3: Table S18).
Level of evidence
Overall, the quality of the evidence for the results of the GRADE system assessment was rated as very low (Additional file 2: Fig. S6). Heterogeneity was the main factor leading to the limited quality of the evidence.
Discussion
The present study determined that the global prevalence of type 2 diabetes among NAFLD patients is 28.3%, with the lowest prevalence rate observed in East Asia. The global incidence density of type 2 diabetes among NAFLD is 24.6 per 1000-person year. The epidemiological features of type 2 diabetes among patients with MAFLD were consistent, showing a pooled prevalence of 26.2% and an incidence density of 26.9 per 1000-person year.
According to the World Health Organization (WHO), the prevalence of diabetes rose to approximately 10% (537 million) in 2019 and is expected to reach 783 million by 2045 [420]. Thus, the prevalence of type 2 diabetes is more than double that of the general population. Consistently, a previous meta-analysis has reported a global prevalence of type 2 diabetes associated with NAFLD of 22.5% for the period of 1989–2015 [6], which was lower than the values in the present study (28.3%). Although several studies have demonstrated that NAFLD is associated with a nearly two-fold increased risk of type 2 diabetes incident, compared to that in non-NAFLD individuals [15, 16], the global incidence density of type 2 diabetes in NAFLD patients remains unclear. A meta-analysis of 33 studies from 2000 to June 2020 has shown a crude incidence of 18.1% among NAFLD patients with type 2 diabetes [17]. This figure was somewhat higher than the value reported in the present findings (14.9%), which might be attributed to the untransformed rate calculation.
Based on the present meta-analysis, the epidemiological features of type 2 diabetes in NAFLD patients are highly heterogenous across geographic regions. Higher prevalence of type 2 diabetes in NAFLD patients was observed in Africa (especially North Africa), South America, and Australia, while lower rates were found in Europe and Asia. As well, results from AAPC indicated that the increased trends of type 2 diabetes among NAFLD were significant in Australia, South America, and Southeast Asia. Consistently with our findings, South America has been reported to have the highest prevalence of NAFLD in 2019, while Europe and Asia showed lower rates [421]. The highest prevalence of age-standardized diabetes worldwide was observed in the Middle East and North Africa (12.2%), and the lowest prevalence of diabetes was found in Europe (6.3%) [422]. Furthermore, higher incidence density of type 2 diabetes among NAFLD populations was noted in North America, while lower incidence was observed in Australia. Regarding to the AAPC, an increased trend of incidence density has been found in East Asia, whereas a downward trend was observed in Europe. With the rapid social and economic development of middle socio-demographic index countries and developing countries in East Asia such as China, great changes have taken place in diet structure and lifestyles. For instance, smoking has been reported to increase the prevalence of NAFLD and advanced liver fibrosis [423]. Soft drinks, the leading source of added sugar worldwide, have been linked to obesity, insulin resistance, and NAFLD [424]. However, basic infrastructure is insufficient to support a healthy lifestyle, and current health services are unable to identify the onset of diabetes in people with NAFLD early and intervene in a timely manner. In addition, population aging accelerates the occurrence of these chronic diseases. According to the WHO, there were 1 billion people worldwide over the age of 60 years in 2019, and this number will rise to 1.4 billion by 2030 and 2.1 billion by 2050 [425]. Both NAFLD and type 2 diabetes are closely associated with aging. The worldwide prevalence of NAFLD has increased from 25.5% in or before 2005 to 37.8% in 2016 or later [426]. Additionally, 135.6 million people aged 65–99 years were living with diabetes in 2019. It is estimated that the number of people over the age of 65 years with diabetes will reach 195.2 million by 2030 and 276.2 million by 2045 [427]. Thus, the increasing disease burden of type 2 diabetes among NAFLD patients is closely related to aging. Intensive measures should be planned and implemented in less developed countries to prevent further increases in the burden of disease and to strengthen health services.
The prevalence and incidence of type 2 diabetes among NAFLD patients also vary depending on the diagnostic methods for NAFLD. Although liver biopsy remains the gold standard for diagnosis of fatty liver, it has limitations due to sampling variability, invasiveness, and high cost [428]. Numerous non-invasive biomarkers, including liver index (serum markers) and imaging modalities, are preferred for practical applications. Consistent with published literature [6], the present study found that liver index can underestimate the prevalence rate of type 2 diabetes, while hospital records may overestimate it. Studies have shown that analyses using liver enzymes (blood tests only) to estimate the prevalence of NAFLD consistently yield lower results than those using imaging studies. In North America, for example, the prevalence of NAFLD diagnosed via ultrasound is 24%, but only 13% if a blood test is used [6]. Additionally, aminotransferase levels may be only mildly elevated in NAFLD individuals, so that up to 78% of patients with NAFLD may actually have normal liver enzyme levels [429]. These findings can explain why publication bias was no longer present when studies using blood tests as the sole means of diagnosing NAFLD were removed in the sensitivity analysis. However, after excluding studies using liver index or hospital records for NAFLD diagnosis, the sensitivity analyses results were still consistent with those of the main analyses, which may be attributed to the lack of studies on liver index and hospital records for NAFLD diagnosis. Clearly, these data also support the importance of selecting the most accurate method to diagnose NAFLD, such as imaging or histology [430].
Results from secondary analysis indicated a significantly higher prevalence of type 2 diabetes in non-lean NAFLD patients, compared to lean NAFLD patients. These findings were consistent with observations in the general population. As reported by a previous systematic review and meta-analysis, a similar phenomenon has been observed in the general population. A study has reported that the overall prevalence of lean NAFLD was 19.2% among the NAFLD population and 5.1% in the general population [431]. Except for the fact that obesity is a relevant risk factor for type 2 diabetes, the observed difference in the prevalence of type 2 diabetes between lean and non-lean NAFLD patients may be related to the differences in genetic variations, as well as in gut microbiota among different ethnic populations. Compared to non-lean NAFLD individuals, lean NAFLD individuals showed better metabolic and histological characteristics. In addition, a previous microbiome study has also indicated a significant difference in fecal and blood microbiota profiles between lean and non-lean NAFLD patients [432]. Additionally, the rate of type 2 diabetes in NAFLD populations has also suggested a sex difference, with the condition being more common in females than males. However, it is worth noting that the sex differences in the prevalence of type 2 diabetes and NAFLD are often thought to depend on the patients’ reproductive stage of life [433, 434]. Specifically, there are more cases of type 2 diabetes or NAFLD in males before the age of puberty, whereas postmenopausal females have a higher prevalence of type 2 diabetes or NAFLD. Studies on the sex subgroup populations are needed to better understand these differences. In addition, published evidence has reported that there were several direct links (e.g., insulin resistance, immune injury) between Covid-19 and the metabolic and endocrine systems. Not only are patients with metabolic dysfunction, such as NAFLD and diabetes, at an increased risk of developing severe Covid-19 but also infection with SARS-CoV-2 might lead to new-onset diabetes or aggravation of pre-existing metabolic disorders [10, 435]. Moreover, only two studies included adults Covid-19 patients from China and Mexico have reported that a much higher prevalence of NAFLD was observed in Covid patients than that reported in the general population (25%). Of which, these patients also had significantly higher proportions of diabetes than the non-NAFLD cases [302, 353]. On the contrary, research from the UK Biobank has revealed no causal relationship between NAFLD and Covid-19 using Mendelian Randomization Analysis [436]. Since the study samples we included were not comprehensive and representative enough, we found the pooled prevalence of type 2 diabetes in NAFLD individuals was lower among Covid-19 patients than in non-Covid-19 patients. Further studies were still warranted to explore the impact of Covid-19 on metabolic disorders.
According to the present results, the prevalence of type 2 diabetes in MAFLD patients was lower than that in NAFLD patients, with the highest prevalence in Europe and the lowest prevalence in Asia. In fact, observational data meta-analysis has reported that Europe has the highest pooled prevalence of MAFLD, followed by Asia and North America [437]. It is worth noting that there are slight differences in prevalence between NAFLD and MAFLD in published epidemiological studies, where the prevalence for MAFLD is often higher than that for NAFLD [437]. Evidence from the 2017–2018 National Health and Nutrition Examination Survey, which is a cohort study conducted in a representative sample of the general US population, has shown that the prevalence values for NAFLD and MAFLD based on imaging diagnose were 37.1 and 39.1%, respectively [438]. Since there is limited evidence regarding the prevalence of type 2 diabetes in MAFLD, further studies are still needed. Moreover, research related to the incidence density of type 2 diabetes among MAFLD patients has only been published in China. Thus, the incidence results are not directly comparable to the global values. Given the fact that the MAFLD criteria are more practical and have higher ability for identifying at-high-risk patients than that of the NAFLD criteria [251], more studies from diverse geographic locations are necessary to obtain a more comprehensive understanding of the incidence of type 2 diabetes in MAFLD patients worldwide.
A key strength of the present analysis is that it first presents a comprehensive overview of the global epidemiological features of type 2 diabetes among NAFLD and MAFLD patients. This work provides essential data to develop policies to address this rising health concern. However, several limitations warrant consideration. First, significant heterogeneity was present in the included studies, which contribute to a low evaluation of evidence quality by GRADE system. However, the quality of initial evidence of observational studies is low. Unexplored factors, such as comorbid conditions of NAFLD or MAFLD, may have contributed to the heterogeneity. Second, some variations arising from different diagnostic methodologies should be acknowledged. The use of liver enzymes to estimate the prevalence of NAFLD may underestimate the true prevalence of NAFLD, compared to liver biopsy and imaging modalities [6]. Another limitation is the scarcity of data on the incidence of type 2 diabetes among MAFLD patients from numerous countries, preventing a complete global overview. Moreover, given that we mainly focused on studies about NAFLD, those with alcohol drinking were excluded. This could underestimate the epidemiological rate of MAFLD. In addition, studies we included in the present study might not represent the geographical areas to which they belong, further national and representative studies are still warranted.
Conclusions
The present meta-analysis results are important for primary care physicians, specialists, and health policy makers, given the increasing burden of type 2 diabetes among patients with NAFLD and/or MAFLD over the last few years. With more than one-fifth of NAFLD or MAFLD adults affected by type 2 diabetes globally, it is crucial to focus on the development and complications of NAFLD and implement strategies to mitigate its impact.
Availability of data and materials
The study-specific summary data included in the meta-analysis can be obtained from the corresponding authors at yxia@cmu.edu.cn.
Abbreviations
- AAPC:
-
Average annual percent change
- CI:
-
Confidence interval
- Covid-19:
-
Corona Virus Disease 2019
- HCC:
-
Hepatocellular carcinoma
- MAFLD:
-
Metabolic-associated fatty liver disease
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- WHO:
-
World Health Organization
References
Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet (London, England). 2022;400(10365):1803–20.
IDF diabetes Atlas. 9th edn. Brussels, Belgium: International Diabetes Federation: International Diabetes Federation; 2019 Available from: https://www.diabetesatlas.org.
Tinajero MG, Malik VS. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol Metab Clin North Am. 2021;50(3):337–55.
Ogurtsova K, Guariguata L, Barengo NC, Ruiz PL-D, Sacre JW, Karuranga S, et al. IDF diabetes Atlas Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. 2022;183:109118.
Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, MD). 2016;64(1):73–84.
Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology (Baltimore, MD). 2016;64(1):19–22.
Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, et al. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J Diabetes Res. 2020;2020:3920196.
Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18(9):599–612.
Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022;10(4):284–96.
Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70(3):531–44.
Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology (Baltimore, MD). 2012;56(3):943–51.
Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43(8):617–49.
Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care. 2018;41(2):372–82.
Morrison AE, Zaccardi F, Khunti K, Davies MJ. Causality between non-alcoholic fatty liver disease and risk of cardiovascular disease and type 2 diabetes: A meta-analysis with bias analysis. Liver Int. 2019;39(3):557–67.
Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31(5):936–44.
Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2021;70(5):962–9.
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73(1):202–9.
Nan Y, An J, Bao J, Chen H, Chen Y, Ding H, et al. The Chinese Society of Hepatology position statement on the redefinition of fatty liver disease. J Hepatol. 2021;75(2):454–61.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals Int Med. 2009;151(4):264–9.
Sutton AJ, Song F, Gilbody SM, Abrams KR. Modelling publication bias in meta-analysis: a review. Stat Methods Med Res. 2000;9(5):421–45.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Hu J, Dong Y, Chen X, Liu Y, Ma D, Liu X, et al. Prevalence of suicide attempts among Chinese adolescents: A meta-analysis of cross-sectional studies. Compr Psychiatry. 2015;61:78–89.
Higgins J, Green SR. Cochrane Handbook for Systematic Review of Interventions. 2011. Version 5.1.0. http://handbook-5-1.cochrane.org.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6.
Shimada M, Hashimoto E, Kaneda H, Noguchi S, Hayashi N. Nonalcoholic steatohepatitis: risk factors for liver fibrosis. Hepatol Res. 2002;24(4):429–38.
Shi YW, Wang QY, Zhao XY, Sun YM, Kong YY, Ou XJ, et al. Non-obese patients with nonalcoholic fatty liver disease may use a lower liver stiffness cut-off to assess fibrosis stages. J Dig Dis. 2020;21(5):279–86.
Kichian K, McLean R, Gramlich LM, Bailey RJ, Bain VG. Nonalcoholic fatty liver disease in patients investigated for elevated liver enzymes. Can J Gastroenterol. 2003;17(1):38–42.
Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clinical Gastroenterol Hepatol. 2004;2(3):262–5.
Han E, Kim MK, Im SS, Jang BK, Kim HS. Non-alcoholic fatty liver disease and sarcopenia is associated with the risk of albuminuria independent of insulin resistance, and obesity. J Diabetes Complications. 2022;36(8):108253.
Bahcecioglu IH, Koruk M, Yilmaz O, Bolukbas C, Bolukbas F, Tuncer I, et al. Demographic and clinicopathological characteristics of nonalcoholic fatty liver disease in the East-Southeastern Anatolia regions in Turkey. Med Princ Pract. 2006;15(1):62–8.
Nonomura A, Enomoto Y, Takeda M, Tamura T, Kasai T, Yoshikawa T, et al. Clinical and pathological features of non-alcoholic steatohepatitis. Hepatol Res. 2005;33(2):116–21.
Ong JP, Elariny H, Collantes R, Younoszai A, Chandhoke V, Reines HD, et al. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg. 2005;15(3):310–5.
Singh H, Pollock R, Uhanova J, Kryger M, Hawkins K, Minuk GY. Symptoms of obstructive sleep apnea in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2005;50(12):2338–43.
VoPham T, Kim NJ, Berry K, Mendoza JA, Kaufman JD, Ioannou GN. PM(2.5) air pollution exposure and nonalcoholic fatty liver disease in the Nationwide Inpatient Sample. Environ Res. 2022;213:113611.
Thomas CE, Yu YC, Luu HN, Wang R, Paragomi P, Behari J, et al. Neutrophil-lymphocyte ratio in relation to risk of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. Cancer Med. 2023;12(3):3589–600.
Lima ML, Mourão SC, Diniz MT, Leite VH. Hepatic histopathology of patients with morbid obesity submitted to gastric bypass. Obes Surg. 2005;15(5):661–9.
Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology (Baltimore, MD). 2005;41(2):372–9.
Arun J, Clements RH, Lazenby AJ, Leeth RR, Abrams GA. The prevalence of nonalcoholic steatohepatitis is greater in morbidly obese men compared to women. Obes Surg. 2006;16(10):1351–8.
Wong VW, Hui AY, Tsang SW, Chan JL, Tse AM, Chan KF, et al. Metabolic and adipokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006;4(9):1154–61.
Wong VW, Hui AY, Tsang SW, Chan JL, Wong GL, Chan AW, et al. Prevalence of undiagnosed diabetes and postchallenge hyperglycaemia in Chinese patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2006;24(8):1215–22.
Riley TR 3rd, Kahn A. Risk factors and ultrasound can predict chronic hepatitis caused by nonalcoholic fatty liver disease. Dig Dis Sci. 2006;51(1):41–4.
Fan JG, Li F, Cai XB, Peng YD, Ao QH, Gao Y. Effects of nonalcoholic fatty liver disease on the development of metabolic disorders. J Gastroenterol Hepatol. 2007;22(7):1086–91.
Khurram M, Ashraf MM. A clinical and biochemical profile of biopsy-proven non-alcoholic Fatty liver disease subjects. J Coll Physicians Surg Pak JCPSP. 2007;17(9):531–4.
Zhang L, Pu K, Chai Z, Dixon JB. Family history of coronary heart disease is associated with a higher incidence of nonalcoholic fatty liver disease Central obesity the likely link. Obes Res Clin Pract. 2007;1(2):I–ii.
Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57(10):1441–7.
De Lusong MA, Labio E, Daez L, Gloria V. Non-alcoholic fatty liver disease in the Philippines: comparable with other nations? World J Gastroenterol. 2008;14(6):913–7.
Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(11):1224–9 (1229.e1221-1222).
Bjørnå ER, Engelsen MT, El-Serag HB, Ness-Jensen E. Prevalence and risk factors of nonalcoholic fatty liver disease in a general population, the HUNT study. Scand J Gastroenterol. 2023;58(5):505–11.
Kashyap SR, Diab DL, Baker AR, Yerian L, Bajaj H, Gray-McGuire C, et al. Triglyceride levels and not adipokine concentrations are closely related to severity of nonalcoholic fatty liver disease in an obesity surgery cohort. Obesity (Silver Spring, Md). 2009;17(9):1696–701.
Sung KC, Ryan MC, Wilson AM. The severity of nonalcoholic fatty liver disease is associated with increased cardiovascular risk in a large cohort of non-obese Asian subjects. Atherosclerosis. 2009;203(2):581–6.
Frith J, Day CP, Henderson E, Burt AD, Newton JL. Non-alcoholic fatty liver disease in older people. Gerontology. 2009;55(6):607–13.
Poster exhibition. Hepatology international. 2009;3(1):86–220. PMID: 19669375.
Abu-Freha N, Cohen B, Weissmann S, Hizkiya R, Abu-Hammad R, Taha G, et al. Comorbidities and Outcomes among Females with Non-Alcoholic Fatty Liver Disease Compared to Males. Biomedicines. 2022;10(11):2908.
Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7(2):234–8.
Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology (Baltimore, MD). 2010;52(3):913–24.
Doycheva I, Zhang T, Amjad W, Thuluvath PJ. Diabetes and Hepatocellular Carcinoma: Incidence Trends and Impact of Liver Disease Etiology. J Clin Exp Hepatol. 2020;10(4):296–303.
Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503–8.
McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265–9.
Gokulakrishnan K, Anjana RM, Indulekha K, Anuradha S, Mohan V. Association of hypoadiponectinemia with non-alcoholic fatty liver disease in urban south Indians–(CURES - 81). Indian J Med Res. 2010;132:271–7.
Liu CC, Hung CL, Shih SC, Ko HJ, Lu YT, Wu YJ, et al. Age-related Differences in the Clinical Presentation, Associated Metabolic Abnormality, and Estimated Cardiovascular Risks from Nonalcoholic Fatty Liver Disease: A Cross-sectional Study from Health Evaluation Center in Taiwan. Int J Gerontol. 2010;4(4):184–91.
Manchanayake J, Chitturi S, Nolan C, Farrell GC. Postprandial hyperinsulinemia is universal in non-diabetic patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2011;26(3):510–6.
Bianchi G, Bertaccini G, et al. 337 NON ALCOHOLIC FATTY LIVER DISEASE AND AGING. PREVALENCE AND ASSOCIATED FACTORS. J Hepatol. 2010;52:S141. https://search.ebscohost.com/login.aspx?direct=true&db=edsbl&AN=RN268932148&lang=zh-cn&site=eds-live.
Shams ME, Al-Gayyar MM, Barakat EA. Type 2 Diabetes Mellitus-Induced Hyperglycemia in patients with NAFLD and normal LFTs: Relationship to Lipid Profile, Oxidative Stress and Pro-Inflammatory Cytokines. Sci Pharm. 2011;79(3):623–34.
Touzin NT, Bush KN, Williams CD, Harrison SA. Prevalence of colonic adenomas in patients with nonalcoholic fatty liver disease. Ther Adv Gastroenterol. 2011;4(3):169–76.
Uslusoy HS, Nak SG, Gülten M. Noninvasive predictors for liver fibrosis in patients with nonalcoholic steatohepatitis. World J Hepatol. 2011;3(8):219–27.
Nakamura A, Yoneda M, Fujita K, Tajima K, Kikuchi K, Nakajima A, et al. Impact of glucose tolerance on the severity of non-alcoholic steatohepatitis. J Diabetes Investig. 2011;2(6):483–9.
Valenti L, Nobili V, Al-Serri A, Rametta R, Leathart JB, Zappa MA, et al. The APOC3 T-455C and C-482T promoter region polymorphisms are not associated with the severity of liver damage independently of PNPLA3 I148M genotype in patients with nonalcoholic fatty liver. J Hepatol. 2011;55(6):1409–14.
Loosen SH, Roderburg C, Demir M, Qvartskhava N, Keitel V, Kostev K, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with an increased incidence of osteoporosis and bone fractures. Z Gastroenterol. 2022;60(8):1221–7.
Machida T, Obara T, Miyazaki M, Inoue J, Mano N. Trends in drug prescriptions for type 2 diabetes, hypertension, and dyslipidemia among adults with non-alcoholic fatty liver disease. Ann Hepatol. 2022;27(4):100699.
Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology (Baltimore, MD). 2012;56(2):605–13.
Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–31.
Cotrim HP, Parise ER, Oliveira CP, Leite N, Martinelli A, Galizzi J, et al. Nonalcoholic fatty liver disease in Brazil Clinical and histological profile. Annals Hepatol. 2011;10(1):33–7.
Oladunjoye O, Oladunjoye AO, Dhital R, Poudel D, Oladiran OD, Oke IO, et al. A Retrospective Study of Hospitalizations in the USA: Proportion of Hospitalizations With Non-Alcoholic Fatty Liver Disease in Non-Obese Population. Cureus. 2021;13(9):e17869.
Tabibian JH, Lazo M, Durazo FA, Yeh HC, Tong MJ, Clark JM. Nonalcoholic fatty liver disease across ethno-racial groups: do Asian-American adults represent a new at-risk population? J Gastroenterol Hepatol. 2011;26(3):501–9.
Zein CO, Unalp A, Colvin R, Liu YC, McCullough AJ. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54(4):753–9.
Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, et al. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med. 2011;9:85.
Chandok N, Minuk G, Wengiel M, Uhanova J. Serum ferritin levels do not predict the stage of underlying non-alcoholic fatty liver disease. J Gastrointestin Liver Dis. 2012;21(1):53–8.
Sun L, Lü SZ. Association between non-alcoholic fatty liver disease and coronary artery disease severity. Chin Med J. 2011;124(6):867–72.
He S, Bao W, Shao M, Wang W, Wang C, Sun J, et al. Risk factors for non-alcoholic fatty liver disease in a Chinese population. Acta Gastro-Enterol Belg. 2011;74(4):503–8.
Francque S, De Maeght S, Adler M, Deltenre P, de Galocsy C, Orlent H, et al. High prevalence of advanced fibrosis in association with the metabolic syndrome in a Belgian prospective cohort of NAFLD patients with elevated ALT Results of the Belgian NAFLD registry. Acta Gastro-enterol Belg. 2011;74(1):9–16.
Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107(2):253–61.
Vuppalanchi R, Gould RJ, Wilson LA, Unalp-Arida A, Cummings OW, Chalasani N, et al. Clinical significance of serum autoantibodies in patients with NAFLD: results from the nonalcoholic steatohepatitis clinical research network. Hep Intl. 2012;6(1):379–85.
Bambha K, Belt P, Abraham M, Wilson LA, Pabst M, Ferrell L, et al. Ethnicity and nonalcoholic fatty liver disease. Hepatology (Baltimore, MD). 2012;55(3):769–80.
Zhang X, Wong VW, Yip TC, Tse YK, Liang LY, Hui VW, et al. Colonoscopy and risk of colorectal cancer in patients with nonalcoholic fatty liver disease: a retrospective territory-wide cohort study. Hepatol Commun. 2021;5(7):1212–23.
Swellam M, Hamdy N. Association of nonalcoholic fatty liver disease with a single nucleotide polymorphism on the gene encoding leptin receptor. IUBMB Life. 2012;64(2):180–6.
Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine. 2012;91(6):319–27.
Valantinas J, Apanaviciene DA, Maroziene L, Sveikata A. The prevalence of metabolic risk factors among outpatients with diagnosed nonalcoholic fatty liver disease in Lithuania. Med Sci Monit. 2012;18(5):Ph57-62.
Gupta AC, Misra R, Sakhuja P, Singh Y, Basir SF, Sarin SK. Association of adiponectin gene functional polymorphisms (-11377C/G and +45T/G) with nonalcoholic fatty liver disease. Gene. 2012;496(1):63–7.
Fracanzani AL, Valenti L, Russello M, Miele L, Bertelli C, Bellia A, et al. Gallstone disease is associated with more severe liver damage in patients with non-alcoholic fatty liver disease. PLoS ONE. 2012;7(7):e41183.
Hatziagelaki E, Karageorgopoulos DE, Chounta A, Tsiavou A, Falagas ME, Dimitriadis G. Predictors of impaired glucose regulation in patients with non-alcoholic fatty liver disease. Exp Diabetes Res. 2012;2012:351974.
Shi L, Liu ZW, Li Y, Gong C, Zhang H, Song LJ, et al. The prevalence of nonalcoholic fatty liver disease and its association with lifestyle/dietary habits among university faculty and staff in Chengdu. Biomed Environ Sci BES. 2012;25(4):383–91.
Al-hamoudi W, El-Sabbah M, Ali S, Altuwaijri M, Bedewi M, Adam M, et al. Epidemiological, clinical, and biochemical characteristics of Saudi patients with nonalcoholic fatty liver disease: a hospital-based study. Ann Saudi Med. 2012;32(3):288–92.
Goh SC, Ho EL, Goh KL. Prevalence and risk factors of non-alcoholic fatty liver disease in a multiracial suburban Asian population in Malaysia. Hep Intl. 2013;7(2):548–54.
Kumar R, Rastogi A, Sharma MK, Bhatia V, Garg H, Bihari C, et al. Clinicopathological characteristics and metabolic profiles of non-alcoholic fatty liver disease in Indian patients with normal body mass index: Do they differ from obese or overweight non-alcoholic fatty liver disease? Indian J Endocrinol Metab. 2013;17(4):665–71.
Patel NS, Peterson MR, Lin GY, Feldstein A, Schnabl B, Bettencourt R, et al. Insulin Resistance Increases MRI-Estimated Pancreatic Fat in Nonalcoholic Fatty Liver Disease and Normal Controls. Gastroenterol Res Pract. 2013;2013:498296.
Abrams GA, Kunde SS, Lazenby AJ, Clements RH. Portal fibrosis and hepatic steatosis in morbidly obese subjects: A spectrum of nonalcoholic fatty liver disease. Hepatol (Baltimore, MD). 2004;40(2):475–83.
Ha Y, Hwang SG, Rim KS. The Association between Vitamin D Insufficiency and Nonalcoholic Fatty Liver Disease: A Population-Based Study. Nutrients. 2017;9(8):806.
Reddy SK, Hyder O, Marsh JW, Sotiropoulos GC, Paul A, Alexandrescu S, et al. Prevalence of nonalcoholic steatohepatitis among patients with resectable intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2013;17(4):748–55.
Hashiba M, Ono M, Hyogo H, Ikeda Y, Masuda K, Yoshioka R, et al. Glycemic variability is an independent predictive factor for development of hepatic fibrosis in nonalcoholic fatty liver disease. PLoS ONE. 2013;8(11):e76161.
Bakhshipour A, Kaykhaei MA, Moulaei N, Mashhadi MA. Prevalence of coeliac disease in patients with non-alcoholic fatty liver disease. Arab J Gastroenterol. 2013;14(3):113–5.
Ghamar Chehreh ME, Vahedi M, Pourhoseingholi MA, Ashtari S, Khedmat H, Amin M, et al. Estimation of diagnosis and treatment costs of non-alcoholic Fatty liver disease: a two-year observation. Hepat Mon. 2013;13(5):e7382.
Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, et al. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD). Dig Dis Sci. 2013;58(10):3017–23.
Cheah WL, Lee PY, Chang CT, Mohamed HJ, Wong SL. Prevalence of ultrasound diagnosed nonalcoholic fatty liver disease among rural indigenous community of Sarawak and its association with biochemical and anthropometric measures. Southeast Asian J Trop Med Public Health. 2013;44(2):309–17.
Zelber-Sagi S, Lotan R, Shibolet O, Webb M, Buch A, Nitzan-Kaluski D, et al. Non-alcoholic fatty liver disease independently predicts prediabetes during a 7-year prospective follow-up. Liver Int. 2013;33(9):1406–12.
Chang Y, Jung HS, Yun KE, Cho J, Cho YK, Ryu S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol. 2013;108(12):1861–8.
Nakahara T, Hyogo H, Yoneda M, Sumida Y, Eguchi Y, Fujii H, et al. Type 2 diabetes mellitus is associated with the fibrosis severity in patients with nonalcoholic fatty liver disease in a large retrospective cohort of Japanese patients. J Gastroenterol. 2014;49(11):1477–84.
Hyogo H, Yamagishi S, Maeda S, Fukami K, Ueda S, Okuda S, et al. Serum asymmetric dimethylarginine levels are independently associated with procollagen III N-terminal peptide in nonalcoholic fatty liver disease patients. Clin Exp Med. 2014;14(1):45–51.
Angulo P, George J, Day CP, Vanni E, Russell L, De la Cruz AC, et al. Serum ferritin levels lack diagnostic accuracy for liver fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12(7):1163-1169.e1161.
Loomba R, Wong R, Fraysse J, Shreay S, Li S, Harrison S, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Aliment Pharmacol Ther. 2020;51(11):1149–59.
Li Y, Liu L, Wang B, Wang J, Chen D. Hematocrit is associated with fibrosis in patients with nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2014;26(3):332–8.
Pulixi EA, Tobaldini E, Battezzati PM, D’Ingianna P, Borroni V, Fracanzani AL, et al. Risk of obstructive sleep apnea with daytime sleepiness is associated with liver damage in non-morbidly obese patients with nonalcoholic fatty liver disease. PLoS ONE. 2014;9(4):e96349.
Del Ben M, Polimeni L, Baratta F, Bartimoccia S, Carnevale R, Loffredo L, et al. Serum Cytokeratin-18 Is Associated with NOX2-Generated Oxidative stress in patients with Nonalcoholic Fatty Liver. Int J Hepatol. 2014;2014:784985.
Ybarra J, Fernández S, Sánchez-Hernández J, Romeo JH, Ballesta-Lopez C, Guell J, et al. Serum alanine aminotransferase predicts interventricular septum thickness and left ventricular mass in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2014;26(6):654–60.
Catanzaro R, Calabrese F, Occhipinti S, Anzalone MG, Italia A, Milazzo M, et al. Nonalcoholic fatty liver disease increases risk for gastroesophageal reflux symptoms. Dig Dis Sci. 2014;59(8):1939–45.
Pemmasani G, Yandrapalli S, Aronow W. Sex differences in cardiovascular diseases and associated risk factors in non-alcoholic steatohepatitis. Am J Cardiovasc Dis. 2020;10(4):362–6.
Liu YL, Patman GL, Leathart JB, Piguet AC, Burt AD, Dufour JF, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61(1):75–81.
Rakha EA, Adamson L, Bell E, Neal K, Ryder SD, Kaye PV, et al. Portal inflammation is associated with advanced histological changes in alcoholic and non-alcoholic fatty liver disease. J Clin Pathol. 2010;63(9):790–5.
Gianotti G, Cenni A, Bianchi G, Masetti M, Zappoli P, Muscari A, et al. Diastolic dysfunction and cardiovascular risk in old subjects: possible association with NAFLD? Arch Gerontol Geriatr. 2014;58(2):188–95.
Feng RN, Du SS, Wang C, Li YC, Liu LY, Guo FC, et al. Lean-non-alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World J Gastroenterol. 2014;20(47):17932–40.
Andrade GC, Fujise LH, Santana JEF, Oliveira F, Silva Rde C. Non-alcoholic fatty liver disease (NAFLD) in different populations A clinical and epidemiological study sample of São José do Rio Preto. Rev Assoc Med Bras (1992). 2016;62(3):218–26.
Cavalcante LN, Stefano JT, Machado MV, Mazo DF, Rabelo F, Sandes KA, et al. Genetic ancestry analysis in non-alcoholic fatty liver disease patients from Brazil and Portugal. World J Hepatol. 2015;7(10):1433–8.
Shil BC, Saha M, Ahmed F, Dhar SC. Nonalcoholic Fatty Liver Disease: Study of Demographic and Predictive Factors. Euroasian J Hepato-gastroenterol. 2015;5(1):4–6.
Hung CS, Tseng PH, Tu CH, Chen CC, Liao WC, Lee YC, et al. Nonalcoholic Fatty liver disease is associated With QT prolongation in the general population. J Am Heart Assoc. 2015;4(7):e001820.
Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent Association Between Improvement of Nonalcoholic Fatty Liver Disease and Reduced Incidence of Type 2 Diabetes. Diabetes Care. 2015;38(9):1673–9.
Chang CC, Wu CL, Su WW, Shih KL, Tarng DC, Chou CT, et al. Interferon gamma-induced protein 10 is associated with insulin resistance and incident diabetes in patients with nonalcoholic fatty liver disease. Sci Rep. 2015;5:10096.
Long MT, Wang N, Larson MG, Mitchell GF, Palmisano J, Vasan RS, et al. Nonalcoholic fatty liver disease and vascular function: cross-sectional analysis in the Framingham heart study. Arterioscler Thromb Vasc Biol. 2015;35(5):1284–91.
Kwak MS, Kim D, Chung GE, Kim W, Kim YJ, Yoon JH. Cholecystectomy is independently associated with nonalcoholic fatty liver disease in an Asian population. World J Gastroenterol. 2015;21(20):6287–95.
Praveenraj P, Gomes RM, Kumar S, Karthikeyan P, Shankar A, Parthasarathi R, et al. Prevalence and Predictors of Non-Alcoholic Fatty Liver Disease in Morbidly Obese South Indian Patients Undergoing Bariatric Surgery. Obes Surg. 2015;25(11):2078–87.
Käräjämäki AJ, Pätsi OP, Savolainen M, Kesäniemi YA, Huikuri H, Ukkola O. Non-alcoholic fatty liver disease as a predictor of atrial fibrillation in middle-aged population (OPERA Study). PLoS ONE. 2015;10(11):e0142937.
Abdel-Razik A, Mousa N, Shabana W, Yassen AH, Abdelsalam M, Wahba MM, et al. De novo Portal Vein Thrombosis in Non-Cirrhotic Non-Alcoholic Fatty Liver Disease: A 9-Year Prospective Cohort Study. Front Med. 2021;8:650818.
Idilman IS, Tuzun A, Savas B, Elhan AH, Celik A, Idilman R, et al. Quantification of liver, pancreas, kidney, and vertebral body MRI-PDFF in non-alcoholic fatty liver disease. Abdom Imaging. 2015;40(6):1512–9.
Du T, Yu X, Yuan G, Zhang J, Sun X. Combined influence of nonalcoholic fatty liver and body size phenotypes on diabetes risk. Cardiovasc Diabetol. 2015;14:144.
Tateishi R, Okanoue T, Fujiwara N, Okita K, Kiyosawa K, Omata M, et al. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort study. J Gastroenterol. 2015;50(3):350–60.
Huang JF, Yeh ML, Yu ML, Huang CF, Dai CY, Hsieh MY, et al. Hyperuricemia inversely correlates with disease severity in Taiwanese nonalcoholic steatohepatitis patients. PLoS ONE. 2015;10(10):e0139796.
Kumar MS, Singh A, Jaryal AK, Ranjan P, Deepak KK, Sharma S, et al. Cardiovascular Autonomic Dysfunction in Patients of Nonalcoholic Fatty Liver Disease. Int J Hepatol. 2016;2016:5160754.
Ballestri S, Nascimbeni F, Romagnoli D, Lonardo A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features. Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol Res. 2016;46(11):1074–87.
Pelusi S, Petta S, Rosso C, Borroni V, Fracanzani AL, Dongiovanni P, et al. Renin-Angiotensin System Inhibitors, Type 2 Diabetes and Fibrosis Progression: An Observational Study in Patients with Nonalcoholic Fatty Liver Disease. PLoS ONE. 2016;11(9):e0163069.
Wu KT, Kuo PL, Su SB, Chen YY, Yeh ML, Huang CI, et al. Nonalcoholic fatty liver disease severity is associated with the ratios of total cholesterol and triglycerides to high-density lipoprotein cholesterol. J Clin Lipidol. 2016;10(2):420-425.e421.
Tapper EB, Lai M. Weight loss results in significant improvements in quality of life for patients with nonalcoholic fatty liver disease: A prospective cohort study. Hepatol (Baltimore, MD). 2016;63(4):1184–9.
Uhanova J, Minuk G, Lopez Ficher F, Chandok N. Nonalcoholic Fatty Liver Disease in Canadian First Nations and Non-First Nations Patients. Can J Gastroenterol Hepatol. 2016;2016:6420408.
Goh GB, Issa D, Lopez R, Dasarathy S, Dasarathy J, Sargent R, et al. The development of a non-invasive model to predict the presence of non-alcoholic steatohepatitis in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31(5):995–1000.
Petrović G, Bjelaković G, Benedeto-Stojanov D, Nagorni A, Brzački V, Marković-Živković B. Obesity and metabolic syndrome as risk factors for the development of non-alcoholic fatty liver disease as diagnosed by ultrasound. Vojnosanit Pregl. 2016;73(10):910–20.
Hagström H, Nasr P, Ekstedt M, Kechagias S, Önnerhag K, Nilsson E, et al. Low to moderate lifetime alcohol consumption is associated with less advanced stages of fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2017;52(2):159–65.
Conti F, Vukotic R, Foschi FG, Domenicali M, Giacomoni P, Savini S, et al. Transient elastography in healthy subjects and factors influencing liver stiffness in non-alcoholic fatty liver disease: An Italian community-based population study. Dig Liver Dis. 2016;48(11):1357–63.
Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66(1):123–31.
Fracanzani AL, Pisano G, Consonni D, Tiraboschi S, Baragetti A, Bertelli C, et al. Epicardial Adipose Tissue (EAT) thickness is associated with cardiovascular and liver damage in Nonalcoholic fatty liver disease. PLoS ONE. 2016;11(9):e0162473.
Treeprasertsuk S, Piyachaturawat P, Soontornmanokul T, Komolmit P, Wisedopas-Klaikaew N, Tangkijavanich P. Accuracy of noninvasive scoring systems to assess advanced liver fibrosis in Thai patients with nonalcoholic fatty liver disease. Asian Biomed. 2016;10:S49–55-S55.
Wong VW, Wong GL, Yeung JC, Fung CY, Chan JK, Chang ZH, et al. Long-term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: A prospective cohort study. Hepatol (Baltimore, MD). 2016;63(3):754–63.
Dichtel LE, Corey KE, Misdraji J, Bredella MA, Schorr M, Osganian SA, et al. The Association Between IGF-1 Levels and the Histologic Severity of Nonalcoholic Fatty Liver Disease. Clin Transl Gastroenterol. 2017;8(1): e217.
Toshikuni N, Tsuchishima M, Fukumura A, Arisawa T, Tsutsumi M. Associations of fatty liver disease with Hypertension, Diabetes, and Dyslipidemia: comparison between alcoholic and nonalcoholic Steatohepatitis. Gastroenterol Res Pract. 2017;2017:9127847.
Chan WK, Treeprasertsuk S, Imajo K, Nakajima A, Seki Y, Kasama K, et al. Clinical features and treatment of nonalcoholic fatty liver disease across the Asia Pacific region-the GO ASIA initiative. Aliment Pharmacol Ther. 2018;47(6):816–25.
van den Berg EH, Gruppen EG, James RW, Bakker SJL, Dullaart RPF. Serum paraoxonase 1 activity is paradoxically maintained in nonalcoholic fatty liver disease despite low HDL cholesterol. J Lipid Res. 2019;60(1):168–75.
Wen X, Wang S, Taveira TH, Akhlaghi F. Required warfarin dose and time in therapeutic range in patients with diagnosed Nonalcoholic Fatty Liver Disease (NAFLD) or Nonalcoholic Steatohepatitis (NASH). PLoS ONE. 2021;16(9):e0251665.
Lee HW, Kim BK, Kim SU, Park JY, Kim DY, Ahn SH, et al. Prevalence and predictors of significant fibrosis among Subjects with Transient Elastography-Defined Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2017;62(8):2150–8.
Petta S, Eslam M, Valenti L, Bugianesi E, Barbara M, Cammà C, et al. Metabolic syndrome and severity of fibrosis in nonalcoholic fatty liver disease: An age-dependent risk profiling study. Liver Int. 2017;37(9):1389–96.
Singh A, Kumar MS, Jaryal AK, Ranjan P, Deepak KK, Sharma S, et al. Diabetic status and grade of nonalcoholic fatty liver disease are associated with lower baroreceptor sensitivity in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2017;29(8):956–61.
Weinstein G, Zelber-Sagi S, Preis SR, Beiser AS, DeCarli C, Speliotes EK, et al. Association of Nonalcoholic Fatty Liver Disease With Lower Brain Volume in Healthy Middle-aged Adults in the Framingham Study. JAMA Neurol. 2018;75(1):97–104.
Morio R, Hyogo H, Hatooka M, Morio K, Kan H, Kobayashi T, et al. The risk of transient postprandial oxyhypoglycemia in nonalcoholic fatty liver disease. J Gastroenterol. 2017;52(2):253–62.
Patel YA, Gifford EJ, Glass LM, McNeil R, Turner MJ, Han B, et al. Risk factors for biopsy-proven advanced non-alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268–78.
Dallio M, Masarone M, Errico S, Gravina AG, Nicolucci C, Di Sarno R, et al. Role of bisphenol A as environmental factor in the promotion of non-alcoholic fatty liver disease: in vitro and clinical study. Aliment Pharmacol Ther. 2018;47(6):826–37.
Jun DW, Kim SG, Park SH, Jin SY, Lee JS, Lee JW, et al. External validation of the non-alcoholic fatty liver disease fibrosis score for assessing advanced fibrosis in Korean patients. J Gastroenterol Hepatol. 2017;32(5):1094–9.
Kim S, Chang Y, Sung E, Kim CH, Yun KE, Jung HS, et al. Non-alcoholic fatty liver disease and the development of nephrolithiasis: A cohort study. PLoS ONE. 2017;12(10): e0184506.
Tasneem AA, Luck NH, Majid Z. Factors predicting non-alcoholic steatohepatitis (NASH) and advanced fibrosis in patients with non-alcoholic fatty liver disease (NAFLD). Trop Doct. 2018;48(2):107–12.
Bae JC, Kim SK, Han JM, Kwon S, Lee DY, Kim J, et al. Additive effect of non-alcoholic fatty liver disease on the development of diabetes in individuals with metabolic syndrome. Diabetes Res Clin Pract. 2017;129:136–43.
Abdeldyem SM, Goda T, Khodeir SA, Abou Saif S, Abd-Elsalam S. Nonalcoholic fatty liver disease in patients with acute ischemic stroke is associated with more severe stroke and worse outcome. J Clin Lipidol. 2017;11(4):915–9.
Kim SS, Cho HJ, Kim HJ, Kang DR, Berry JR, Kim JH, et al. Nonalcoholic fatty liver disease as a sentinel marker for the development of diabetes mellitus in non-obese subjects. Dig Liver Dis. 2018;50(4):370–7.
Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: A long-term follow-up study. Hepatol Commun. 2018;2(1):48–57.
Peng H, Wang M, Pan L, Cao Z, Yao Z, Chen Q, et al. Associations of serum multivitamin levels with the risk of non-alcoholic fatty liver disease A population-based cross-sectional study in U.S. adults. Front Nut. 2022;9:962705.
Janovsky C, Cesena FH, Valente VAT, Conceição RDO, Santos RD, Bittencourt MS. Association between Thyroid-Stimulating Hormone Levels and Non-Alcoholic Fatty Liver Disease Is Not Independent from Metabolic Syndrome Criteria. Eur Thyroid J. 2018;7(6):302–7.
Yoneda M, Yamamoto T, Honda Y, Imajo K, Ogawa Y, Kessoku T, et al. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: a retrospective nationwide claims database study in Japan. J Gastroenterol. 2021;56(11):1022–32.
Shahab O, Biswas R, Paik J, Bush H, Golabi P, Younossi ZM. Among Patients With NAFLD, Treatment of Dyslipidemia Does Not Reduce Cardiovascular Mortality. Hepatol Commun. 2018;2(10):1227–34.
Patel P, Hossain F, Horsfall LU, Banh X, Hayward KL, Williams S, et al. A pragmatic approach identifies a high Rate of Nonalcoholic fatty liver disease with advanced Fibrosis in diabetes clinics and at-risk populations in primary care. Hepatol Commun. 2018;2(8):893–905.
Akuta N, Kawamura Y, Arase Y, Saitoh S, Fujiyama S, Sezaki H, et al. Hepatocellular carcinoma is the most common liver-related complication in patients with histopathologically-confirmed NAFLD in Japan. BMC Gastroenterol. 2018;18(1):165.
Kwon YJ, Kim JH, Jung DH. Association between nonalcoholic fatty liver disease and intraocular pressure in Korean adults. J Glaucoma. 2018;27(12):1099–104.
Qin S, Wang S, Wang X, Wang J. Liver stiffness assessed by transient elastography as a potential indicator of chronic kidney disease in patients with nonalcoholic fatty liver disease. J Clin Lab Anal. 2019;33(2):e22657.
Chung GE, Youn J, Kim YS, Lee JE, Yang SY, Lim JH, et al. Dietary patterns are associated with the prevalence of nonalcoholic fatty liver disease in Korean adults. Nutrition. 2019;62:32–8.
De Silva S, Li W, Kemos P, Brindley JH, Mecci J, Samsuddin S, et al. Non-invasive markers of liver fibrosis in fatty liver disease are unreliable in people of South Asian descent. Front Gastroenterol. 2018;9(2):115–21.
Koo BK, Um SH, Seo DS, Joo SK, Bae JM, Park JH, et al. Growth differentiation factor 15 predicts advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease. Liver Int. 2018;38(4):695–705.
Mazzotti A, Caletti MT, Brodosi L, Di Domizio S, Forchielli ML, Petta S, et al. An internet-based approach for lifestyle changes in patients with NAFLD: Two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69(5):1155–63.
Kimura T, Tanaka N, Fujimori N, Sugiura A, Yamazaki T, Joshita S, et al. Mild drinking habit is a risk factor for hepatocarcinogenesis in non-alcoholic fatty liver disease with advanced fibrosis. World J Gastroenterol. 2018;24(13):1440–50.
Labenz C, Huber Y, Kalliga E, Nagel M, Ruckes C, Straub BK, et al. Predictors of advanced fibrosis in non-cirrhotic non-alcoholic fatty liver disease in Germany. Aliment Pharmacol Ther. 2018;48(10):1109–16.
Pardhe BD, Shakya S, Bhetwal A, Mathias J, Khanal PR, Pandit R, et al. Metabolic syndrome and biochemical changes among non-alcoholic fatty liver disease patients attending a tertiary care hospital of Nepal. BMC Gastroenterol. 2018;18(1):109.
Tuttolomondo A, Petta S, Casuccio A, Maida C, Corte VD, Daidone M, et al. Reactive hyperemia index (RHI) and cognitive performance indexes are associated with histologic markers of liver disease in subjects with non-alcoholic fatty liver disease (NAFLD): a case control study. Cardiovasc Diabetol. 2018;17(1):28.
Li H, Hu B, Wei L, Zhou L, Zhang L, Lin Y, et al. Non-alcoholic fatty liver disease is associated with stroke severity and progression of brainstem infarctions. Eur J Neurol. 2018;25(3):577-e534.
Ooi GJ, Burton PR, Bayliss J, Raajendiran A, Earnest A, Laurie C, et al. Effect of Body Mass Index, Metabolic Health and Adipose Tissue inflammation on the severity of non-alcoholic fatty liver disease in Bariatric surgical patients: a prospective study. Obes Surg. 2019;29(1):99–108.
de la Aller Fuente R, Mora Cuadrado N, Tafur C, de la López Gómez JJ, Gómez Cuesta S, García Sánchez MC, et al. Histopathological differences in patients with biopsyproven nonalcoholic fatty liver disease with and without type 2 diabetes. Endocrinol Diabetes nutr. 2018;65(6):354–60.
Rabelo F, Stefano JT, Cavaleiro AM, Lima RVC, de Campos Mazo DF, Carrilho FJ, et al. Association between the CYBA and NOX4 genes of NADPH oxidase and its relationship with metabolic syndrome in non-alcoholic fatty liver disease in Brazilian population. Hepatobiliary Pancreat Dis Int. 2018;17(4):330–5.
Nigam P, Pandey RP, Pawar M, Indurkar M. CLINICAL PROFILE OF NAFLD PATIENTS AND ITS CORRELATION WITH METABOLIC SYNDROME. J Evol Med Dent Sci. 2018;7(4):433–6.
Omrani HR, Golmohamadi S, Asadi N. Non-Alcoholic Fatty Liver Disease and Associated Risk Factors among Hemodialysis Patients. 2018. https://doi.org/10.5742/MEWFM.2018.93354. https://search.ebscohost.com/login.aspx?direct=true&db=edsaml&AN=edsaml.117456&lang=zh-cn&site=eds-live.
Gordon SC, Fraysse J, Li S, Ozbay AB, Wong RJ. Disease Severity Is Associated With Higher Healthcare Utilization in Nonalcoholic Steatohepatitis Medicare Patients. Am J gastroenterol. 2020;115(4):562–74.
Shah AS, Khan S, Rahim H, Chishti KA, Khan AG. Prevalence of non alcoholic fatty liver and Non alcoholic Steatohepatitis in Peshawar Cantonment, Khyber Pakhtunkhwa Pakistan. Pak J Pharm Sci. 2018;31(1):193–8.
Chen HJ, Yang HY, Hsueh KC, Shen CC, Chen RY, Yu HC, et al. Increased risk of osteoporosis in patients with nonalcoholic fatty liver disease: A population-based retrospective cohort study. Medicine. 2018;97(42):e12835.
Alam S, Anam K, Islam S, Mustafa G, Mamun AA, Ahmad N. Clinical, anthropometric, biochemical and histological character of nonalcoholic fatty liver disease without Insulin Resistance. J Clin Exp Hepatol. 2019;9(2):176–81.
Hamaguchi M, Hashimoto Y, Obora A, Kojima T, Fukui M. Non-alcoholic fatty liver disease with obesity as an independent predictor for incident gastric and colorectal cancer: a population-based longitudinal study. BMJ Open Gastroenterol. 2019;6(1):e000295.
Khammas ASA, Hassan HA, Salih SQM, Kadir H, Ibrahim RM, Nasir NNM, et al. Prevalence and risk factors of sonographically detected non alcoholic fatty liver disease in a screening centre in Klang Valley, Malaysia: an observational cross-sectional study. Porto Biomed J. 2019;4(2):e31.
Moolla A, Motohashi K, Marjot T, Shard A, Ainsworth M, Gray A, et al. A multidisciplinary approach to the management of NAFLD is associated with improvement in markers of liver and cardio-metabolic health. Front Gastroenterol. 2019;10(4):337–46.
Nabi O, Lacombe K, Boursier J, Mathurin P, Zins M, Serfaty L. Prevalence and Risk Factors of Nonalcoholic Fatty Liver Disease and Advanced Fibrosis in General Population: the French Nationwide NASH-CO Study. Gastroenterol. 2020;159(2):791-793.e792.
Shida T, Oshida N, Oh S, Okada K, Shoda J. Progressive reduction in skeletal muscle mass to visceral fat area ratio is associated with a worsening of the hepatic conditions of non-alcoholic fatty liver disease. Diabetes Metab Syndr Obes. 2019;12:495–503.
Tsou P, Wu CJ. Serum Vitamin E Levels of Adults with Nonalcoholic Fatty Liver Disease: An Inverse Relationship with All-Cause Mortality in Non-Diabetic but Not in Pre-Diabetic or Diabetic Subjects. J Clin Med. 2019;8(7):1057.
Azuma S, Asahina Y, Kakinuma S, Azuma K, Miyoshi M, Inoue E, et al. Diabetic Retinopathy as a Risk Factor Associated with the Development of Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease. Dig Dis. 2019;37(3):247–54.
Blackett JW, Verna EC, Lebwohl B. Increased Prevalence of Colorectal Adenomas in Patients with Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study. Dig Dis. 2020;38(3):222–30.
Cho HJ, Hwang S, Park JI, Yang MJ, Hwang JC, Yoo BM, et al. Improvement of Nonalcoholic fatty liver disease reduces the risk of type 2 Diabetes Mellitus. Gut and liver. 2019;13(4):440–9.
Jaafar RF, Hajj Ali AM, Zaghal AM, Kanso M, Habib SG, Halaoui AF, et al. Fibroscan and low-density lipoprotein as determinants of severe liver fibrosis in diabetic patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2019;31(12):1540–4.
Kim D, Kim W, Joo SK, Kim JH, Harrison SA, Younossi ZM, et al. Predictors of nonalcoholic steatohepatitis and significant fibrosis in non-obese nonalcoholic fatty liver disease. Liver Int. 2019;39(2):332–41.
Maev IV, Samsonov AA, Palgova LK, Pavlov CS, Shirokova E, Starostin KM. Real-world comorbidities and treatment patterns among patients with non-alcoholic fatty liver disease receiving phosphatidylcholine as adjunctive therapy in Russia. BMJ Open Gastroenterol. 2019;6(1):e000307.
Udelsman BV, Corey KE, Lindvall C, Gee DW, Meireles OR, Hutter MM, et al. Risk factors and prevalence of liver disease in review of 2557 routine liver biopsies performed during bariatric surgery. Surg Obes Relat Dis. 2019;15(6):843–9.
Ciardullo S, Monti T, Sala I, Grassi G, Mancia G, Perseghin G. Nonalcoholic Fatty Liver Disease and Advanced Fibrosis in US Adults Across Blood Pressure Categories. Hypertension. 2020;76(2):562–8.
Yılmaz Y, Kanı HT, Demirtaş C, Kaya E, Sapmaz AF, Qutranji L, et al. Growing burden of nonalcoholic fatty liver disease in Turkey: A single-center experience. Turkish J Gastroenterol. 2019;30(10):892–8.
Han J, Wang Y, Yuan Z, Liu L, Zhao M, Guan Q, et al. Nonalcoholic fatty liver disease represents a greater metabolic burden in patients with atherosclerosis: A cross-sectional study. Medicine. 2019;98(11):e14896.
Mahamid M, Khoury T, Mahamid B, Sbeit W, Mari A, Nseir W. The interplay between abdominal aortic aneurysm, metabolic syndrome and fatty liver disease: a retrospective case-control study. Diabetes Metabolic Syndr Obes. 2019;12:1743–9.
Zhou X, Li Y, Zhang X, Guan YY, Puentes Y, Zhang F, et al. Independent markers of nonalcoholic fatty liver disease in a gentrifying population-based Chinese cohort. Diabetes Metab Res Rev. 2019;35(5):e3156.
Wu J, Zeng C, Yang Z, Li X, Lei G, Xie D, et al. Association Between Dietary Selenium Intake and the Prevalence of Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study. J Am Coll Nutr. 2020;39(2):103–11.
Gawrieh S, Wilson LA, Cummings OW, Clark JM, Loomba R, Hameed B, et al. Histologic Findings of Advanced Fibrosis and Cirrhosis in Patients With Nonalcoholic Fatty Liver Disease Who Have Normal Aminotransferase Levels. Am J Gastroenterol. 2019;114(10):1626–35.
Barb D, Bril F, Kalavalapalli S, Cusi K. Plasma Fibroblast Growth Factor 21 Is Associated With Severity of Nonalcoholic Steatohepatitis in Patients With Obesity and Type 2 Diabetes. J Clin Endocrinol Metab. 2019;104(8):3327–36.
Zhang X, Zhou X, Han X, Fu Z, Wang L, Li Y, et al. The Morbidity and Comorbidity of Nonalcoholic Fatty Liver Disease and Different Glucose Intolerance Strata in a Community-Based Chinese Population. Metab Syndr Relat Disord. 2020;18(6):284–90.
Luo K, Bian J, Wang Q, Wang J, Chen F, Li H, et al. Association of obesity with chronic kidney disease in elderly patients with nonalcoholic fatty liver disease. Turkish J Gastroenterol. 2019;30(7):611–5.
Simon TG, Trejo MEP, McClelland R, Bradley R, Blaha MJ, Zeb I, et al. Circulating Interleukin-6 is a biomarker for coronary atherosclerosis in nonalcoholic fatty liver disease: Results from the Multi-Ethnic Study of Atherosclerosis. Int J Cardiol. 2018;259:198–204.
Li Y, He F, He Y, Pan X, Wu Y, Hu Z, et al. Dose-response association between physical activity and non-alcoholic fatty liver disease: a case-control study in a Chinese population. BMJ Open. 2019;9(3):e026854.
Tu S, Zhao R, Fang H, Wang L, Shao A, Sheng J. Association between Non-Alcoholic Fatty Liver Disease and Intracerebral Hemorrhage. Cell Transplant. 2019;28(8):1033–8.
Önnerhag K, Dreja K, Nilsson PM, Lindgren S. Increased mortality in non-alcoholic fatty liver disease with chronic kidney disease is explained by metabolic comorbidities. Clin Res Hepatol Gastroenterol. 2019;43(5):542–50.
Weinstein G, Davis-Plourde K, Himali JJ, Zelber-Sagi S, Beiser AS, Seshadri S. Non-alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle-aged adults: The Framingham Study. Liver Int. 2019;39(9):1713–21.
Ulasoglu C, Enc FY, Kaya E, Yilmaz Y. Characterization of Patients with Biopsy-Proven Non-Alcoholic Fatty Liver Disease and Normal Aminotransferase Levels. J Gastrointestin Liver Dis. 2019;28(4):427–31.
Zhao H, Hu B. Risk factors for Non-alcoholic Fatty Liver Disease in Chinese population: a five-year follow-up study. Iran Red Crescent Med J. 2019;21(7):e91064.
Tada T, Toyoda H, Sone Y, Yasuda S, Miyake N, Kumada T, et al. Type 2 diabetes mellitus: A risk factor for progression of liver fibrosis in middle-aged patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2019;34(11):2011–8.
Niriella MA, Kasturiratne A, Pathmeswaran A, De Silva ST, Perera KR, Subasinghe S, et al. Lean non-alcoholic fatty liver disease (lean NAFLD): characteristics, metabolic outcomes and risk factors from a 7-year prospective, community cohort study from Sri Lanka. Hep Intl. 2019;13(3):314–22.
Rong HJ, Meng CR, Chen ZY, Xue L. Biomarkers of non-alcoholic fatty liver disease in a Chinese population: a cross-sectional survey. Int J Clin Exp Med. 2019;12(2):1827–34.
Poynard T, Peta V, Deckmyn O, Pais R, Ngo Y, Charlotte F, et al. Performance of liver biomarkers, in patients at risk of nonalcoholic steato-hepatitis, according to presence of type-2 diabetes. Eur J Gastroenterol Hepatol. 2020;32(8):998–1007.
McGhee-Jez AE, Chervoneva I, Yi M, Ahuja A, Nahar R, Shah S, et al. Nonalcoholic fatty liver disease after Pancreaticoduodenectomy for a cancer diagnosis. J Pancreat Cancer. 2021;7(1):23–30.
Tahir F, Majid Z, Majid B, Ahmed J, Zaman A, Tariq M, et al. Anthropometric Measurements and Lifestyle Characteristics of Individuals with Non-alcoholic Fatty Liver Disease. Cureus. 2020;12(2):e7016.
Bian H, Zhu X, Xia M, Yan H, Chang X, Li X, et al. Impact of type 2 diabetes on nonalcoholic steatohepatitis and advanced fibrosis in patients with nonalcoholic fatty liver disease. Endocr Pract. 2020;26(4):444–53-453.
Clough GF, Chipperfield AJ, Thanaj M, Scorletti E, Calder PC, Byrne CD. Dysregulated Neurovascular Control Underlies Declining Microvascular Functionality in People With Non-alcoholic Fatty Liver Disease (NAFLD) at Risk of Liver Fibrosis. Front Physiol. 2020;11:551.
Kogiso T, Sagawa T, Kodama K, Taniai M, Hashimoto E, Tokushige K. Development and course of diabetes according to genetic factors and diabetes treatment among patients with nonalcoholic fatty liver disease. Nutrition. 2021;83:111080.
An JN, Joo SK, Koo BK, Kim JH, Oh S, Kim W. Portal inflammation predicts renal dysfunction in patients with nonalcoholic fatty liver disease. Hep Intl. 2020;14(5):798–807.
Lisboa QC, Nardelli MJ, Pereira PA, Miranda DM, Ribeiro SN, Costa RSN, et al. PNPLA3 and TM6SF2 polymorphisms in Brazilian patients with nonalcoholic fatty liver disease. World J Hepatol. 2020;12(10):792–806.
Qiao ZP, Zheng KI, Zhu PW, Gao F, Zheng MH. Lower levels of plasma NT-proBNP are associated with higher prevalence of NASH in patients with biopsy-proven NAFLD. Nutr Metab Cardiovasc Dis. 2020;30(10):1820–5. PMID: 32636124.
Hirose S, Matsumoto K, Tatemichi M, Tsuruya K, Anzai K, Arase Y, et al. Nineteen-year prognosis in Japanese patients with biopsy-proven nonalcoholic fatty liver disease: Lean versus overweight patients. PLoS ONE. 2020;15(11):e0241770.
Ishiba H, Sumida Y, Seko Y, Tanaka S, Yoneda M, Hyogo H, et al. Type IV Collagen 7S is the most accurate test for identifying advanced Fibrosis in NAFLD with type 2 diabetes. Hepatol Commun. 2021;5(4):559–72.
Xia W, Yang N, Li Y. Analysis of Risk Factors for Adverse Cardiovascular Events in Elderly Patients with Acute Myocardial Infarction and Non-Alcoholic Fatty Liver Disease (NAFLD). Med Sci Monit. 2020;26:e922913.
Afolabi PR, Scorletti E, Calder PC, Byrne CD. Factors independently associated with cardiorespiratory fitness in patients with non-alcoholic fatty liver disease. Liver Int. 2020;40(12):2998–3007.
Ma C, Liu Y, He S, Zeng J, Li P, Ma C, et al. C-Peptide: A Mediator of the Association Between Serum Uric Acid to Creatinine Ratio and Non-Alcoholic Fatty Liver Disease in a Chinese Population With Normal Serum Uric Acid Levels. Front Endocrinol. 2020;11:600472.
Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49(4):608–12.
Ahmed HS, Pedersen N, Jayanna MB, Ten Eyck P, Sanchez A, Murali AR. Predictive Factors and Time to Development of Hepatic Decompensation in Patients with Non-alcoholic Fatty Liver Disease. J Gen Intern Med. 2020;35(5):1523–9.
Giraldi L, Miele L, Aleksovska K, Manca F, Leoncini E, Biolato M, et al. Mediterranean diet and the prevention of non-alcoholic fatty liver disease: results from a case-control study. Eur Rev Med Pharmacol Sci. 2020;24(13):7391–8.
Linge J, Ekstedt M, Dahlqvist LO. Adverse muscle composition is linked to poor functional performance and metabolic comorbidities in NAFLD. JHEP Rep. 2021;3(1):100197.
Li CH, Chou YT, Shen WC, Lu FH, Yang YC, Wu JS, et al. Increased risks of different grades of non-alcoholic fatty liver disease in prediabetic subjects with impaired fasting glucose and glucose tolerance, including the isolated glycosylated hemoglobin levels of 5.7–6.4% in a Chinese population. J Diabetes Investig. 2020;11(5):1336–43.
Su Y, Liu Z, Yang L, Li Y, Jiang S, Yao H, et al. PPARγ gene Pro12Ala variants reduce the risk of obese individuals to non-alcoholic fatty liver: A study in Uygur Chinese population residing in Northwestern China. Clin Res Hepatol Gastroenterol. 2020;44(6):894–904.
Sun DQ, Jin Y, Wang TY, Zheng KI, Rios RS, Zhang HY, et al. MAFLD and risk of CKD. Metabolism. 2021;115:154433.
Miller A, McNamara J, Hummel SL, Konerman MC, Tincopa MA. Prevalence and staging of non-alcoholic fatty liver disease among patients with heart failure with preserved ejection fraction. Sci Rep. 2020;10(1):12440.
Sinn DH, Kang D, Chang Y, Ryu S, Cho SJ, Paik SW, et al. Non-alcoholic fatty liver disease and the incidence of myocardial infarction: A cohort study. J Gastroenterol Hepatol. 2020;35(5):833–9.
Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver international : official journal of the International Association for the Study of the Liver. 2020;40(9):2082–9.
Rahman MM, Kibria MG, Begum H, Haque M, Sultana N, Akhter M, et al. Prevalence, risk factors and metabolic profile of the non-obese and obese non-alcoholic fatty liver disease in a rural community of South Asia. BMJ Open Gastroenterol. 2020;7(1):e000535.
Wang X, Han F, Ji Z, Shen L, Shen Y. Association between Non-alcoholic Fatty Liver Disease and the Severity of Coronary Artery Stenosis in Eastern Chinese Population. Hepat Mon. 2021;21(11):e122772.
Iritani S, Akuta N, Kawamura Y, Kajiwara A, Kasuya K, Fujiyama S, et al. Non-invasive predictors of prognosis of Asian patients with histopathologically-confirmed lean nonalcoholic fatty liver disease. BMC Gastroenterol. 2020;20(1):368.
Stefano JT, Guedes LV, de Souza AAA, Vanni DS, Alves VAF, Carrilho FJ, et al. Usefulness of collagen type IV in the detection of significant liver fibrosis in nonalcoholic fatty liver disease. Ann Hepatol. 2021;20:100253.
Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, et al. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology. 2020;71(3):808–19.
Björkström K, Widman L, Hagström H. Risk of hepatic and extrahepatic cancer in NAFLD: A population-based cohort study. Liver Int. 2022;42(4):820–8.
Boga S, Koksal AR, Sen İ, Kurul Yeniay M, Yilmaz Ozguven MB, Serin E, et al. Liver and pancreas “Castor and Pollux” regarding the relationship between hepatic steatosis and pancreas exocrine insufficiency. Pancreatology. 2020;20(5):880–6.
Miele L, Grattagliano I, Lapi F, Dajko M, De Magistris A, Liguori A, et al. Non-alcoholic fatty liver disease and the risk of fibrosis in Italian primary care services: GPS-NAFLD Study: GPS-NAFLD Study. Liver Int. 2022;42(12):2632–45.
Ng CH, Chan KE, Chin YH, Zeng RW, Tsai PC, Lim WH, et al. The effect of diabetes and prediabetes on the prevalence, complications and mortality in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2022;28(3):565–74.
Adrian T, Sørensen IMH, Knop FK, Bro S, Ballegaard ELF, Nordestgaard BG, et al. Prevalence of non-alcoholic fatty liver disease in patients with chronic kidney disease: a cross-sectional study. Nephrol Dial Transplant. 2022;37(10):1927–34.
Dai W, Zhang Z, Zhao S. The risk of type 2 diabetes and Coronary artery disease in non-obese Patients with non-alcoholic fatty liver disease: a cohort study. Front cardiovasc med. 2021;8:680664.
Hu PF, Zeng X, Zou ZY, Tang W, Guo YB, Yuan ZL, et al. The presence of NAFLD in nonobese subjects increased the risk of metabolic abnormalities than obese subjects without NAFLD: a population-based cross-sectional study. Hepatobiliary Surg Nutr. 2021;10(6):811–24.
Alswat K, Sanai FM, Al-Hamoudi W, Ismail M, Dahlan Y, AlGhamdi HS, et al. Clinical and Metabolic Characteristics of Non-Alcoholic Fatty Liver Disease Patients in Saudi Arabia: Data from the Systematic Observatory Liver Disease (SOLID) Registry. Diabetes Metab Syndr Obes. 2021;14:1167–75.
Eldafashi N, Darlay R, Shukla R, McCain MV, Watson R, Liu YL, et al. A PDCD1 Role in the Genetic Predisposition to NAFLD-HCC? Cancers. 2021;13(6):1412.
Higuera-de-la-Tijera F, Córdova-Gallardo J, Buganza-Torio E, Barranco-Fragoso B, Torre A, Parraguirre-Martínez S, et al. Hepamet Fibrosis Score in Nonalcoholic Fatty Liver Disease Patients in Mexico: Lower than Expected Positive Predictive Value. Dig Dis Sci. 2021;66(12):4501–7.
Johnson PC, Cochet AA, Gore RS, Harrison SA, Magulick JP, Aden JK, et al. Early Cardiac Dysfunction in Biopsy-proven Nonalcoholic Fatty Liver Disease. Korean J Gastroenterol. 2021;78(3):161–7.
Satapathy SK, Tran QT, Kovalic AJ, Bontha SV, Jiang Y, Kedia S, et al. Clinical and Genetic Risk Factors of Recurrent Nonalcoholic Fatty Liver Disease After Liver Transplantation. Clin Transl Gastroenterol. 2021;12(2):e00302.
Yang RX, Zou ZS, Zhong BH, Deng H, He FP, Shi JP, et al. The pathologic relevance of metabolic criteria in patients with biopsy-proven nonalcoholic fatty liver disease and metabolic dysfunction associated fatty liver disease: A multicenter cross-sectional study in China. Hepatobiliary Pancreat Dis Int. 2021;20(5):426–32.
Chen LW, Huang MS, Shyu YC, Chien RN. Gamma-glutamyl transpeptidase elevation is associated with metabolic syndrome, hepatic steatosis, and fibrosis in patients with nonalcoholic fatty liver disease: A community-based cross-sectional study. Kaohsiung J Med Sci. 2021;37(9):819–27.
Fujii H, Fukumoto S, Enomoto M, Uchida-Kobayashi S, Kimura T, Tamori A, et al. The FibroScan-aspartate aminotransferase score can stratify the disease severity in a Japanese cohort with fatty liver diseases. Sci Rep. 2021;11(1):13844.
Anstee QM, Hallsworth K, Lynch N, Hauvespre A, Mansour E, Kozma S, et al. Real-world management of non-alcoholic steatohepatitis differs from clinical practice guideline recommendations and across regions. JHEP Rep. 2022;4(1):100411.
Siddiqui B, Kamran M, Tikmani SS, Azmat R, Mushtaq Z, Zafar SB, et al. Frequency and risk factors of non-alcoholic fatty liver disease in Helicobacter pylori-infected dyspeptic patients: A cross-sectional study. SAGE Open Med. 2021;9:20503121211025420.
Yao VJH, Sun M, Rahman AA, Samuel Z, Chan J, Zheng E, et al. Comparative analysis of metabolic risk factors for progression of non-alcoholic fatty liver disease. Clin Exp Hepatol. 2021;7(2):241–7.
Moon JH, Koo BK, Kim W. Non-alcoholic fatty liver disease and sarcopenia additively increase mortality: a Korean nationwide survey. J Cachexia Sarcopenia Muscle. 2021;12(4):964–72.
Song E, Kim JA, Roh E, Yu JH, Kim NH, Yoo HJ, et al. Long Working Hours and Risk of Nonalcoholic Fatty Liver Disease: Korea National Health and Nutrition Examination Survey VII. Front Endocrinol. 2021;12:647459.
Funuyet-Salas J, Pérez-San-Gregorio M, Martín-Rodríguez A, Romero-Gómez M. Quality of Life and Coping in Nonalcoholic Fatty Liver Disease: Influence of Diabetes and Obesity. Int J Environ Res Public health. 2021;18(7):3503.
Nguyen AL, Park H, Nguyen P, Sheen E, Kim YA, Nguyen MH. Rising Inpatient Encounters and Economic Burden for Patients with Nonalcoholic Fatty Liver Disease in the USA. Dig Dis Sci. 2019;64(3):698–707.
Hu Z, Li Y, Yang Y, Yu W, Xie W, Song G, et al. Serum lipids mediate the relationship of multiple polyaromatic hydrocarbons on non-alcoholic fatty liver disease: A population-based study. Sci Total Environ. 2021;780:146563.
Vittal A, Shapses M, Sharma B, Sharma D, Sun Q, Sampson M, et al. Lipoprotein Insulin Resistance Index Reflects Liver Fat Content in Patients With Nonalcoholic Fatty Liver Disease. Hepatol Commun. 2021;5(4):589–97.
Bayliss J, Ooi GJ, De Nardo W, Shah YJH, Montgomery MK, McLean C, et al. Ectodysplasin A Is Increased in Non-Alcoholic Fatty Liver Disease, But Is Not Associated With Type 2 Diabetes. Front Endocrinol. 2021;12:642432.
Roberts SK, Majeed A, Glenister K, Magliano D, Lubel JS, Bourke L, et al. Prevalence of non-alcoholic fatty liver disease in regional Victoria: a prospective population-based study. Med J Aust. 2021;215(2):77–82.
Baikpour M, Ozturk A, Dhyani M, Mercaldo ND, Pierce TT, Grajo JR, et al. Portal Venous Pulsatility Index: A Novel Biomarker for Diagnosis of High-Risk Nonalcoholic Fatty Liver Disease. AJR Am J Roentgenol. 2020;214(4):786–91.
Gerber L, Otgonsuren M, Mishra A, Escheik C, Birerdinc A, Stepanova M, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther. 2012;36(8):772–81.
Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009;104(4):861–7.
Pereira E, Paula DP, de Araujo BP, da Fonseca MJM, Diniz M, Daliry A, et al. Advanced glycation end product: A potential biomarker for risk stratification of non-alcoholic fatty liver disease in ELSA-Brasil study. World J Gastroenterol. 2021;27(29):4913–28.
Emamat H, Ghalandari H, Totmaj AS, Tangestani H, Hekmatdoost A. Calcium to magnesium intake ratio and non-alcoholic fatty liver disease development: a case-control study. BMC Endocr Disord. 2021;21(1):51.
Irvine KM, Okano S, Patel PJ, Horsfall LU, Williams S, Russell A, et al. Serum matrix metalloproteinase 7 (MMP7) is a biomarker of fibrosis in patients with non-alcoholic fatty liver disease. Sci Rep. 2021;11(1):2858.
Lum JHM, Cheah MCC, Leow WQ, Wan WK, Lim TKH, Chow WC, et al. Clinical profile of non-alcoholic fatty liver disease in nonobese patients. J Gastroenterol Hepatol. 2021;36(1):257–61.
Zhu K, Guo Y, Zhao C, Kang S, Li J, Wang J, et al. Etiology Exploration of Non-alcoholic Fatty Liver Disease From Traditional Chinese Medicine Constitution Perspective: A Cross-Sectional Study. Front Public Health. 2021;9:635818.
Zhang J, Xu Q, Lai F, Chen N, Lin M, Liu Y, et al. Joint associations of metabolically healthy abdominal obesity and non-alcoholic fatty liver disease with prediabetes and diabetes in Chinese adults. BMJ Open Diabetes Res Care. 2021;9(1):e002362.
Belinchón-Romero I, Bellot P, Romero-Pérez D, Herraiz-Romero I, Marco F, Frances R, et al. Non-alcoholic fatty liver disease is associated with bacterial translocation and a higher inflammation response in psoriatic patients. Sci Rep. 2021;11(1):8593.
Labenz C, Kostev K, Armandi A, Galle PR, Schattenberg JM. Impact of thyroid disorders on the incidence of non-alcoholic fatty liver disease in Germany. United Eur Gastroenterol J. 2021;9(7):829–36.
Huang R, Fan JG, Shi JP, Mao YM, Wang BY, Zhao JM, et al. Health-related quality of life in Chinese population with non-alcoholic fatty liver disease: a national multicenter survey. Health Qual Life Outcomes. 2021;19(1):140.
Khayyat YM. Lean non-alcoholic fatty liver disease and associated metabolic disturbance: A Saudi Arabian cross-sectional study. Physiol Rep. 2021;9(14):e14949.
García-Compeán D, Villarreal-Pérez JZ, Cavazos MEO, Lavalle-Gonzalez FJ, Borjas-Almaguer OD, Del Cueto-Aguilera AN, et al. Prevalence of liver fibrosis in an unselected general population with high prevalence of obesity and diabetes mellitus Time for screening? Annals Hepatol. 2020;19(3):258–64.
Sang C, Yan H, Chan WK, Zhu X, Sun T, Chang X, et al. Diagnosis of Fibrosis Using Blood Markers and Logistic Regression in Southeast Asian Patients With Non-alcoholic Fatty Liver Disease. Front Med. 2021;8:637652.
Gerber Y, VanWagner LB, Yaffe K, Terry JG, Rana JS, Reis JP, et al. Non-alcoholic fatty liver disease and cognitive function in middle-aged adults: the CARDIA study. BMC Gastroenterol. 2021;21(1):96.
Blomdahl J, Nasr P, Ekstedt M, Kechagias S. Moderate alcohol consumption is associated with advanced fibrosis in non-alcoholic fatty liver disease and shows a synergistic effect with type 2 diabetes mellitus. Metabol Clin Exp. 2021;115:154439.
Zhang X, Heredia NI, Balakrishnan M, Thrift AP. Prevalence and factors associated with NAFLD detected by vibration controlled transient elastography among US adults: Results from NHANES 2017–2018. PLoS ONE. 2021;16(6):e0252164.
Simon TG, Roelstraete B, Sharma R, Khalili H, Hagström H, Ludvigsson JF. Cancer risk in patients with biopsy-confirmed nonalcoholic fatty liver disease: a population-based cohort study. Hepatology. 2021;74(5):2410–23.
Wang G, Wu S, Wu C, Zhang Q, Wu F, Yu B, et al. Association between non-alcoholic fatty liver disease with the susceptibility and outcome of COVID-19: A retrospective study. J Cell Mol Med. 2021;25(24):11212–20.
Wang B, Zhao Z, Liu S, Wang S, Chen Y, Xu Y, et al. Impact of diabetes on subclinical atherosclerosis and major cardiovascular events in individuals with and without non-alcoholic fatty liver disease. Diabetes Res Clin Pract. 2021;177:108873.
Nass KJ, van den Berg EH, Faber KN, Schreuder T, Blokzijl H, Dullaart RPF. High prevalence of apolipoprotein B dyslipoproteinemias in non-alcoholic fatty liver disease: The lifelines cohort study. Metabol Clin Exp. 2017;72:37–46.
Younossi ZM, Felix S, Jeffers T, Younossi E, Nader F, Pham H, et al. Performance of the Enhanced Liver Fibrosis Test to Estimate Advanced Fibrosis Among Patients With Nonalcoholic Fatty Liver Disease. JAMA Netw Open. 2021;4(9):e2123923.
Rodriguez LA, Shiboski SC, Bradshaw PT, Fernandez A, Herrington D, Ding J, et al. Predicting Non-Alcoholic Fatty Liver Disease for Adults Using Practical Clinical Measures: Evidence from the Multi-ethnic Study of Atherosclerosis. J Gen Intern Med. 2021;36(9):2648–55.
Baratta F, Ferro D, Pastori D, Colantoni A, Cocomello N, Coronati M, et al. Open issues in the transition from NAFLD to MAFLD: the experience of the Plinio study. Int J Environ Res Public Health. 2021;18(17):8993.
van Kleef LA, Ayada I, Alferink LJM, Pan Q, de Knegt RJ. Metabolic dysfunction-associated fatty liver disease improves detection of high liver stiffness: The Rotterdam Study. Hepatology. 2022;75(2):419–29.
Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: which has closer association with all-cause and cause-specific mortality?-results from NHANES III. Front Med. 2021;8:693507.
Wu W, Xiang J, Chen X. Association between diabetes Mellitus and all-cause and Cardiovascular mortality among individuals with ultrasound-defined non-alcoholic fatty liver disease. Front Endocrinol. 2021;12:773342.
Younes R, Govaere O, Petta S, Miele L, Tiniakos D, Burt A, et al. Caucasian lean subjects with non-alcoholic fatty liver disease share long-term prognosis of non-lean: time for reappraisal of BMI-driven approach? Gut. 2022;71(2):382–90.
Ghumman NA, Qamar MA, Khurrum N, Khan ZA, Mustafa ZU, Irshad F. Clinical Symptomatology and Laboratory Diagnosis of Nonalcoholic Chronic Fatty Liver Disease. Pak J Med Sci. 2021;15:2371–2.
Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75(6):1284–91.
Park H, Yoon EL, Cho S, Jun DW, Nah EH. Diabetes is the strongest risk factor of hepatic fibrosis in lean patients with non-alcoholic fatty liver disease. Gut. 2022;71(5):1035–6.
Alsuhaibani KA, Althunayyan FS, Alsudays AM, Alharbi AA, Aljarallah BM. Nonalcoholic fatty liver disease in lean and obese patients in Saudi patients from a single center. J Family Med Prim Care. 2021;10(9):3381–6.
Alexopoulos AS, Crowley MJ, Wang Y, Moylan CA, Guy CD, Henao R, et al. Glycemic control predicts severity of Hepatocyte Ballooning and Hepatic Fibrosis in nonalcoholic fatty liver Disease. Hepatology. 2021;74(3):1220–33.
Pinyopornpanish K, Khoudari G, Saleh MA, Angkurawaranon C, Pinyopornpanish K, Mansoor E, et al. Hepatocellular carcinoma in nonalcoholic fatty liver disease with or without cirrhosis: a population-based study. BMC Gastroenterol. 2021;21(1):394.
Holmer M, Ekstedt M, Nasr P, Zenlander R, Wester A, Tavaglione F, et al. Effect of common genetic variants on the risk of cirrhosis in non-alcoholic fatty liver disease during 20 years of follow-up. Liver Int. 2022;42(12):2769–80.
Grupper A, Rabinowich A, Ben Shabat I, Tzadok R, Schwartz D, Schwartz IF, et al. Nonalcoholic fatty liver disease before Kidney Transplantation Correlates with new onset diabetes and poor Metabolic outcomes. Am J Nephrol. 2022;53(8–9):636–45.
Golabi P, Paik JM, Harring M, Younossi E, Kabbara K, Younossi ZM. Prevalence of high and moderate risk Nonalcoholic fatty liver disease among adults in the United States, 1999–2016. Clin Gastroenterol Hepatol. 2022;20(12):2838-2847.e2837.
Elhence A, Anand A, Biswas S, Vaishnav M, Yadav R, Das P, et al. Compensated advanced chronic liver disease in nonalcoholic fatty liver disease: two-step strategy is better than Baveno Criteria. Dig Dis Sci. 2023;68(3):1016–25.
Damjanovska S, Karb DB, Tripathi A, Asirwatham J, Delozier S, Perez JA, et al. Accuracy of Ultrasound Elastography and Fibrosis-4 Index (FIB-4) in Ruling Out Cirrhosis in obese non-alcoholic fatty liver Disease (NAFLD) Patients. Cureus. 2022;14(9):e29445.
Ferrari-Cestari M, Okano S, Patel PJ, Horsfall LU, Keshvari S, Hume DA, et al. Serum CC-Chemokine Ligand 2 Is associated with visceral adiposity but not fibrosis in patients with non-alcoholic fatty liver disease. Dig Dis. 2023;41(3):439–46.
Thrift AP, Nguyen TH, Pham C, Balakrishnan M, Kanwal F, Loomba R, et al. The Prevalence and Determinants of NAFLD and MAFLD and Their Severity in the VA Primary Care Setting. Clin Gastroenterol Hepatol. 2023;21(5):1252-1260.e1255.
Singh SP, Panigrahi MK, Patel A, Viswanathan L, Rath MM, Kar SK, et al. Comparison of Clinical, Biochemical, and Histopathologic Profiles between NAFLD in Asian-Indians and United States Adults. Eur J Hepato-gastroenterol. 2022;12(Suppl 1):S15-s18.
Wang J, Sun H, Wang Y, An Y, Liu J, Wang G. Glucose metabolism status modifies the relationship between lipoprotein(a) and carotid plaques in individuals with fatty liver disease. Front Endocrinol. 2022;13:947914.
Seko Y, Kawanaka M, Fujii H, Iwaki M, Hayashi H, Toyoda H, et al. Age-dependent effects of diabetes and obesity on liver-related events in non-alcoholic fatty liver disease: Subanalysis of CLIONE in Asia. J Gastroenterol Hepatol. 2022;37(12):2313–20.
Sayiner M, Arshad T, Golabi P, Paik J, Farhat F, Younossi ZM. Extrahepatic manifestations and healthcare expenditures of non-alcoholic fatty liver disease in the Medicare population. Hep Intl. 2020;14(4):556–66.
Blank V, Petroff D, Boehlig A, Heinze A, Karlas T, Berg T, et al. Clinical implications of hepatic structure and function evaluation based on vibration-controlled transient elastography and liver maximum function capacity test in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2022;34(6):686–92.
Bharath Kumar C, Goel A, Jaleel R, David D, Zachariah U, Ramachandran J, et al. Prevalence of Risk Factors for Nonalcoholic Fatty Liver Disease in Middle-Aged and Elderly Patients With Cryptogenic Cirrhosis. J Clin Exp Hepatol. 2022;12(2):492–502.
Boehlig A, Gerhardt F, Petroff D, van Boemmel F, Berg T, Blank V, et al. Prevalence of Pruritus and Association with Anxiety and Depression in Patients with Nonalcoholic Fatty Liver Disease. Biomedicines. 2022;10(2):451.
Antwi SO, Craver EC, Nartey YA, Sartorius K, Patel T. Metabolic risk factors for Hepatocellular Carcinoma in patients with nonalcoholic fatty liver disease: a prospective study. Cancers. 2022;14(24):6234.
Andrade TG, Xavier LCD, Souza FF, Araújo RC. Risk predictors of advanced hepatic fibrosis in patients with nonalcoholic fatty liver disease - a survey in a university hospital in Brazil. Arch Endocrinol Metabol. 2022;66(6):823–30.
Chen Y, Su H, Xue H, Wang T, Qian T, Liao C, et al. Correlation between serum total bile acid and nonalcoholic fatty liver disease: A cross-sectional study. Saudi J Gastroenterol. 2022;28(4):304–11.
Kim H, Lee CJ, Ahn SH, Lee KS, Lee BK, Baik SJ, et al. MAFLD Predicts the Risk of Cardiovascular Disease Better than NAFLD in Asymptomatic Subjects with Health Check-Ups. Dig Dis Sci. 2022;67(10):4919–28.
Truong E, Yeo YH, Cook-Wiens G, Muthiah M, Yang JD, Sundaram V, et al. Nonalcoholic fatty liver disease prevalence and severity in Asian Americans from the national health and nutrition examination surveys 2017–2018. Hepatol Commun. 2022;6(9):2253–61.
Wang Q, Zhu M, Li H, Chen P, Wang M, Gu L, et al. Hyperferritinemia Correlates to Metabolic Dysregulation and Steatosis in Chinese Biopsy-Proven Nonalcoholic Fatty Liver Disease Patients. Diabetes Metabol Syndr Obes. 2022;15:1543–52.
Trifan A, Rotaru A, Stafie R, Stratina E, Zenovia S, Nastasa R, et al. Clinical and laboratory characteristics of normal weight and obese individuals with non-alcoholic fatty liver disease. Diagnostics (Basel, Switzerland). 2022;12(4):801.
Gray ME, Bae S, Ramachandran R, Baldwin N, VanWagner LB, Jacobs DR Jr, et al. Dietary Patterns and Prevalent NAFLD at Year 25 from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Nutrients. 2022;14(4):854.
Younossi ZM, Stepanova M, Myers RP, Younossi I, Henry L. The potential role of fatigue in identifying patients with NASH and advanced Fibrosis who experience disease Progression. Clin Gastroenterol Hepatol. 2023;21(4):970-977.e971.
Sun T, Deng Y, Geng X, Fang Q, Li X, Chen L, et al. Plasma Alkylresorcinol Metabolite, a Biomarker for Whole-Grain intake, is inversely associated with risk of nonalcoholic fatty liver disease in a case-control study of Chinese adults. J Nutr. 2022;152(4):1052–8.
Saitta C, Caruso A, Maimone S, Filomia R, Cacciola I, Caccamo G, et al. Antibody to hepatitis B virus core antigen positivity is a predictor of non-alcoholic fatty liver disease severity. Intern Emerg Med. 2022;17(6):1609–16.
Rinella ME, Satapathy SK, Brandman D, Smith C, Elwir S, Xia J, et al. Factors impacting survival in those transplanted for NASH Cirrhosis: data from the NailNASH Consortium. Clin Gastroenterol Hepatol. 2023;21(2):445-455.e442.
Karamfilova V, Assyov Y, Nedeva I, Gateva A, Ivanova I, Cherkezov N, et al. Increased Serum Pentraxin 3 Is Associated with Prediabetes and Type 2 Diabetes in Obese Patients with Nonalcoholic Fatty Liver Disease. Metab Syndr Relat Disord. 2022;20(2):132–6.
Sripongpun P, Kim WR, Mannalithara A, Charu V, Vidovszky A, Asch S, et al. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. Hepatology. 2023;77(1):256–67.
Klaric KA, Parai JL, Kepron CA, Walker AE, Milroy CM. Postmortem survey of haemoglobin A1c, non-alcoholic steatohepatitis and liver fibrosis within a general population. J Clin Pathol. 2023;76(9):606–11.
Ito T, Nguyen VH, Tanaka T, Park H, Yeh ML, Kawanaka M, et al. Poor Diagnostic Efficacy of Noninvasive tests for advanced Fibrosis in obese or younger than 60 Diabetic NAFLD patients. Clin Gastroenterol Hepatol. 2023;21(4):1013-1022.e1016.
Giammarino AM, Qiu H, Bulsara K, Khan S, Jiang Y, Da BL, et al. Community Socioeconomic Deprivation Predicts Nonalcoholic Steatohepatitis. Hepatol Commun. 2022;6(3):550–60.
Yu C, Wang M, Zheng S, Xia M, Yang H, Zhang D, et al. Comparing the Diagnostic Criteria of MAFLD and NAFLD in the Chinese Population: A Population-based Prospective Cohort Study. J Clin Transl Hepatol. 2022;10(1):6–16.
Jiang SX, Trasolini R, Heer M, Cox B, Galts C, Marquez V, et al. Non-alcoholic fatty liver disease (NAFLD) in Filipino North American patients: Results from a multi-ethnic cohort. Canadian liver journal. 2022;5(1):4–13.
Pariente A, Rosa I, Dharancy S, Hanslik B. Nonalcoholic fatty liver disease: opinions, population served, and management from a sample of French hepato(gastroentero)logists (PERSEPT study). Eur J Gastroenterol Hepatol. 2022;34(7):791–800.
Kotronen A, Yki-Järvinen H, Männistö S, Saarikoski L, Korpi-Hyövälti E, Oksa H, et al. Non-alcoholic and alcoholic fatty liver disease - two diseases of affluence associated with the metabolic syndrome and type 2 diabetes: the FIN-D2D survey. BMC Public Health. 2010;10:237.
Moctezuma-Velázquez P, Miranda-Zazueta G, Ortiz-Brizuela E, Garay-Mora JA, González-Lara MF, Tamez-Torres KM, et al. NAFLD determined by Dallas Steatosis Index is associated with poor outcomes in COVID-19 pneumonia: a cohort study. Intern Emerg Med. 2022;17(5):1355–62.
Zeina AR, Kopelman Y, Mari A, Ahmad HS, Artul S, Khalaila AS, et al. Pulmonary embolism risk in hospitalized patients with nonalcoholic fatty liver disease: A case-control study. Medicine. 2022;101(45):e31710.
van den Berg EH, Amini M, Schreuder TC, Dullaart RP, Faber KN, Alizadeh BZ, et al. Prevalence and determinants of non-alcoholic fatty liver disease in lifelines: A large Dutch population cohort. PLoS ONE. 2017;12(2):e0171502.
Zhao J, Fan H, Wang T, Yu B, Mao S, Wang X, et al. TyG index is positively associated with risk of CHD and coronary atherosclerosis severity among NAFLD patients. Cardiovasc Diabetol. 2022;21(1):123.
Masterton G, Hayes P. P31 Pattern and significance of biochemical abnormalities in referrals to a NAFLD clinic. 2010. https://doi.org/10.1136/gut.2010.223362.57. https://search.ebscohost.com/login.aspx?direct=true&db=edsbas&AN=edsbas.83BB32A5&lang=zh-cn&site=eds-live.
Deckmyn O, Poynard T, Bedossa P, Paradis V, Peta V, Pais R, et al. Clinical Interest of Serum Alpha-2 Macroglobulin, Apolipoprotein A1, and Haptoglobin in Patients with Non-Alcoholic Fatty Liver Disease, with and without Type 2 Diabetes, before or during COVID-19. Biomedicines. 2022;10(3):699.
Reddy SK, Zhan M, Alexander HR, El-Kamary SS. Nonalcoholic fatty liver disease is associated with benign gastrointestinal disorders. World J Gastroenterol. 2013;19(45):8301–11.
Li F, Ye J, Sun Y, Lin Y, Wu T, Shao C, et al. Distinct Dose-Dependent Association of Free Fatty Acids with Diabetes Development in Nonalcoholic Fatty Liver Disease Patients. Diabetes Metab J. 2021;45(3):417–29.
Alvarez CS, Graubard BI, Thistle JE, Petrick JL, McGlynn KA. Attributable Fractions of Nonalcoholic Fatty Liver Disease for Mortality in the United States: Results From the Third National Health and Nutrition Examination Survey With 27 Years of Follow-up. Hepatology. 2020;72(2):430–40.
Maurice JB, Goldin R, Hall A, Price JC, Sebastiani G, Morse CG, et al. Increased body mass index and type 2 diabetes are the main predictors of nonalcoholic fatty liver disease and advanced Fibrosis in Liver Biopsies of Patients With Human Immunodeficiency Virus Monoinfection. Clin Infect Dis. 2021;73(7):e2184–93.
Huang J, Ou W, Wang M, Singh M, Liu Y, Liu S, et al. MAFLD Criteria Guide the Subtyping of Patients with Fatty Liver Disease. Risk Manag Healthcare Policy. 2021;14:491–501.
Deng Y, Zhao Q, Gong R. Association Between Metabolic Associated Fatty Liver Disease and Chronic Kidney Disease: A Cross-Sectional Study from NHANES 2017–2018. Diabetes Metabol Syndr Obes. 2021;14:1751–61.
Fan J, Luo S, Ye Y, Ju J, Zhang Z, Liu L, et al. Prevalence and risk factors of metabolic associated fatty liver disease in the contemporary South China population. Nutr Metab. 2021;18(1):82.
Lee GH, Phyo WW, Loo WM, Kwok R, Ahmed T, Shabbir A, et al. Validation of genetic variants associated with metabolic dysfunction-associated fatty liver disease in an ethnic Chinese population. World J Hepatol. 2020;12(12):1228–38.
Li N, Xie G, Zhou B, Qu A, Meng H, Liu J, et al. Serum Adropin as a Potential Biomarker for Predicting the Development of Type 2 Diabetes Mellitus in Individuals With Metabolic Dysfunction-Associated Fatty Liver Disease. Front Physiol. 2021;12:696163.
Tang LJ, Ma HL, Eslam M, Wong GL, Zhu PW, Chen SD, et al. Among simple non-invasive scores, Pro-C3 and ADAPT best exclude advanced fibrosis in Asian patients with MAFLD. Metabol Clin Exp. 2022;128:154958.
Wang X, Wu S, Yuan X, Chen S, Fu Q, Sun Y, et al. Metabolic Dysfunction-associated Fatty Liver Disease and Mortality Among Chinese Adults: a Prospective Cohort Study. J Clin Endocrinol Metab. 2022;107(2):e745–55.
Rodriguez-Duque JC, Calleja JL, Iruzubieta P, Hernández-Conde M, Rivas-Rivas C, Vera MI, et al. Increased risk of MAFLD and Liver Fibrosis in inflammatory bowel disease independent of classic metabolic risk Factors. Clinical Gastroenterol Hepatol. 2023;21(2):406-414.e407.
Park H, Yoon EL, Kim M, Lee J, Kim JH, Cho S, et al. Comparison of diagnostic performance between FIB-4 and NFS in metabolic-associated fatty liver disease era. Hepatol Res. 2022;52(3):247–54.
Semmler G, Wernly S, Bachmayer S, Leitner I, Wernly B, Egger M, et al. Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)-Rather a Bystander Than a Driver of Mortality. J Clin Endocrinol Metab. 2021;106(9):2670–7.
Yang S, Cheng J, Zhang R, Sun H, Zhang H, Lyu S, et al. Metabolic dysfunction-associated fatty liver disease and liver fibrosis: Prevalence and associated factors in the middle-aged and older US population. Hepatol Res. 2022;52(2):176–86.
Kim M, Yoon EL, Cho S, Lee CM, Kang BK, Park H, et al. Prevalence of advanced hepatic fibrosis and comorbidity in metabolic dysfunction-associated fatty liver disease in Korea. Liver Int. 2022;42(7):1536–44.
Alam S, Noor EASM, Chowdhury ZR, Alam M, Kabir J. Nonalcoholic steatohepatitis in nonalcoholic fatty liver disease patients of Bangladesh. World J Hepatol. 2013;5(5):281–7.
Salgado Alvarez GA, Pinto Galvez SM, Garcia Mora U, Cano Contreras AD, Durán Rosas C, Priego-Parra BA, et al. Higher cardiovascular risk scores and liver fibrosis risk estimated by biomarkers in patients with metabolic-dysfunction-associated fatty liver disease. World J Hepatol. 2022;14(8):1633–42.
Fukunaga S, Nakano D, Tsutsumi T, Kawaguchi T, Eslam M, Yoshinaga S, et al. Lean/normal-weight metabolic dysfunction-associated fatty liver disease is a risk factor for reflux esophagitis. Hepatol Res. 2022;52(8):699–711.
Guo Y, Yang J, Ma R, Zhang X, Guo H, He J, et al. Metabolic Dysfunction-Associated Fatty Liver Disease Is Associated with the Risk of Incident Cardiovascular Disease: A Prospective Cohort Study in Xinjiang. Nutrients. 2022;14(12):2361.
Drożdż K, Nabrdalik K, Kwiendacz H, Hendel M, Olejarz A, Tomasik A, et al. Risk factors for cardiovascular disease in patients with metabolic-associated fatty liver disease: a machine learning approach. Cardiovasc Diabetol. 2022;21(1):240.
Matsubayashi Y, Fujihara K, Yamada-Harada M, Mitsuma Y, Sato T, Yaguchi Y, et al. Impact of metabolic syndrome and metabolic dysfunction-associated fatty liver disease on cardiovascular risk by the presence or absence of type 2 diabetes and according to sex. Cardiovasc Diabetol. 2022;21(1):90.
Jamali R, Veisi S, Jamali A, Yaseri M. Relationship Between Cardiovascular Disease Risk Factors and Quality of Life in Patients with Metabolic-Associated Fatty Liver Disease. Hepat Mon. 2022;22(1):e124229.
Peng D, Yu Z, Wang M, Shi J, Sun L, Zhang Y, et al. Association of Metabolic Dysfunction-Associated Fatty Liver Disease With Left Ventricular Diastolic Function and Cardiac Morphology. Front Endocrinol. 2022;13:935390.
Lei F, Qin JJ, Song X, Liu YM, Chen MM, Sun T, et al. The prevalence of MAFLD and its association with atrial fibrillation in a nationwide health check-up population in China. Front Endocrinol. 2022;13:1007171.
Vítor Macedo S, Marta F, Sofia X, Pedro Boal C, Joana M, Carla M, et al. The New FibroScan-AST (FAST) Score: Enhancing Diabetes Mellitus Impact on Metabolic-Associated Fatty Liver Disease. GE Port J Gastroenterol. 2023;30(6):422–9.
Su W, Chen M, Xiao L, Du S, Xue L, Feng R, et al. Association of metabolic dysfunction-associated fatty liver disease, type 2 diabetes mellitus, and metabolic goal achievement with risk of chronic kidney disease. Front Public Health. 2022;10:1047794.
Seo JY, Cho EJ, Kim MJ, Kwak MS, Yang JI, Chung SJ, et al. The relationship between metabolic dysfunction-associated fatty liver disease and low muscle mass in an asymptomatic Korean population. J Cachexia Sarcopenia Muscle. 2022;13(6):2953–60.
Tang LJ, Li G, Eslam M, Zhu PW, Chen SD, Leung HH, et al. N-terminal propeptide of type 3 collagen-based sequential algorithm can identify high-risk steatohepatitis and fibrosis in MAFLD. Hep Intl. 2023;17(1):190–201.
Wang Y, Yu Y, Zhang H, Chen C, Wan H, Chen Y, et al. Cardiovascular and renal burdens among patients with MAFLD and NAFLD in China. Front Endocrinol. 2022;13:968766.
Yang J, Luo S, Li R, Ju J, Zhang Z, Shen J, et al. Sleep Factors in Relation to Metabolic Dysfunction-Associated Fatty Liver Disease in Middle-Aged and Elderly Chinese. J Clin Endocrinol Metab. 2022;107(10):2874–82.
Zhang YC, Lyu ZY, Ma B, Li LM, Wang W, Sheng C, et al. A new risk stratification strategy for fatty liver disease by incorporating MAFLD and fibrosis score in a large US population. Hep Intl. 2022;16(4):835–45.
Chen X, Goh GB, Huang J, Wu Y, Wang M, Kumar R, et al. Validation of Non-invasive Fibrosis Scores for Predicting Advanced Fibrosis in Metabolic-associated Fatty Liver Disease. J Clin Transl Hepatol. 2022;10(4):589–94.
Tang X, Shi Y, Du J, Hu K, Zhou T, Chen L, et al. Clinical outcome of non-alcoholic fatty liver disease: an 11-year follow-up study. BMJ Open. 2022;12(6):e054891.
Cai X, Wang M, Liu S, Yuan Y, Hu J, Zhu Q, et al. Establishment and validation of a nomogram that predicts the risk of type 2 diabetes in obese patients with non-alcoholic fatty liver disease: a longitudinal observational study. Am J Transl Res. 2022;14(7):4505–14.
Kim Y, Chang Y, Ryu S, Wild SH, Byrne CD. NAFLD improves risk prediction of type 2 diabetes: with effect modification by sex and menopausal status. Hepatology. 2022;76(6):1755–65.
Zhang H, Wang Y, Chen C, Wang B, Chen J, Tan X, et al. Non-alcoholic fatty liver disease, sleep behaviors, and incident type 2 diabetes. J Gastroenterol Hepatol. 2022;37(8):1633–40.
Arase Y, Suzuki F, Ikeda K, Kumada H, Tsuji H, Kobayashi T. Multivariate analysis of risk factors for the development of type 2 diabetes in nonalcoholic fatty liver disease. J Gastroenterol. 2009;44(10):1064–70.
Chung GE, Cho EJ, Yoon JW, Yoo JJ, Chang Y, Cho Y, et al. Nonalcoholic fatty liver disease increases the risk of diabetes in young adults A nationwide population-based study in Korea. Metabol Clin Exp. 2021;123:154866.
Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med. 2021;385(17):1559–69.
Wei Z, Ren Z, Hu S, Gao Y, Sun R, Lv S, et al. Development and validation of a simple risk model to predict major cancers for patients with nonalcoholic fatty liver disease. Cancer Med. 2020;9(3):1254–62.
Li WD, Fu KF, Li GM, Lian YS, Ren AM, Chen YJ, et al. Comparison of effects of obesity and non-alcoholic fatty liver disease on incidence of type 2 diabetes mellitus. World J Gastroenterol. 2015;21(32):9607–13.
Liu C, Wu Y, Duan W, Xu W. Cigarette Smoking Increases the Risk of Type 2 Diabetes Mellitus in Patients with Non-Alcoholic Fatty Liver Disease A Population-Based Cohort Study. Exp Clin Endocrinol Diabetes. 2022;130(12):793–800.
Fukuda T, Hamaguchi M, Kojima T, Hashimoto Y, Ohbora A, Kato T, et al. The impact of non-alcoholic fatty liver disease on incident type 2 diabetes mellitus in non-overweight individuals. Liver Int. 2016;36(2):275–83.
Nasr P, Fredrikson M, Ekstedt M, Kechagias S. The amount of liver fat predicts mortality and development of type 2 diabetes in non-alcoholic fatty liver disease. Liver Int. 2020;40(5):1069–78.
Xia Y, Cao L, Zhang Q, Liu L, Zhang S, Meng G, et al. Adherence to a vegetable dietary pattern attenuates the risk of non-alcoholic fatty liver disease in incident type 2 diabetes: The TCLSIH cohort study. J Intern Med. 2022;291(4):469–80.
Lee CH, Han KD, Kim DH, Kwak MS. The Repeatedly Elevated Fatty Liver Index Is Associated With Increased Mortality: A Population-Based Cohort Study. Front Endocrinol. 2021;12:638615.
Bae JC, Han JM, Cho JH, Kwon H, Park SE, Park CY, et al. The persistence of fatty liver has a differential impact on the development of diabetes: The Kangbuk Samsung Health Study. Diabetes Res Clin Pract. 2018;135:1–6.
Tokita Y, Maejima Y, Shimomura K, Takenoshita S, Ishiyama N, Akuzawa M, et al. Non-alcoholic Fatty Liver Disease Is a Risk Factor for Type 2 Diabetes in Middle-aged Japanese Men and Women. Int Med (Tokyo, Japan). 2017;56(7):763–71.
Zheng X, Cao C, He Y, Wang X, Wu J, Hu H. Association between nonalcoholic fatty liver disease and incident diabetes mellitus among Japanese: a retrospective cohort study using propensity score matching. Lipids Health Dis. 2021;20(1):59.
Wong VW, Chan HL, Hui AY, Chan KF, Liew CT, Chan FK, et al. Clinical and histological features of non-alcoholic fatty liver disease in Hong Kong Chinese. Aliment Pharmacol Ther. 2004;20(1):45–9.
Bahrami H, Daryani NE, Mirmomen S, Kamangar F, Haghpanah B, Djalili M. Clinical and histological features of nonalcoholic steatohepatitis in Iranian patients. BMC Gastroenterol. 2003;3:27.
Seko Y, Yano K, Takahashi A, Okishio S, Kataoka S, Okuda K, et al. FIB-4 Index and Diabetes Mellitus Are Associated with Chronic Kidney Disease in Japanese Patients with Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2019;21(1):171.
Friis-Liby I, Aldenborg F, Jerlstad P, Rundström K, Björnsson E. High prevalence of metabolic complications in patients with non-alcoholic fatty liver disease. Scand J Gastroenterol. 2004;39(9):864–9.
Hui AY, Wong VW, Chan HL, Liew CT, Chan JL, Chan FK, et al. Histological progression of non-alcoholic fatty liver disease in Chinese patients. Aliment Pharmacol Ther. 2005;21(4):407–13.
Lee J, Cho YK, Kang YM, Kim HS, Jung CH, Kim HK, et al. The Impact of NAFLD and Waist Circumference Changes on Diabetes Development in Prediabetes Subjects. Sci Rep. 2019;9(1):17258.
Barritt ASt, Dellon ES, Kozlowski T, Gerber DA, Hayashi PH. The influence of nonalcoholic fatty liver disease and its associated comorbidities on liver transplant outcomes. J Clin Gastroenterol. 2011;45(4):372–8.
Sinn DH, Kang D, Cho SJ, Paik SW, Guallar E, Cho J, et al. Lean non-alcoholic fatty liver disease and development of diabetes: a cohort study. Eur J Endocrinol. 2019;181(2):185–92.
Ming J, Xu S, Gao B, Liu G, Ji Y, Yang F, et al. Non-alcoholic fatty liver disease predicts type 2 diabetes mellitus, but not prediabetes, in Xi’an, China: a five-year cohort study. Liver Int. 2015;35(11):2401–7.
Miyake T, Matsuura B, Furukawa S, Ishihara T, Yoshida O, Miyazaki M, et al. Fatty liver with metabolic disorder, such as metabolic dysfunction-associated fatty liver disease, indicates high risk for developing diabetes mellitus. J Diabetes Investig. 2022;13(7):1245–52.
Cheng YM, Wang CC, Kao JH. Metabolic associated fatty liver disease better identifying patients at risk of liver and cardiovascular complications. Hep Intl. 2023;17(2):350–6.
IDF diabetes atlas. 9th ed. Brussels: International Diabetes Federation: International Diabetes Federation.; 2021 Available from: http://www.diabetesatlas.org.
Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69(6):2672–82.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843.
Jung HS, Chang Y, Kwon MJ, Sung E, Yun KE, Cho YK, et al. Smoking and the Risk of Non-Alcoholic Fatty Liver Disease: A Cohort Study. Am J Gastroenterol. 2019;114(3):453–63.
Meng G, Zhang B, Yu F, Li C, Zhang Q, Liu L, et al. Soft drinks consumption is associated with nonalcoholic fatty liver disease independent of metabolic syndrome in Chinese population. Eur J Nutr. 2018;57(6):2113–21.
. Ageing World Health Organization2019 Available from: https://www.who.int/health-topics/ageing#tab=tab_1.
Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(9):851–61.
Sinclair A, Saeedi P, Kaundal A, Karuranga S, Malanda B, Williams R. Diabetes and global ageing among 65–99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2020;162:108078.
Piazzolla VA, Mangia A. Noninvasive Diagnosis of NAFLD and NASH. Cells. 2020;9(4):1005.
Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp Diabetes Res. 2012;2012:145754.
Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–73.
Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–52.
Yun Y, Kim HN, Lee EJ, Ryu S, Chang Y, Shin H, et al. Fecal and blood microbiota profiles and presence of nonalcoholic fatty liver disease in obese versus lean subjects. PLoS ONE. 2019;14(3):e0213692.
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53.
Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology (Baltimore, MD). 2019;70(4):1457–69.
Steenblock C, Schwarz PEH, Ludwig B, Linkermann A, Zimmet P, Kulebyakin K, et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9(11):786–98.
Li J, Tian A, Zhu H, Chen L, Wen J, Liu W, et al. Mendelian randomization analysis reveals no causal relationship between nonalcoholic fatty liver disease and severe COVID-19. Clin Gastroenterol Hepatol. 2022;20(7):1553-1560.e1578.
Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2023;21(3):619-629.e617.
Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021;41(6):1290–3.
Acknowledgements
We acknowledge all authors’ hard work.
Meta registration
The study protocol, with all relevant tools, is publicly available at PROSPERO, CRD42022335692 (https://www.crd.york.ac.uk/prospero/#myprospero).
Funding
This work was supported by the 345 Talent Project of Shengjing Hospital of China Medical University [grant number M0294], the National Natural Science Foundation of China [grant numbers 82003456, 82103811], the Yong Elite Scientist Sponsorship Program by Cast [grant number 2020QNRC001], the Beijing Chao-Yang Hospital Golden Seeds Foundation [grant number CYJZ202102], and Tianjin Health Research Project [grant number TJWJ2022QN043].
Author information
Authors and Affiliations
Contributions
L.M.C., Y.X., and Y.H.L. conceived the study. H.Y.L., J.G.J., W.Q.L., M.Y.S., Y.H.Z., W.D., and Y.L.L. conducted the literature search, independently screened the studies, extracted data, and discussed results with L.K.C. and Y.H.Z. L.M.C. conducted the meta-analysis. L.M.C. and Y.A. drafted the manuscript. All authors provided critical input during the writing and revision of the paper. Y.X., Y.H.L., and L.K.C. supervised the study. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors read and approved the final manuscript.
Authors’ Twitter handles
Twitter handles: @YangXia623134.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary methods.
Additional file 2: Figures S1-S6. Fig. S1.
Flow chart. Fig. S2. The prevalence of type 2 diabetes among patients with NAFLD-stratified by publication year. Fig. S3. The prevalence of type 2 diabetes among patients with MAFLD across 12 countries. Fig. S4. The prevalence of type 2 diabetes among patients with MAFLD-stratified by age, region, publication year, sample size and quality grade. Fig. S5. The incidence density of type 2 diabetes among patients with NAFLD-stratified by publication year. Fig. S6. Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) instrument.
Additional file 3: Tables S1-S18. Table S1.
Characteristics of the included studies in the prevalence of type 2 diabetes among NAFLD patients. Table S2. Characteristics of studies reporting the prevalence of type 2 diabetes in patients with NAFLD: source of heterogeneity. Table S3. Average annual percent change (%) in the prevalence of type 2 diabetes among NAFLD populations globally and by region. Table S4. The prevalence of type 2 diabetes among patients with NAFLD-stratified by age, region, publication year, sample size and diagnosis of NAFLD (excluding index and hospital record of NAFLD). Table S5. Secondary analysis of the prevalence of type 2 diabetes among patients with NAFLD. Table S6. Univariable and multivariable meta-regression analyses on the prevalence of type 2 diabetes among patients with NAFLD. Table S7. Characteristics of the included studies in the prevalence of type 2 diabetes among MAFLD patients. Table S8. Characteristics of studies reporting the prevalence of type 2 diabetes in patients with MAFLD: source of heterogeneity. Table S9. Average annual percent change (%) in the prevalence of type 2 diabetes among MAFLD populations globally and by region. Table S10. Univariable and multivariable meta-regression analyses on the prevalence of type 2 diabetes among patients with MAFLD. Table S11. Characteristics of the included studies in the incidence density of type 2 diabetes among NAFLD patients. Table S12. Characteristics of studies reporting the incidence density of type 2 diabetes in patients with NAFLD: source of heterogeneity. Table S13. The incidence density of type 2 diabetes among patients with NAFLD-stratified by age, region, publication year, sample size and diagnosis of NAFLD (excluding index and hospital record of NAFLD). Table S14. Average annual percent change (%) in the incidence density of type 2 diabetes among NAFLD populations globally and by region. Table S15. Univariable and multivariable meta-regression analyses on the incidence density of type 2 diabetes among patients with NAFLD. Table S16. Characteristics of the include studies in the incidence density of type 2 diabetes among MAFLD patients. Table S17. Characteristics of studies reporting the incidence density of type 2 diabetes in patients with MAFLD: source of heterogeneity. Table S18. The incidence density of type 2 diabetes among patients with MAFLD.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cao, L., An, Y., Liu, H. et al. Global epidemiology of type 2 diabetes in patients with NAFLD or MAFLD: a systematic review and meta-analysis. BMC Med 22, 101 (2024). https://doi.org/10.1186/s12916-024-03315-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03315-0