Abstract
Background
The reconstructive tracheal options for extensive lesions still remain limited and although a valid substitute is required unfortunately, the biomechanical tracheal characteristics do not allow an easy replacement. In this study we reviewed the described options and investigated, in human cadaver model, whether thigh fascia can be used, as an alternative to forearm fascia, as recipient site for trachea graft heterotopical allotransplantation.
Anatomical study
In three fresh cadavers, 3 tracheal graft, 6 radial forearm (RF) fascial flaps and 6 antero-lateral thigh (ALT) fascial flaps were harvested. For each flap we simulated the heteretopical transplantation of the trachea in each fascial flap, and the harvesting of the composite graft as a free flap. The composite graft was finally decomposed at bench and the pedicle was injected to confirm fascial vascularization. The main measured outcomes were: flap fascia vascularization after pedicle injection, average time of flap harvesting, number of perforators included in ALT fascial flap and diameter of the vessels for anastomosis. Difficulties were noted, in order to compare RF flap and ALT flap.
Results
Fascia vascularization was confirmed in all cases by pedicle injection. The main difficulty with radial flap was to harvest the fascial layer due to its thinness and its strong adherence to palmaris longus tendon, while the main difficulty with ALT flap was to prevent any traction on the perforators. The average time of flap harvesting and graft inset (by a junior plastic surgeon) was 1 h and 30 min for radial forearm flap and 2 h and 10 min for ALT flap.
Conclusion
Despite many different techniques proposed in the literature, tracheal heterotopical allotransplantation still seemed the most promising, and ALT flap promised be a feasible alternative for heterotopical transplantation of trachea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reconstructive options of trachea still remain limited. Usually minor defects, less than half in adults and less than one third in children, can be treated with segmental resection and anastomosis, while extensive lesions need substitute tissues [1, 2].
Tracheal allotransplantation was successfully described by Delaere in 6 cases [3]. Since the trachea lacks an identifiable vascular pedicle, the technique applied a reconstructive method called prelamination, first described in 1994 by Pribaz and Fine [4], and often used for total nasal or ear reconstruction. According to Delaere’s procedure, firstly the donor trachea is heterotopically transplanted in a radial forearm (RF) fascial flap, and then the composite free flap is transferred, as a vascular unit to replace the tracheal defect, after waiting for at least three months in order to determine the revascularization of the graft from surrounding tissues [3].
Our report investigates the preliminary results of a possible alternative technique for vascularized tracheal allotransplantation. Thus, human cadaver models were used to evaluate whether antero-lateral thigh (ALT) fascia could be used, instead of RF fascia, as recipient site for trachea graft in the heterotopical allotransplantation framework.
Evolution of operative techniques
The ideal substitute should have particular features: 1) resistent to dislocation and stenosis; 2) flexible longitudinally, 3) limiting the accumulation of secretions and bacteria [1].
Grillo analyzed and classified various tracheal substitutes into four groups: 1) foreign materials, 2) tissue engineered parts, 3) autologous tissues, 4) tracheal transplantation [5].
Different foreign materials have been used in animal models to replace the trachea, such as titanium mesh, polyurethane and polytetrafluoroethylene (PTFE), a non-biodegradable polymer used widely in reconstructive vascular surgery [6,7,8]. Unfortunately, complications have been registered such as emigration, infection and extrusion due to the use of these non-resorbable materials.
Tissue engineering attempted to find an organic solution to reliably replace the trachea [9,10,11]. Recently, Scierski implanted a cylindrical tracheal implant, made of polymers containing various forms of carbon fibers, impregnated with polysulfone (PSU), in 10 ovines [12]. After 24 weeks it was found that vascularized connective tissue covered the inner surfaces, whilst another type of tissue, which resembled histological structure of normal tracheal wall, covered the outer surfaces.
Autologous tissues have been used as grafts and as flaps; multiple tissues were used as grafts, such as bronchial patches, dermis, pericardium, and aortic grafts. In 2000, Martinod implanted a thoracic aortic patch with temporary stenting, achieving metaplasia of the aortic tissue, and introducing a new era of in situ regeneration for tissue engineered trachea replacement [13].
Prelamination method, first described in 1994 by Pribaz and Fine [4], was applied to tracheal reconstruction. This was a staged procedure in which additional tissue (a cartilage graft) was shaped and added to an existing flap (radial flap or omentum flap), and, after revascularization of the graft from surrounding tissues (about 3 weeks), the composite free flap was transferred as a vascular unit to replace the tracheal defect [2].
The donor trachea was used to replace the original trachea as a non-vascularized graft, without encouraging results. In fact, mucosa was identified as the main antigenic source by Beigel et al. [14] and Bujia et al. [15]. Experimental trials involving de-epithelization, merthiolate treatment and cryopreservation to inhibit immunogenicity, while maintaining structural integrity, resulted in failures. Although rejection was avoided, the failure has been due to fibrosis, stenosis or necrosis [2].
The vascular supply to trachea has different sources from the thyroid, esophagus, pharynx, larynx and other surrounding tissue, this pattern does not allow performing a standard vascularized organ transplantation. In a relatively recent study on swine model [16], the trachea was harvested with the surrounding organs and heterotopically transplanted as a visceral compartment supplied from branches of the carotid artery and internal jugular vein. The animals were sacrificed after 14 days and the graft appeared well vascularized and healthy.
Nowadays, investigators mainly focused on indirect revascularization of donor trachea by prelamination. For these purposes, donor trachea was wrapped in a soft tissue flap, after revascularization of the graft from surrounding tissues (about 3 weeks), the prelaminated composite free flap was transferred [3, 17]. These methods have produced encouraging results, but they were time-consuming processes due to the staged nature of the grafting.
Xu et al. [17] used the greater omentum as a tracheal transplantation carrier in 3 patients. The main disadvantage of the procedure was the need for abdominal surgery.
Delaere et al. [3] reported 6 successful transplantations with a three-step procedure:
firstly, a heterotopical transplantation of the donor trachea in the recipient forearm fascial flap was done with concomitant administration of immunosuppression therapy; secondly, after 3 months, the graft was revascularized from surrounding tissues and donor respiratory mucosa was completely regenerated, then donor respiratory epithelium was replaced with recipient buccal mucosa graft. Finally, after ingrowth of the recipient mucosal graft, the orthotopic transplantation of the composite graft as a free flap could be performed while the immunosuppression therapy was gradually stopped.
The main advantage of this procedure was the limited administration of immunosuppression therapy compared to the other composite tissue allotransplantations (CTA). In fact, Delaere described [18] the withdrawal of immunosuppression in a safe manner and some tricks such as the second procedure of removal of donor respiratory epithelium and replacement with recipient buccal mucosa graft. These allowed all immunogenic non-cartilagineous tissues to be gradually replaced by cells from recipient mucosa, the only permanent donor tissue was the cartilage, not causing allorejection.
At present, indirect revascularization of donor trachea seemed to be the best clinical option.
Anatomical study
The study was conducted at the Anatomy Laboratory of the “Centre Hospitalier Universitaire” in Liege, Belgium, three fresh cadavers were used.
In each cadaver a tracheal segment, composed by 10 to 12 rings, was harvested. Then two RF fascial flaps (6 in total) and two ALT fascial flaps (6 in total) were harvested by the same junior plastic surgeon.
All trachea grafts were healthy and not calcified, each graft was used to simulate heterotopical transplantation 4 times, for the 4 flaps of the cadaver.
For each flap, we simulated the heterotopical transplantation of the trachea graft in the fascial flap, then the composite graft as a free flap was harvested. The composite graft was finally decomposed at bench and the pedicle was injected.
Fascial vascularization after pedicle injection, average time of flap harvesting, number of perforators included in ALT fascial flap, diameter of the vessels for anastomosis and notes on the difficulties were collected.
Harvesting technique
To harvest the tracheal graft, a skin incision was made between the two mastoid processes, passing above the jugular notch. A flap of skin and platysma was elevated to the lower border of the mandible. Dissection of sternohyoid and sternothyroid muscles exposed the laryngo-tracheal complex. Trachea was then separated from esophagus posteriorly and thyroid anteriorly. A 10 rings segment in 1 cadaver and 12 rings segments in 2 cadavers were harvested. The trachea was then treated at bench, removing the membranous part and the anterior pre-tracheal fascia. The inner mucosa was left attached.
The RF fascial flap was harvested as described by Delaere [3]. Trachea was then positioned on the forearm and wrapped with the fascia. Trachea was fixed at both margins to the skin with sutures to maintain its length. Skin was then sutured to tracheal edges.
To simulate the harvest of the composite graft a random rectangular skin flap, as long as the graft and wide as the posterior tracheal wall, to reconstruct was drawn laterally or medially to one edge of the composite graft. The flap was elevated, rotated in order to reconstruct the tracheal posterior wall and sutured to the other edge. The composite flap was then dissected within radial pedicle.
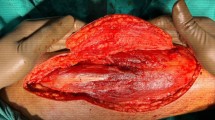
ALT fascial flap was harvested with a similar technique. A skin incision was made medially to the cutaneous projection of the intermuscular septum, between rectus femoralis and vastus lateralis muscles, described by a line from the anterior superior iliac spine to the patella lateral margin. The incision was made through the skin and subcutaneous tissue until the fascia; supra-fascial dissection was then performed. All perforators were identified on the supra-fascial layer and ligated distally, this made the later identification in the sub-fascial layer very easy. An incision was made through the fascia on the medial aspect of the thigh and the dissection continued laterally in the subfascial plane, preserving all possible perforators. The intermuscular septum between vastus lateralis and rectus femoralis muscles was then opened to identify the descending branch of the lateral circumflex femoral artery and the origin of the perforators. The best possible perforator was then selected and marked. The fascia was incised in a rectangular shape, centered on the selected perforator; the dimension was set on that of the tracheal graft (Fig. 1). The lateral edges of the fascia were entirely incised, but not the cranial and caudal ones to avoid any possible tension on the vessels (Fig. 2). The fascial flap was wrapped around the trachea and the lateral margins were then sutured to tracheal edges (Fig. 3). Superior and inferior boarders of the trachea were also fixed to the skin with sutures, to maintain the graft length (Fig. 4). The margins of skin incision were then sutured to trachea in order to obtain the same result of forearm in setting (Fig. 5) and thigh incision was sutured.
To simulate the harvest of the composite graft, a random rectangular skin flap, as long as the graft and wide as the posterior tracheal wall, to reconstruct was drawn laterally or medially to one edge of the composite graft. The flap was elevated, rotated in order to reconstruct the tracheal posterior wall and sutured to the other edge.
The fascia was then completely detached and the septum was opened to isolate the vascular pedicle until its origin.
Our experience
In all cases we obtained a composite graft of trachea and fascia and fascia vascularization was confirmed by pedicle injection.
The main problem encountered during RF dissection was the difficulty to harvest the fascial flap due to the thinness of the fascia and its strong adherence to palmaris longus tendon. However, ALT fascia was clearly thicker and easier to dissect, while the main problem encountered during ALT flap dissection was to prevent any traction on the perforators.
In 2 ALT flaps we found 2 perforators on the same vertical axis, and preserved them both.
The average time of flap harvesting and graft inset was 1 h and 30 min for radial forearm flap and 2 h and 10 min for ALT flap.
The average diameter of the artery for anastomosis was 2.5 mm (range 2 to 3 mm) for ALT flap and 3 mm (range: 2.0 to 4.0 mm) for RF flap. In 3 of the 6 ALT flaps, the 2 venae comitantes joined in one larger vein, the average diameter of the vein for anastomosis was 2 mm (range 2 to 3.5 mm) for ALT flap and 1.7 mm (range: 1.5 to 2 mm) for RF flap.
Discussion
The aim of our work was to prove the technical feasibility of preparing the composite graft using the ALT fascial flap as a carrier, instead of RF fascial flap.
The thinness of RF fascia and its strong adherence with the palmaris longus tendon determine a difficult dissection. This issue might be partly solved by harvesting the fascial flap quite distant from the elbow, in order to find the muscular part of the palmaris longus instead of its tendinous part.
ALT fascia flap was harvested easily in all reported cases. Fascia is very strong and easy to dissect, due to its low adherence to subcutaneous and muscular planes. The pedicle could be only visualized inside the intermuscular septum, without dissecting it, in order to reduce vascular damage due to tension on the pedicle. Leaving the fascia partially attached on the axis of the perforators allowed reduction of tension on the perforators, while the sutures between trachea and skin reduced the tension on the vascular pedicle, maintaining the own length. The well-known problem linked to vascular anatomy of variability in ALT flap could be solved by preoperative angio-tomography study. Graft harvesting was quite simple and harvesting time difference between the two methods (average 40 min) was acceptable. In both methods the pedicle was long enough to safely perform.
anastomoses to neck vessels without tension, furthermore the vessels caliber were adequate in all flaps.
The use of ALT flap in-vivo could be a solution to the well-known drawbacks of the RF donor site - sacrifice of a major artery of the limb, the unpleasant visible scar in the forearm region and possible scar related symptoms such as pain and numbness [19]. The comparison between these two flaps and the superiority of perforator flaps about donor site morbidity is well known [20]. Moreover, according to Delaere’s procedure, the patient will have the heterotopic trachea allograft in place for at least three months, before its transfer, and we would like to highlight that a wound and a dressing on the thigh is much easier to live with and less disturbing, than on the forearm.
Conclusion
Despite many different techniques proposed in the literature, tracheal heterotopical allotransplantation still seems the most promising, and ALT seems to be a feasible alternative for heterotopical transplantation of trachea.
Change history
21 July 2022
Missing Open Access funding informati on has been added in the Funding Note.
References
Abouarab AA, Elsayed HH, Elkhayat H, Mostafa A, Cleveland DC, Nori AE. Current solutions for long-segment tracheal reconstruction. Ann Thorac Cardiovasc Surg. 2017;23:66–75. https://doi.org/10.5761/atcs.ra.16-00251.
Kucera KA, Doss AE, Dunn SS, Clemson LA, Zwischenberger JB. Tracheal replacements: part 1. ASAIO J. 2007;53:497–505. https://doi.org/10.1097/MAT.0b013e3180616b5d.
Delaere P, Van Raemdonck D, Vranckx J. Tracheal transplantation. Intensive Care Med. 2019;45:391–3. https://doi.org/10.1007/s00134-018-5445-9.
Pribaz JJ, Fine NA. Prelamination: defining the prefabricated flap--a case report and review. Microsurgery. 1994;15:618–23. https://doi.org/10.1002/micr.1920150903.
Grillo HC. In: surgery of the Trachea and bronchi: tracheal replacement. Hamilton, ON, Canada: BC Decker Inc; 2004. p. 839–54.
Schultz P, Vautier D, Charpiot A, Lavalle P, Debry C. Development of tracheal prostheses made of porous titanium: a study on sheep. Eur Arch Otorhinolaryngol. 2007;264:433–8. https://doi.org/10.1007/s00405-006-0195-7.
Schauwecker HH, Gerlach J, Planck H, Bücherl ES. Isoelastic polyurethane prosthesis for segmental trachea replacement in beagle dogs. Artif Organs. 1989;13:216–8. https://doi.org/10.1111/j.1525-1594.1989.tb02866.x.
Cull DL, Lally KP, Mair EA, Daidone M, Parsons DS. Tracheal reconstruction with polytetrafluoroethylene graft in dogs. Ann Thorac Surg. 1990;50:899–901. https://doi.org/10.1016/0003-4975(90)91116-s.
Edgerton MT, Zovickian A. Reconstruction of the trachea and infraglottic larynx. Plast Reconstr Surg (1946). 1954;13:167–192. https://doi.org/10.1097/00006534-195403000-00003.
Spinazzola AJ, Graziano JL, Neville WE. Experimental reconstruction of the tracheal carina. J Thorac Cardiovasc Surg. 1969;58:1–13.
Aronstam EM, Nims RM, Winn DF Jr. Studies in segmental replacement of the thoracic trachea. J Surg Res. 1961;1:108–10. https://doi.org/10.1016/s0022-4804(61)80005-x.
Ścierski W, Lisowska G, Namysłowski G, et al. Reconstruction of ovine trachea with a biomimetic composite biomaterial. Biomed Res Int. 2018. https://doi.org/10.1155/2018/2610637.
Martinod E, Seguin A, Pfeuty K, et al. Long-term evaluation of the replacement of the trachea with an autologous aortic graft. Ann Thorac Surg. 2003;75:1572–8. https://doi.org/10.1016/s0003-4975(03)00120-6.
Beigel A, Steffens-Knutzen R, Müller B, Schumacher U, Stein H. Tracheal transplantation. III. Demonstration of transplantation antigens on the tracheal mucosa of inbred rat strains. Arch Otorhinolaryngol. 1984;241:1–8. https://doi.org/10.1007/BF00457910.
Bujia J, Wilmes E, Hammer C, Kastenbauer E. Tracheal transplantation: demonstration of HLA class II subregion gene products on human trachea. Acta Otolaryngol. 1990;110:149–54. https://doi.org/10.3109/00016489009122530.
Jacobson AS, Roden DF, Lee EQ, et al. Tracheal replacement revisited: use of a vascularized tracheal transplant in a porcine model. Laryngoscope. 2018;128:S1–9. https://doi.org/10.1002/lary.27671.
Xu L, Zhang S, Li J, et al. Human tracheal allotransplant with greater omentum for revascularization. Exp Clin Transplant. 2014;12:448–53.
Delaere P, Vranckx J, Verleden G, De Leyn P, Van Raemdonck D. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med. 2010;362:138–45. https://doi.org/10.1056/NEJMoa0810653.
Timmons MJ, Missotten FE, Poole MD, Davies DM. Complications of radial forearm flap donor sites. Br J Plast Surg. 1986;39:176–8. https://doi.org/10.1016/0007-1226(86)90078-0.
Huang C-H, Chen H-C, Huang Y-L, Mardini S, Feng G-M. Comparison of the radial forearm flap and the thinned anterolateral thigh cutaneous flap for reconstruction of tongue defects: an evaluation of donor-site morbidity. Plast Reconstr Surg. 2004;114:1704–10. https://doi.org/10.1097/01.prs.0000142476.36975.07.
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement. No funding was obtained for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics committee approval
EC approval not applicable as it is a review article.
Informed consent
Not applicable being a review article and no patient/cadaver identifiable material is being presented.
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
All procedures performed in this study involving human participants (cadavers) were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sgarzani, R., Meccariello, G., Iannella, G. et al. Tracheal reconstruction: mini-review and anatomical study on the use of antero lateral thigh fascial flap for heterotopical transplantation. Indian J Thorac Cardiovasc Surg 38, 506–510 (2022). https://doi.org/10.1007/s12055-022-01354-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-022-01354-x