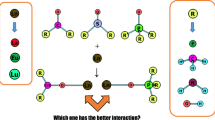
Abstract
Nucleophilicity and CO2 fixation ability of the ylides of phosphorous, sulfur and nitrogen have been assessed using DFT calculations at M06-2X/6-311++G(d,p)//M06-2X/6-31+G(d) level. Nucleophilicity of 54 ylides and their CO2 adducts have been evaluated using a theoretical nucleophilicity index (N) which shows good linear correlation (R2 = 0.87) with Mayr’s experimental nucleophilicity parameter for the S-ylides. Two key geometrical parameters, condensed Fukui function (fk−) and Gibbs free energy of reaction (ΔGr) for the ylide-CO2 adducts have also been calculated. In absence of steric effect around the ylidic C-atom and any other secondary interaction, binding energy of the CO2-adducts of all the three types of ylides has a good linear correlation with the nucleophilicity. Nucleophilicity of a P-ylide increases when electron-donating groups are introduced as substituents on both P and the ylidic C-atom. An electron-withdrawing group on the same sites reduces nucleophilicity. Free ylides have higher nucleophilicity than their CO2 adducts.
Graphic abstract










Similar content being viewed by others
References
Beach R H, Sulser T B, Crimmins A, Cenacchi N, Cole J, Fukagawa N K, Mason-D’Croz D, Myers S, Sarofim M C, Smith M and Ziska L H 2019 Combining the effects of increased atmospheric carbon dioxide on protein, iron, and zinc availability and projected climate change on global diets: a modelling study Lancet Planet. Heal. 3 e307
Fiorani G, Guo W and Kleij A W 2015 Sustainable conversion of carbon dioxide: The advent of organocatalysis Green Chem. 17 1375
Riduan S N and Zhang Y 2010 Recent developments in carbon dioxide utilization under mild conditions Dalton Trans. 39 3347
Liu Q, Wu L, Jackstell R and Beller M 2015 Using carbon dioxide as a building block in organic synthesis Nat. Commun. 6 5933
Yang L and Wang H 2014 Recent advances in carbon dioxide capture, fixation, and activation by using N-heterocyclic carbenes ChemSusChem 7 962
Appel A M, Bercaw J E, Bocarsly A B, Dobbek H, DuBois D L, Dupuis M, et al. 2013 Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation Chem. Rev. 113 6621
Wang S and Xi C 2019 Recent advances in nucleophile-triggered CO2-incorporated cyclization leading to heterocycles Chem. Soc. Rev. 48 382
Leitner W 1996 The coordination chemistry of carbon dioxide and its relevance for catalysis: A critical survey Coord. Chem. Rev. 153 257
Roy L, Ghosh B and Paul A 2017 Lewis acid promoted hydrogenation of CO2 and HCOO– by amine boranes: mechanistic insight from computational approach J. Phys. Chem. A 121 5204
Zhou H and Lu X 2017 Lewis base-CO2 adducts as organocatalysts for CO2 transformation Sci. Chin. Chem. 60 904
van Ausdall B R, Glass J L, Wiggins K M, Aarif A M and Louie J 2009 A systematic investigation of factors influencing the decarboxylation of imidazolium carboxylates J. Org. Chem. 74 7935
Wang Y B, Wang Y M, Zhang W Z and Lu X B 2013 Fast CO2 sequestration, activation, and catalytic transformation using N-heterocyclic olefins J. Am. Chem. Soc. 135 11996
Zhou H, Wang G X, Zhang W Z and Lu X B 2015 CO2 Adducts of phosphorus ylides: highly active organocatalysts for carbon dioxide transformation ACS Catal. 5 6773
Ajitha M J and Suresh C H 2012 Assessment of stereoelectronic factors that influence the CO2 fixation ability of N-heterocyclic carbenes: A DFT study J. Org. Chem. 77 1087
Anila S and Suresh C H 2021 Guanidine as a strong CO2 adsorbent: a DFT study on cooperative CO2 adsorption Phys. Chem. Chem. Phys. 23 13662
Crocker R D and Nguyen T V 2016 The resurgence of the highly ylidic N-heterocyclic olefins as a new class of organocatalysts Chem. Eur. J. 22 2208
Dong L, Wen J and Li W 2015 A theoretical investigation of substituent effects on the stability and reactivity of N-heterocyclic olefin carboxylates Org. Biomol. Chem. 13 8533
Li W, Yang N and Lyu Y 2016 Theoretical insights into the catalytic mechanism of N-heterocyclic olefins in carboxylative cyclization of propargyl alcohol with CO2 J. Org. Chem. 81 5303
Domingo L R and Pérez P 2011 The nucleophilicity N index in organic chemistry Org. Biomol. Chem. 9 7168
Domingo L R, Chamorro E and Pérez P 2008 Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study J. Org. Chem. 73 4615
Domingo L R and Sáez J A 2009 Understanding the mechanism of polar Diels-Alder reactions Org. Biomol. Chem. 7 3576
Matthews C N, Driscoll J S and Birum G H 1966 Mesomeric phosphonium inner salts Chem. Commun. 736
Sabet-Sarvestani H, Izadyar M, Eshghi H and Noroozi-Shad N 2018 Understanding the thermodynamic and kinetic performances of the substituted phosphorus ylides as a new class of compounds in carbon dioxide activation Energy 145 329
Sabet-Sarvestani H, Izadyar M and Eshghi H 2017 Phosphorus ylides as a new class of compounds in CO2 activation: Thermodynamic and kinetic studies J. CO2 Util. 21 459
Geerlings P, De Proft F and Langenaeker W 2003 Conceptual density functional theory Chem. Rev. 103 1793
Domingo L R, Ríos-Gutiérrez M and Pérez P 2016 Applications of the conceptual density functional theory indices to organic chemistry reactivity Molecules 21 748
Chattaraj P K, Sarkar U and Roy D R 2006 Electrophilicity index Chem. Rev. 106 2065
Jupp A R, Johnstone T C and Stephan D W 2018 The global electrophilicity index as a metric for Lewis acidity Dalton Trans. 47 7029
Roy R K, Krishnamurti S, Geerlings P and Pal S 1998 Local softness and hardness based reactivity descriptors for predicting intra- and intermolecular reactivity sequences: Carbonyl compounds J. Phys. Chem. A 102 3746
Chattaraj P K, Maiti B and Sarkar U 2003 Philicity: A unified treatment of chemical reactivity and selectivity J. Phys. Chem. A 107 4973
Contreras R, Andrés J, Safont V S, Campodonico P and Santos J G 2003 A theoretical study on the relationship between nucleophilicity and ionization potentials in solution phase J. Phys. Chem. A 107 5588
Chattaraj P K, Duley S and Domingo L R 2012 Understanding local electrophilicity/nucleophilicity activation through a single reactivity difference index Org. Biomol. Chem. 10 2855
Berger G 2013 Using conceptual density functional theory to rationalize regioselectivity: A case study on the nucleophilic ring-opening of activated aziridines Comput. Theor. Chem. 1010 11
Yang W and Mortier W J 1986 The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines J. Am. Chem. Soc. 108 5708
Deuri S and Phukan P 2012 A DFT study on nucleophilicity and site selectivity of nitrogen nucleophiles Comput. Theor. Chem. 980 49
Deuri S and Phukan P 2012 A stepwise mechanism for the uncatalyzed Michael addition of acetylacetone to methyl vinyl ketone Int. J. Quantum Chem. 112 801
Baruah B, Deuri S and Phukan P 2014 Reactivity and regioselectivity in the ring opening of 2-substituted non-activated aziridines: A density functional theory based analysis Comput. Theor. Chem. 1027 197
Deka K and Phukan P 2016 DFT analysis of the nucleophilicity of substituted pyridines and prediction of new molecules having nucleophilic character stronger than 4-pyrrolidino pyridine J. Chem. Sci. 128 633
Deuri S and Phukan P 2010 A density functional theory study on π-nucleophilicity and electron-transfer oxidation of silyl enol ethers and ketene silyl acetals J. Mol. Struct.: THEOCHEM 945 64
Pathak D, Deuri S and Phukan P 2016 Theoretical insights on the interaction of N-heterocyclic carbenes with tetravalent silicon reagents J. Phys. Chem. A 120 128
Appel R, Hartmann N and Mayr H 2010 Scope and limitations of cyclopropanations with sulfur ylides J. Am. Chem. Soc. 132 17894
Appel R and Mayr H 2010 Nucleophilic reactivities of sulfur ylides and related carbanions: Comparison with structurally related organophosphorus compounds Chem. Eur. J. 16 8610
Petz W, Köhler K, Mörschel P and Neumüller B 2005 The coordination chemistry of the ylide adduct O2CC(PPh3)2; preparation, crystal structures, and spectroscopic properties of [Cl3In{O2CC(PPh3)2}], [Cl2Sn{O2CC(PPh3)2}], and [I2In{O2CC(PPh3)2}2]I Zeitschrift Anorg. Allg. Chemie. 631 1779
Becke A 1993 Density functional thermochemistry iii the role of exact exchange J. Chem. Phys. 98 5648
Lee C, Yang W and Parr R G 1988 Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density Phys. Rev. B 37 785
Lynch B J, Fast P L, Harris M and Truhlar D G 2000 Adiabatic connection for kinetics J. Phys. Chem. A 104 4811
Lynch B J, Zhao Y and Truhlar D G 2003 Effectiveness of diffuse basis functions for calculating relative energies by density functional theory J. Phys. Chem. A 107 1384
Zhao Y and Truhlar D G 2008 The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function Theor. Chem. Acc. 120 215
Zhao Y and Truhlar D G 2008 Density functionals with broad applicability in chemistry Acc. Chem. Res. 41 157
Ditchfield R, Hehre W J and Pople J A 1971 Self-consistent molecular-orbital methods. IX. An extended gaussian-type basis for molecular-orbital studies of organic molecules J. Chem. Phys. 54 724
Hehre W J, Ditchfield K and Pople J A 1972 Self-consistent molecular orbital methods. XII. Further extensions of gaussian-type basis sets for use in molecular orbital studies of organic molecules J. Chem. Phys. 56 2257
Hariharan P C and Pople J A 1974 Accuracy of AHn equilibrium geometries by single determinant molecular orbital theory Mol. Phys. 27 209
Gordon M S 1980 The isomers of silacyclopropane Chem. Phys. Lett. 76 163
Hariharan P C and Pople J A 1973 The influence of polarization functions on molecular orbital hydrogenation energies Theor. Chim. Acta 28 213
Blaudeau J P, McGrath M P and Curtiss L A and Radom L1997Extension of Gaussian-2 (G2) theory to molecules containing third-row atoms K and Ca J. Chem. Phys. 107 5016
Francl M M and Pietro W J, Hehre W J, Binkley J S, Gordon M S, DeFrees D J and Pople J A 1982 Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements J. Chem. Phys. 77 3654
Binning R C and Curtiss L A 1990 Compact contracted basis sets for third-row atoms: Ga–Kr J. Comput. Chem. 11 1206
Rassolov V A, Pople J A, Ratner M A and Windus T L 1998 6–31G* basis set for atoms K through Zn J. Chem. Phys. 109 1223
Rassolov V A, Ratner M A, Pople J A, Redfern P C and Curtiss L A 2001 6–31G* basis set for third-row atoms J. Comput. Chem. 22 976
Tomasi J, Mennucci B and Cammi R 2005 Quantum mechanical continuum solvation models Chem. Rev. 105 2999
Hirshfeld F L 1977 Bonded-atom fragments for describing molecular charge densities Theor. Chim. Acta 44 129
Kolandaivel P, Praveena G and Selvarengan P 2005 Study of atomic and condensed atomic indices for reactive sites of molecules J. Chem. Sci. 117 591
Dennington R D, Keith T A and Millam J M 2008 GaussView 5.0.8, Gaussian
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji H, Caricato M, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G, Sonnenberg J L, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J A Jr, Peralta J E, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J C, Iyengar S S, Tomasi J, Cossi M, Rega N, Millam J M, Klene M, Knox J E, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador P, Dannenberg J J, Dapprich S, Daniels A D, Farkas O, Foresman J B, Ortiz J V, Cioslowski J, and Fox D J, Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford CT, 2009
Acknowledgements
Financial support from SERB, India (Grant No. EMR/2016/007883) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pathak, D., Deuri, S. & Phukan, P. Nucleophilicity and CO2 fixation ability of phosphorus, nitrogen and sulfur ylides: insights on stereoelectronic factors from DFT study. J Chem Sci 133, 127 (2021). https://doi.org/10.1007/s12039-021-01983-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-021-01983-6