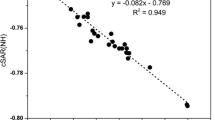
Abstract
Some commonly used 3-substituted, 4-substituted and 3,4,5-substituted pyridines were examined using DFT to predict the nucleophilicity behavior based on four different methods known in the literature. HOMO-LUMO energy calculations were done using DFT/B3LYP/6-311G + (d,p) level of theory. To establish the most suitable nucleophilicity scale for all the ranges of pyridines covered herein, either Hammett substituent constant (σ) or experimental nucleophilicity values were computed. On the basis of this study, some new 4-substituted pyridines with enhanced nucleophilicity have been proposed. Nucleophilic behaviour of a few predicted molecules was found to be better than that of 4-pyrrolidino pyridine.

Four methods were applied for predicting nucleophilicity of substituted pyridines. Good linear regression coefficient was achieved particularly for 4-substituted pyridines. Based on the outcome of the study, some new molecules having nucleophilic character stronger than 4-pyrrolidino pyridine are predicted.










Similar content being viewed by others
References
Ramsay W 1876 Phil. Mag. 2 269
Wu W, Kong H, Li H, Ho Y, Gao Y, Hao J, Murphy M B, Lam M H, Wong K and Lee C 2011 Eur. J. Org. Chem. 26 5054
Wu J, Kang S, Song B, Hu D, He M, Jin L and Yang S 2012 Chem. Central J. 62 8
Di X, Liu Y, Liu Y, Yu X, Xiao H, Tian X and Gao R 2007 Pesticide Biochem. Physiol. 89 81
Dorn F, Pfiffner A and Schlageter M 1991 ACS Symposium Series 443 506
Lee L F, Stikes G L, Normansell J E, Molyneaux J M, Sing L Y L, Chupp J P, Parrish S K and Kaufmann J E 1991 ACS Symposium Series 443 195
Peine G, Hoffmann P, Seifert G and Schilling G 1985 Biochem. Physiol. Pflanzen 180 1
Harris R L N, Huppatz J L, Phillips J N and Teitei T 1979 In Advances in Pesticide Science H Geissbühler, G T Brooks and P C Kearney (Eds.) (Oxford: Pergamon) p. 99
Willand-Charnley R, Fisher T J, Johnson B M and Dussault P H 2012 Org. Lett. 14 2242
Zhang Y, Zhang Y, Sun Y L, Du X, Shi J Y, Wang W D and Wang W 2012 Chem. Eur. J. 18 6328
Ko K, Nakano K, Watanabe S, Ichikawa Y and Kotsuki H 2009 Tetrahedron Lett. 50 4025
Xu X, Tang Z, Wang Y, Luo S, Cun L and Gong L 2007 J. Org. Chem. 72 9905
List B 2007 Chem. Rev. 107 5413
Cheong P H -Y, Legault C Y, Um J M, Cȩlebi-Ołcu̧m N and Houk K N 2011 Chem. Rev. 111 5042
De Rycke N, Couty F and David O R P 2011 Chem. Eur. J. 17 12852
Geerlings P, De Proft F and Langenaeker W 2003 Chem. Rev. 103 1793
Ingold C K 1934 Chem. Rev. 15 225
Swain C G and Scott C B 1953 J. Am. Chem. Soc. 75 141
Edwards J O 1954 J. Am. Chem. Soc. 76 1540
Edwards J O and Pearson R G 1962 J. Am. Chem. Soc. 84 16
Bunnett J F 1963 Ann. Rev. Phys. Chem. 14 271
Pearson R G, Sobel H and Songstad J 1968 J. Am. Chem. Soc. 90 319
Legon A C and Millen D J 1987 J. Am. Chem. Soc. 109 356
Legon A C and Millen D J 1987 Acc. Chem. Res. 20 39
Brotzel F, Kempf B, Singer T, Zipse H and Mayr H 2007 Chem. Eur. J. 13 336
De Rycke N, Berionni G, Couty F, Mayr H, Goumont R and David O R P 2011 Org. Lett. 13 530
Campodonico P, Santos J G, Andres J and Contreras R 2004 J. Phys. Org. Chem. 17 273
Contreras R, Andres J, Safont V S, Campodonico P and Santos J G 2003 J. Phys. Chem. A 107 5588
Chattaraj P K and Maiti B 2001 J. Phys. Chem. A 105 169
Pratihar S and Roy S 2010 J. Org. Chem. 75 4957
Pratihar S and Roy S 2011 Organometallics 30 3257
Domingo L R and Pérez P 2011 Org. Biomol. Chem. 9 7168
Campodónico P R and Aizman A 2006 Chem. Phys. Lett. 422 204
Deuri S and Phukan P 2012 Comput. Theor. Chem. 980 49
Soliman S M 2012 Comput. Theor. Chem. 994 105
Ess D H, Jones G O and Houk K N 2006 Adv. Synth. Catal. 348 2337
Chattaraj P K, Sarkar U and Roy D R 2006 Chem. Rev. 106 2065
Chattaraj P K and Roy D R 2007 Chem. Rev. 107 PR46
Chattaraj P K, Giri S and Duley S 2011 Chem. Rev. 111 PR43
Parr R G and Pearson R G 1983 J. Am. Chem. Soc. 105 7512
Parr R G, Von Szentpaly L and Liu S 1999 J. Am. Chem. Soc. 121 1922
Parr R G, Donnelly R A, Levy M and Palke W E 1978 J. Chem. Phys. 68 3801
Gázquez J L, Cedillo A and Vela A 2007 J. Phys. Chem. A 111 1966
Domingo L R, Chamorro E and Perez P 2008 J. Org. Chem. 73 4615
Kohn W and Sham L J 1965 Phys. Rev. 140 1133
Becke A D 1993 J. Chem. Phys. 98 5648
Lee C, Yang W and Parr R G 1988 Phys. Rev. B 37 785
Cohen A, Mori-Sánchez P and Yang W 2012 Chem. Rev. 112 289
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Zakrzewski V G, Montgomery J A, Stratmann R E, Burant J C, Dapprich S, Millam J M, Daniels A D, Kudin K N, Strain M C, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson G A, Ayala P Y, Cui Q, Morokuma K, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Cioslowski J, Ortiz J V, Baboul A G, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin R L, Fox D J, Keith T, Al- Laham M A, Peng C Y, Nanayakkara A, Gonzalez C, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Andres J L, Gonzalez C, Head Gordon M, Replogle E S and Pople J A 2004 GAUSSIAN 03, Revision E.01, (Gaussian Inc.: Wallingford, CT)
Swain C G, Swain M S, Powell A L and Alunni S 1983 J. Am. Chem. Soc. 105 502
Um I -H, Lee E -J and Jeon S -E 2002 J. Phys. Org. Chem. 15 561
Kim Y, Cramer C J and Truhlar D G 2009 J. Phys. Chem. A 113 9109
Smith M B and March J 2007 In Advanced Organic Chemistry: Reactions, Mechanism and Structure 6 th edn., (Hoboken- New Jersey: John Wiley)
Acknowledgements
Financial support from UGC (Grant No. 41-206/2012/ SR) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information (SI)
Additional information pertaining to leave-one-out correlation and regression analysis by distribution of molecules into training and test sets is available in Supporting Information at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
DEKA, K., PHUKAN, P. DFT analysis of the nucleophilicity of substituted pyridines and prediction of new molecules having nucleophilic character stronger than 4-pyrrolidino pyridine. J Chem Sci 128, 633–647 (2016). https://doi.org/10.1007/s12039-016-1057-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1057-5