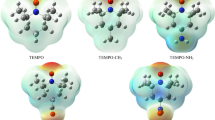
Abstract
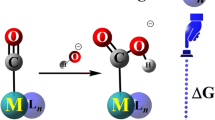
The Lewis acidity of methyltrioxorhenium (MTO) and its monoperoxo [A: MeReO2(η 2–O2)] and bisperoxo [B.H 2 O: MeReO(η 2–O2)2(H2O)] derivatives toward pyridine and its derivatives has been theoretically investigated. These compounds, presented in MTO/H2O2 system as one of the most versatile and useful systems, are believed to be active catalysts in oxidation processes. The excellent linear relationships were observed between the equilibrium constants (for the reactions MTO + N = MTO.N, A + N = A.N, and B.H 2 O + N = B.N + H2O; N = pyridine and its derivatives) and either the pK a values of the pyridine and its derivatives or the Hammett σ constants of the substituents on the pyridine. The slope of the plot of log K eq versus pyridines’ pK a and the Hammett ρ values suggests the acidity order as MTO < A < B.H 2 O.






Similar content being viewed by others
References
Mijs WJ, de Jonge CR (1986) Organic syntheses by oxidation with metal compounds. Plenum Publishing Corporation, New York
Jørgensen KA (1989) Transition-metal-catalyzed epoxidations. Chem Rev 89(3):431–458
Lane BS, Burgess K (2003) Metal-catalyzed epoxidations of alkenes with hydrogen peroxide. Chem Rev 103(7):2457–2474
Mukaiyama T, Yamada T (1995) Recent advances in aerobic oxygenation. Bull Chem Soc Jpn 68(1):17–35
Sica D, Musumeci D, Zollo F, De Marino S (2001) Reactivity of steroidal dienes towards the methyltrioxorhenium/H2O2–urea oxidation system: isolation and characterization of new oxygenated steroids. Eur J Org Chem 19:3731–3739
Sica D, Musumeci D, Zollo F, De Marino S (2001) A new route to polyoxygenate C and D rings of steroids by oxidation, 14-diene steroid with the methyltrioxorhenium–H2O2–urea system. J Chem Soc Perkin Trans 1(16):1889–1896
Musumeci D, Sica D (2002) CH3ReO3-catalyzed oxidation of cholesta-5,7-dien-3β-yl acetate with the urea–hydrogen peroxide adduct under various conditions. Synthesis of the natural epoxy sterol 9α,11α-epoxy-5α-cholest-7-en-3β, 5,6β-triol. Steroids 67(7):661–668
Al-Ajlouni AM, Espenson JH (1995) Epoxidation of styrenes by hydrogen peroxide as catalyzed by methylrhenium trioxide. J Am Chem Soc 117(36):9243–9250
Al-Ajlouni AM, Espenson JH (1996) Kinetics and mechanism of the epoxidation of alkyl-substituted alkenes by hydrogen peroxide, catalyzed by methylrhenium trioxide. J Org Chem 61(12):3969–3976
Adam W, Saha-Möller CR, Weichold O (2000) Epoxidation of trans-cyclooctene by methyltrioxorhenium/H2O2: reaction of trans-epoxide with the monoperoxo complex. J Org Chem 65(16):5001–5004
Saladino R, Neri V, Checconi P, Celestino I, Nencioni L, Palamara AT, Crucianelli M (2013) Synthesis of 2′-deoxy-1′-homo-N-nucleosides with anti-influenza activity by catalytic methyltrioxorhenium (MTO)/H2O2 oxyfunctionalization. Chem Eur J 19(7):2392–2404
Qu S, Dang Y, Wen M, Wang ZX (2013) Mechanism of the methyltrioxorhenium-catalyzed deoxydehydration of polyols: a new pathway revealed. Chem Eur J 19(12):3827–3832
Herrmann WA, Fischer RW, Marz DW (1991) Methyltrioxorhenium as catalyst for olefin oxidation. Angew Chem Int Ed Engl 30(12):1638–1641
Owens GS, Arias J, Abu-Omar MM (2000) Rhenium oxo complexes in catalytic oxidations. Catal Today 55(4):317–363
Herrmann WA, Kühn FE (1997) Organorhenium oxides. Acc Chem Res 30(4):169–180
Espenson JH (1999) Atom-transfer reactions catalyzed by methyltrioxorhenium(VII)—mechanisms and applications. Chem Commun 6:479–488
Romão CC, Kühn FE, Herrmann WA (1997) Rhenium(VII) oxo and imido complexes: synthesis, structures, and applications. Chem Rev 97(8):3197–3246
Kühn FE, Scherbaum A, Herrmann WA (2004) Methyltrioxorhenium and its applications in olefin oxidation, metathesis and aldehyde olefination. J Organomet Chem 689(24):4149–4164
Herrmann WA, Fischer RW, Scherer W, Rauch MU (1993) Methyltrioxorhenium(VII) as catalyst for epoxidations: structure of the active species and mechanism of catalysis. Angew Chem Ed Engl 32(8):1157–1160
Herrmann WA, Fischer RW, Rauch MU, Scherer W (1994) Alkylrhenium oxides as homogeneous epoxidation catalysts: activity, selectivity, stability, deactivation. J Mol Catal 86(1):243–266
Adam W, Mitchell CM (1996) Methyltrioxorhenium(VII)-catalyzed epoxidation of alkenes with the urea/hydrogen peroxide adduct. Angew Chem Int Ed Engl 35(5):533–535
Boehlow TR, Spilling CD (1996) The regio- and stereo-selective epoxidation of alkenes with methyl trioxorhenium and urea–hydrogen peroxide adduct. Tetrahedron Lett 37(16):2717–2720
Rudolph J, Reddy KL, Chiang JP, Sharpless KB (1997) Highly efficient epoxidation of olefins using aqueous H2O2 and catalytic methyltrioxorhenium/pyridine: pyridine-mediated ligand acceleration. J Am Chem Soc 119(26):6189–6190
Copéret C, Adolfsson H, Sharpless KB (1997) A simple and efficient method for epoxidation of terminal alkenes. Chem Commun 16:1565–1566
Herrmann WA, Kratzer RM, Ding H, Thiel WR, Glas H (1998) Methyltrioxorhenium/pyrazole—a highly efficient catalyst for the epoxidation of olefins. J Organomet Chem 555(2):293–295
Adolfsson H, Converso A, Sharpless KB (1999) Comparison of amine additives most effective in the new methyltrioxorhenium-catalyzed epoxidation process. Tetrahedron Lett 40(21):3991–3994
Vaino AR (2000) Sodium percarbonate as an oxygen source for MTO catalyzed epoxidations. J Org Chem 65(13):4210–4212
Ferreira P, Xue W-M, Bencze É, Herdtweck E, Kühn FE (2001) Bidentate Lewis base adducts of methyltrioxorhenium(VII) and their application in catalytic epoxidation. Inorg Chem 40(23):5834–5841
Sabater MaJ, Domine ME, Corma A (2002) Highly stable chiral and achiral nitrogen–base adducts of methyltrioxorhenium(VII) as catalysts in the epoxidation of alkenes. J Catal 210(1):192–197
Kühn FE, Santos AM, Roesky PW, Herdtweck E, Scherer W, Gisdakis P, Yudanov IV, Di Valentin C, Rösch N (1999) Trigonal–bipyramidal Lewis base adducts of methyltrioxorhenium(VII) and their bisperoxo congeners: characterization, application in catalytic epoxidation, and density functional mechanistic study. Chem Eur J 5(12):3603–3615
Santos AM, Kühn FE, Xue W-M, Herdtweck E (2000) Syntheses and characterisation of methyltrioxorhenium adducts of low-valence organometallic Lewis bases. J Chem Soc Dalton Trans 20:3570–3574
Nabavizadeh SM (2003) Adduct formation of methyltrioxorhenium with mono- and bidentate nitrogen donors: formation constants. Inorg Chem 42(13):4204–4208
Nabavizadeh SM, Rashidi M (2006) Lewis acidity of methyltrioxorhenium(VII) (MTO) based on the relative binding strengths of N-donors. J Am Chem Soc 128(1):351–357
Frisch MJ, Rega N, Petersson GA, Trucks GW, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Burant JC, Nakajima T, Honda Y, Kitao O, Schlegel HB, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Millam JM, Bakken V, Adamo C, Jaramillo J, Gomperts R, Scuseria GE, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Iyengar SS, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Robb MA, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Tomasi J, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cheeseman JR, Ortiz JV, Cui Q, Baboul AG, Barone V, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Montgomery JA Jr, Martin RL, Fox DJ, Mennucci B, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Vreven T, Wong MW, Cossi M, Gonzalez C, Pople JA, Kudin KN, Scalmani G (2004) Gaussian 03, Revision C.02. Gaussian, Inc, Wallingford
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82:270–283
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta 28(3):213–222
Ehlers A, Böhme M, Dapprich S, Gobbi A, Höllwarth A, Jonas V, Köhler K, Stegmann R, Veldkamp A, Frenking G (1993) A set of f-polarization functions for pseudo-potential basis sets of the transition metals Sc–Cu, Y–Ag and La–Au. Chem Phys Lett 208(1):111–114
Wang W-D, Espenson JH (1998) Effects of pyridine and its derivatives on the equilibria and kinetics pertaining to epoxidation reactions catalyzed by methyltrioxorhenium. J Am Chem Soc 120(44):11335–11341
Herrmann WA, Kiprof P, Rypdal K, Tremmel J, Blom R, Alberto R, Behm J, Albach RW, Bock H (1991) Multiple bonds between main-group elements and transition metals. 86. Methyltrioxorhenium(VII) and trioxo (5-pentamethylcyclopentadienyl) rhenium(VII): structures, spectroscopy and electrochemistry. J Am Chem Soc 113(17):6527–6537
Hosseini FN, Nabavizadeh SM, Azimi G (2013) Theoretical study of the solvent effect on the methyltrioxorhenium/hydrogen peroxide system. J Solut Chem 42(11):2137–2148
Gonzales JM, Distasio R, Periana RA, Goddard WA, Oxgaard J (2007) Methylrhenium trioxide revisited: mechanisms for nonredox oxygen insertion in an M–CH3 bond. J Am Chem Soc 129(51):15794–15804
Costa PJ, Calhorda MJ, Bossert J, Daniel C, Romão CC (2006) Photochemistry of methyltrioxorhenium revisited: a DFT/TD-DFT and CASSCF/MS-CASPT2 theoretical study. Organometallics 25(22):5235–5241
Wu YD, Sun J (1998) Transition structures of epoxidation by CH3Re(O)2(O2) and CH3Re(O)(O2)2 and their water adducts. J Org Chem 63:1752–1753
Herrmann WA, Fischer RW, Scherer W, Rauch MU (1993) Methyltrioxorhenium(VII) as catalyst for epoxidations: structure of the active species and mechanism of catalysis. Angew Chem Int Ed Engl 32(8):1157–1160
Spencer J, Ganunis TF, Zafar AI, Salata CM, Gupta S, Puppala S, Eppley HJ, Ealy JL, Yoder CH (1991) Enthalpy and entropy contributions to solvent effects on adduct formation. J Phys Chem 95(12):4910–4915
Nabavizadeh SM, Rashidi M (2007) Acidity of osmium tetroxide (OsO4) towards coordination with pyridine and its derivatives. Polyhedron 26(7):1476–1482
Martell AE, Smith RM (1974) Critical stability constants, vol 1. Springer, Berlin
Kerr J, Lide D (2000) CRC handbook of chemistry and physics 1999–2000: a ready-reference book of chemical and physical data. In: CRC handbook of chemistry and physics, 81st edn. CRC Press, Boca Raton
Nabavizadeh SM, Akbari A, Rashidi M (2005) Thermodynamic study of the binding of methyltrioxorhenium with pyridine and its derivatives in benzene solution. Eur J Inorg Chem 12:2368–2375
Hansch C, Leo A, Taft R (1991) A survey of Hammett substituent constants and resonance and field parameters. Chem Rev 91(2):165–195
Gasperini M, Ragaini F (2004) Method of establishing the Lewis acidity of a metal fragment based on the relative binding strengths of Ar-BIAN ligands (Ar-BIAN = bis(aryl) acenaphthenequinonediimine). Organometallics 23(5):995–1001
Szintay G, Horváth A (2000) Temperature dependence study of five-coordinate complex formation of zinc(II) octaethyl and tetraphenylporphyrin. Inorg Chim Acta 310(2):175–182
Glendening E, Reed A, Carpenter J, Weinhold F (2004) NBO Version 3.1. In: Gaussian 03, Revision, D. 01. Gaussian, Inc., Wallingford
Acknowledgments
Financial support of the Shiraz Branch, Islamic Azad University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sedaghatzadeh, V., Hosseini, F.N. Scale of relative Lewis acidities of methyltrioxorhenium and its mono- and bisperoxo derivatives from their equilibria with pyridines; a density functional theory study. Struct Chem 26, 35–45 (2015). https://doi.org/10.1007/s11224-014-0462-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0462-y