Abstract
In this paper, we have theoretically focused on the doping of up to nine Li atoms to the double-ring \(\text {B}_{20}\) nanotubule to reveal the electronic and structural features of the nLi@\(\text {B}_{20}\) (\(\text {n}=1{-}9\)) molecules. The most stable species for each of the nLi@\(\text {B}_{20}\) (\(\text {n}=1{-}9\)) molecules has been reported on the singlet or doublet potential energy surfaces through density functional theory (DFT). The calculated results show that the nLi@\(\text {B}_{20}\) (\(\text {n}=1{-}9\)) molecules have high thermodynamic and chemical stabilities due to high values of the adsorption energy, −2.51 eV to −3.57 eV, and the HOMO–LUMO energy gap, 1.32 eV to 2.34 eV. Additionally, the values reported for deformation of the double-ring \(\text {B}_{20}\) backbone, 0.10 eV to 4.29 eV, increase severely along with increasing number of the Li atoms in the nLi@\(\text {B}_{20}\) (\(\text {n}=1{-}9\)) molecules. The NBO charges of positive values for the Li atoms along with those of negative values for the B atoms confirm the role of electron donor of the Li atom and electron acceptor of the B atom. Finally, we have not found any Li–Li interaction in the nLi@\(\text {B}_{20}\) (\(\text {n}=1{-}9\)) molecules based on AIM analysis. Moreover, all reported Li–B interactions are weak and non-covalent.
Graphical Abstract
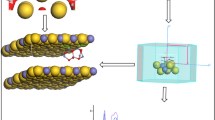
In the present study, we have systematically added up to nine Li atoms to the double-ring \(\text {B}_{20}\) molecule to report electronic and structural properties of the most stable local minima for each nLi@\(\text {B}_{20}\) (\(\hbox {n}=1{-}9\)) molecules on the singlet or doublet potential energy surfaces.







Similar content being viewed by others
References
Pauling L 1960 The nature of the chemical bond and the structure of molecules and crystals: an introduction to modern structural chemistry (New York: Cornell university press)
Marques M A and Botti S 2005 The planar-to-tubular structural transition in boron clusters from optical absorption J. Chem. Phys. 123 014310
Boustani I 1997 New quasi-planar surfaces of bare boron Surf. Sci. 370 355
Cotton A F, Wilkinson G, Bochmann M and Murillo C A 1999 Advanced inorganic chemistry (Singapore: Wiley)
Greenwood N N and Earnshaw A 1997 Chemistry of the Elements 2\(^{nd}\) ed. (Location: Butterworth-Heinemann)
Atiş M, Özdoşan C and Güvenç Z B 2009 Density Functional Study of Physical and Chemical Properties of Nano Size Boron Clusters: \(\text{ B }_{n}\) (n= 13–20) Chin. J. Chem. Phys. 22 380
Fakioğlu E, Yürüm Y and Veziroğlu T N 2004 A review of hydrogen storage systems based on boron and its compounds Int. J. Hydrogen Energ. 29 1371
Demirbaş A 2005 Hydrogen and boron as recent alternative motor fuels Energ. Source 27 741
Mishima O, Tanaka J, Yamaoka S and Fukunaga O 1987 High-temperature cubic boron nitride p-n junction diode made at high pressure Science 238 181
Reisch M S 1987 High-performance fibers find expanding military, industrial uses Chem. Eng. News 65 9
Eremets M I, Struzhkin V V, Mao H K and Hemley R J 2001 Superconductivity in boron Science 293 272
Boustani I 1997 New convex and spherical structures of bare boron clusters J. Solid State Chem. 133 182
Hanley L, Whitten J L and Anderson S L 1988 Collision-induced dissociation and ab initio studies of boron cluster ions: determination of structures and stabilities J. Phys. Chem. 92 5803
La Placa S J, Roland P A and Wynne J J 1992 Boron clusters (\(\text{ B }_{{\rm n}}\), n= 2–52) produced by laser ablation of hexagonal boron nitride Chem. Phys. Lett. 190 163
Zhai H J, Alexandrova A N, Birch K A, Boldyrev A I and Wang L S 2003 Hepta-and Octacoordinate Boron in Molecular Wheels of Eight-and Nine-Atom Boron Clusters: Observation and Confirmation Angew. Chem. Int. Ed. 42 6004
Meng X M, Hu J Q, Jiang Y, Lee C S and Lee S T 2003 Boron nanowires synthesized by laser ablation at high temperature. Chem. Phys. Lett. 370 825
Boustani I, Quandt A, Hernández E and Rubio A 1999 New boron based nanostructured materials J. Chem. Phys. 110 3176
Ciuparu D, Klie R F, Zhu Y and Pfefferle L 2004 Synthesis of pure boron single-wall nanotubes J. Phys. Chem. B. 108 3967
Tai T B, Tam N M and Nguyen M T 2012 The Boron conundrum: the case of cationic clusters Bn\(^{+}\) with n= 2–20 Theor. Chem. Acc. 131 1241
Tai T B, Tam N M and Nguyen M T 2012 Structure of boron clusters revisited, \(\text{ B }_{n}\) with n= 14–20 Chem. Phys. Lett. 530 71
Pham H T, Duong L V and Nguyen M T 2014 Electronic structure and chemical bonding in the double ring tubular boron clusters J. Phys. Chem. C 118 24181
Kiran B, Bulusu S, Zhai H J, Yoo S, Zeng X C and Wang L S 2005 Planar-to-tubular structural transition in boron clusters: B20 as the embryo of single-walled boron nanotubes Proc. Natl Acad. Sci. 102 961
Jian-Bing G, Xiang-Dong Y, Huai-Qian W and Hui-Fang L 2012 Structural, electronic, and magnetic properties of boron cluster anions doped with aluminum: \(\text{ B }_{{\rm n}}\)Al-(2\(\le \) n\(\le \) 9) Chin. Phys. B 21 043102
Romanescu C, Galeev T R, Sergeeva A P, Li W L, Wang L S and Boldyrev A I 2012 Experimental and computational evidence of octa-and nona-coordinated planar iron-doped boron clusters: Fe\(\copyright \) \(\text{ B }_{8}^{-}\) and Fe\(\copyright \) \(\text{ B }_{9}^{-}\) J. Org. Chem. 721 148
Van Duong L and Nguyen M T 2017 Silicon doped boron clusters: how to make stable ribbons? Phys. Chem. Chem. Phys. 19 14913
Piazza Z A, Hu H-S, Li W-L, Zhao Y-F, Li J and Wang L-S 2014 Planar hexagonal \(\text{ B }_{36}\) as a potential basis for extended single-atom layer boron sheets Nature Commun. 5 3113
Romanescu C, Galeev T R, Li W-L, Boldyrev A I and Wang L-S 2011 Aromatic Metal-Centered Monocyclic Boron Rings: Co@\(\text{ B }_{8}^{-}\) and Ru@\(\text{ B }_{9}^{-}\) Angew. Chem. Int. Ed. 50 9334
Piazza Z A, Li W-L, Romanescu C, Sergeeva A P and Wang L-S et al. 2012 A photoelectron spectroscopy and ab initio study of \(\text{ B }_{21}^{-}\): Negatively charged boron clusters continue to be planar at 21 J. Chem. Phys. 136 104310
Popov I A, Piazza Z A, Li W-L, Wang L-S and Boldyrev A I 2013 A combined photoelectron spectroscopy and ab initio study of the quasi-planar \(\text{ B }_{24}^{-}\) Cluster J. Chem. Phys. 139 144307
Sergeeva A P, Piazza Z A, Romanescu C, Li W-L, Boldyrev A I and Wang L-S 2012 \(\text{ B }_{22}^{-}\) and \(\text{ B }_{23}^{-}\): All-Boron Analogues of Anthracene and Phenanthrene J. Am. Chem. Soc. 134 18065
Perdew J P, Burke K and Ernzerhof M 1996 Generalized gradient approximation made simple Phys. Rev. Lett. 77 3865
Perdew J P, Burke K and Ernzerhof M 1997 Generalized gradient approximation made simple Phys. Rev. Lett. 78 1396
Adamo C and Barone V 1999 Toward reliable density functional methods without adjustable parameters: The PBE0 model J. Chem. Phys. 110 6158
Kendall R A, Dunning T H and Harrison R J 1992 Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions J. Chem. Phys. 96 6796
Grimme S, Antony J, Ehrlich S and Krieg S 2010 A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu J. Chem. Phys. 132 154104
Biegler-König F and Schönbohm J 2002 AIM2000 Program Package Ver. 2.0. (Bielefeld: University of Applied Sciences)
Frisch M J et al. 2009 Gaussian 09, Revision A 01, Gaussian, Inc Wallingford CT
Pearson R G 1997 Chemical hardness (New York: Wiley-VCH) p. 198
Landis C R and Weinhold F 2014 The Chemical bond. 1. Fundamental aspects of chemical bonding Frenking G and Shaik S (Eds.) (Weinham: Wiley-VCH)
Landis C R and Weinhold F 2005 Valency and bonding: A natural bond orbital donor-acceptor perspective (Cambridge: Cambridge University Press)
Reed A E, Curtiss L A and Weinhold F 1988 Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint Chem. Rev. 88 899
Bader R F W 1990 In The international series of monographs of chemistry Halpen J and Green M L H (Eds.) (Oxford: Clarendon Press)
Vessally E, Ebrahimi S, Goodarzi M and Mortezapour A 2014 A computational study of the non-covalent bindings in complexes pairing sulfur tetroxide (SO\(_{4}\)(C\(_{2{\rm V}}\))) with the nitrous oxide (NNO) Struct. Chem. 25 1141
Vessally E, Mortezapour A and Goodarzi M 2014 On the intermolecular interactions of isothiocyanic acid (HNCS) with disulfur monoxide (SSO): a first principles approach J. Sulfur Chem. 35 484
Vessally E, Ebrahimi S, Goodarzi M and Seif A 2014 Insight into detailed mechanism of the atmospheric reaction of imidogen with hydroxyl: a computational study Struct. Chem. 25 169
Vessally E, Ebrahimi S, Goodarzi M and Seif A 2013 The location of stationary points in the reaction of fluoroformyloxyl radical \((\text{ FCO }_{2}(\text{ C }_{2\text{ V }}))\) with atomic hydrogen: A computational study on the pathways of the singlet and triplet reaction and intersystem crossing Comput. Theor. Chem. 1022 86
Seif A, Ebrahimi S, Vessally E and Goodarzi M 2013 Comparative study on the stabilities and properties of heterodimers containing the intermolecular interactions of \(\text{ CF }_{2}\text{ Cl }_{2}\) with the isoelectronic and isostructure species of \(\text{ N }_{2}\text{ O }\) and \(\text{ CO }_{2}\) Struct. Chem. 241737
Bagherzadeh R, Vessally E and Goodarzi M 2013 A computational investigation on the potential energy surface of thiosulfeno with O(3P) reaction Struct. Chem. 24 517
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hosseinian, A., Delir Kheirollahi Nezhad, P., Vessally, E. et al. Insight into electronic and structural properties of nLi@\(\text {B}_{20}\) (\(\hbox {n}=1{-}9\)) nanotubules: a computational study. J Chem Sci 130, 130 (2018). https://doi.org/10.1007/s12039-018-1536-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1536-y