Abstract
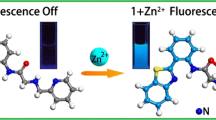
To develop an effective fluorescent chemosensor for relay recognition of Zn2+ and oxalate, a new fluorescent sensor based on binaphthol-quinoline Schiff base L 1 was designed and synthesized. In DMSO-H2O (1/1, v/v, HEPES 10 mM, pH = 7.4) solution, L 1 exhibits highly selective fluorescence turn on response to Zn2+ over other metal ions. The Zn2+ recognition event is barely interfered by other coexisting metal ions except Cu 2+, Co 2+ and Ni 2+. The in situ generated L 1 -Zn\(^{\mathrm {2+}}_{\mathrm {}}\)complex was further used as a chemosensing ensemble for oxalate detection. The complex L 1 -Zn2+ displays high selectivity to oxalate with significant fluorescence quenching through Zn2+ ion displacement approach. In addition, application of L 1 for imaging of Zn2+ and oxalate in living HeLa cells was also examined.

A new binaphthol-quinoline Schiff base-based fluorescent sensor L 1 exhibits highly selective relay recognition of Zn2+ and oxalate through fluorescence “off-on-off” functionality in DMSO-H2O (1/1, v/v, HEPES 10 mM, pH = 7.4) solution.












Similar content being viewed by others
References
Berg J M and Shi Y 1996 Science 271 1081
Walker C F and Black R E 2004 Annu. Rev. Nutr. 24 255
(a) Chen Y, Bai Y, Han Z, He W and Guo Z 2015 Chem. Soc. Rev. 44 4517; (b) Jiang P and Guo Z 2004 Coord. Chem. Rev. 248 205; (c) Xu Z, Yoon J and Spring D R 2010 Chem. Soc. Rev. 39 1996; (d) Bhaumik C, Maity D, Das S and Baitalik S 2012 RSC Adv. 2 2581; (e) Mardanya S, Karmakar S, Das S and Baitalik S 2015 Sens. Actuators, B 206 701; (f) Mondal D, Bar M, Maity D and Baitalik S 2015 J. Phys. Chem. C 119 25429; (g) Ding A, Tang F, Wang T, Tao X and Yang J 2015 J. Chem. Sci. 127 375; (h) Chao D 2016 J. Chem. Sci. 128 133
Capra R H, Strumia M, Vadgama P M and Baruzzi A M 2005 Anal. Chimi. Acta 530 49
Li H, Chai X -S, DeMartini N, Zhan H and Fu S 2008 J. Chromatogr. A 1192 208
Liu Y, Huang J, Wang D, Hou H and You T 2010 Anal. Methods 2 855
(a) Tang L, Park J, Kim H-J, Kim Y, Kim S J, Chin J and Kim K M 2008 J. Am. Chem. Soc. 130 12606; (b) Hu M and Feng G 2012 Chem. Commun. 48 6951; (c) Wang G, Zhu H, Lin Y, Chen Y and Fu N 2015 Sens. Actuators, B 206 624; (d) He C, Qian X, Xu Y, Yang C, Yin L and Zhu W 2011 Dalton Trans. 40 1034
(a) Mummidivarapu V S, Nehra A, Hinge V K and Rao C P 2012 Org. Lett. 14 2968; (b) Yang Y, Yin C, Huo F, Chao J and Zhang Y 2014 Sens. Actuators, B 204 402; (c) Tang L, Zhou P, Huang Z, Zhao J and Cai M 2013 Tetrahedron Lett. 54 5948; (d) Tang L, Cai M, Zhou P, Zhao J, Zhong K, Hou S and Bian Y 2013 RSC Adv. 3 16802; (e) Peng Y, Dong Y-M, Dong M and Wang Y-W 2012 J. Org. Chem. 77 9072; (f) Tang L, Dai X, Cai M, Zhao J, Zhou P and Huang Z 2014 Spectrochim. Acta, Part A 122 656; (g) Kaur N and Alreja P 2015 J. Chem. Sci. 127 1253
Ye F, Zheng Z -J, Deng W -H, Zheng L -S, Deng Y, Xia C -G and Xu L -W 2013 Chem. - Eur. J. 8 2242
Zhu J -F, Yuan H, Chan W -H and Lee A W M 2010 Org. Biomol. Chem. 8 3957
Shi F, Shen J K, Chen D, Fog K, Thirstrup K, Hentzer M, Karlsson J -J, Menon V, Jones K A, Smith K E and Smith G 2011 ACS Med. Chem. Lett. 2 303
Sun Y -Q, Wang P, Liu J, Zhang J and Guo W 2012 Analyst 137 3430
Zhang Y, Guo X, Si W, Jia L and Qian X 2008 Org. Lett. 10 473
Lin W, Yuan L, Cao Z, Feng Y and Long L 2009 Chem. - Eur. J. 15 5096
Lu Q, Hou J, Wang J, Xu B, Zhang J and Yu X 2013 Chin. J. Chem. 31 641
Acknowledgements
We are grateful to the National Natural Science Foundation of China (No. 21176029, 21476029), Liaoning BaiQianWan Talents Program (No. 2012921057), and the Program for Liaoning Excellent Talents in University (LR2015001) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Supplementary Information (Figures S1–S6) is available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
TANG, L., WU, D., HUANG, Z. et al. A fluorescent sensor based on binaphthol-quinoline Schiff base for relay recognition of Zn2+ and oxalate in aqueous media. J Chem Sci 128, 1337–1343 (2016). https://doi.org/10.1007/s12039-016-1124-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1124-y