Abstract
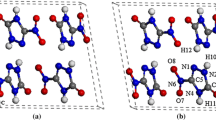
Periodic density functional theory with dispersion correction (DFT-D) was used to study the structural, electronic, and absorption properties of crystalline 5-nitramino-3, 4-dinitropyrazole (NADNP) under hydrostatic compression of 0-140 GPa. The results indicate that the PBE-G06 is the best functional for studying NADNP. As the pressure increases, the lattice of parameters, band gap, density of states and absorption spectra change regularly except for 126 GPa, where NADNP begins to decompose and form a new bond. An analysis of the band gap and density of states indicates that NADNP becomes more and more sensitive under compression. The absorption spectra show that NADNP has relatively high optical activity with increasing pressure.

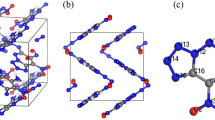
The compression ratios of the relaxed lattice constants (a, b, c) of 5-nitramino-3,4-dinitropyrazole gradually increase in the pressure range of 0-140 GPa.














Similar content being viewed by others
References
Yin P, Parrish D A and Shreeve J M 2015 J. Am. Chem. Soc. 137 4778
Zhu W H, Wei T, Zhang X W and Xiao H M 2009 J. Mol. Struct.: Theochem. 895 131
Qiu L, Xiao H M, Ju X H and Gong X D 2005 Int. J. Quantum Chem. 1 48
Zhu W H and Xiao H M 2007 J. Solid. State Chem. 180 3521
Zhu W H, Xiao J J and Xiao H M 2006 Chem. Phys. Lett. 422 117
Zhu W H, Xiao J J and Xiao H M 2006 J. Phys. Chem. B 110 9856
Peiris S M, Wong C P and Zerilli F J 2004 J. Chem. Phys. 120 8060
Zhu W H, Zhang X W, Zhu W and Xiao H M 2008 Phys. Chem. Chem. Phys. 10 7318
Zhu W H, Zhang X W, Zhu W H and Xiao H M 2009 Theor. Chem. Acc. 124 179
Zhu W H and Xiao H M 2010 Struct. Chem. 21 657
Wu Q, Zhu W H and Xiao H M 2013 J. Phys. Chem. C 117 16830
Zhu W H and Xiao H M 2008 J. Comput. Chem. 29 176
Xiao H M and Ju X H 2004 Int. J. Quantum Chem. 121 12523
Fabbiani F P A and Pulham C R 2006 Chem. Soc. Rev. 35 932
Zhu W H, Xiao J J, Ji G F, Zhao F and Xiao H M 2007 J. Phys. Chem. B 111 12715
Segall M D, Lindan P J D, Prober M J T, Pickard C J, Hasnip P J, Clark S J and Payne M C 2002 J. Phys: Condens. Matter 14 2717
Payne M C, Teter M P, Allan D C, Arias T A and Joannopoulos J D 1992 Rev. Mod. Phys. 64 1045
Vanderbilt D 1990 Phys. Rev. B 41 7892
Perdew J P and Zunger A 1981 Phys. Rev. B 23 5048
Perdew J P, Burke K and Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
Perdew J P and Wang Y 1992 Phys. Rev. B 45 12947
Grimme S 2006 J. Comput. Chem. 27 1787
Tkatchenko A and Scheffler M 2009 Phys. Rev. Lett. 102 073005
Ortmann F, Bechstedt F and Schmidt W G 2006 Phys. Rev. B 73 205101
Appalakondaiah S, Vaitheeswaran G and Lebègue S 2014 Chem. Phys. Lett. 605–606 10
Kresse G and Furthmüller J 1996 Phys. Rev. B 54 11169
Fletcher R 1980 In Practical Methods of Optimization (New York: Wiley)
Saha S, Sinha T P and Mookerjee A 2000 Phys. Rev. B 62 8828
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21273115) and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
XIANG, D., WU, Q., LIU, Z. et al. Pressure-induced changes in the structural and absorption properties of crystalline 5-nitramino-3,4-dinitropyrazole. J Chem Sci 127, 1777–1784 (2015). https://doi.org/10.1007/s12039-015-0938-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0938-3