Abstract
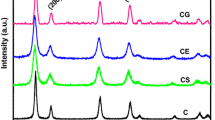
In this work, nanosized Ce0.7M0.3O2−δ (M = Mn, Fe, Co) solid solutions were prepared by a facile coprecipitation method and evaluated for CO oxidation. The physicochemical properties of the synthesized samples were investigated by various characterization techniques, namely, XRD, ICP-OES, BET surface area, SEM-EDX, TEM and HRTEM, Raman, XPS, and H2-TPR. XRD studies confirmed the formation of nanocrystalline single phase Ce0.7M0.3O2−δ solid solutions. ICP-OES analysis confirmed actual amount of metal loadings in the respective catalysts. The BET surface area of Ce0.7M0.3O2−δ samples significantly enhanced after the incorporation of dopants. TEM studies confirmed nanosized nature of the samples and the average particle sizes of Ce0.7M0.3O2−δ were found to be in the range of ∼8–16 nm. Raman studies indicated that the incorporation of dopant ions into the CeO2 lattice promote the formation of more oxygen vacancies. The existence of oxygen vacancies and different oxidation states (Ce3+/Ce4+ and Mn2+/Mn3+, Fe2+/ Fe3+, and Co2+/Co3+) in the doped CeO2 samples were further confirmed from XPS investigation. TPR measurements revealed an enhanced reducibility of ceria after the incorporation of dopants. The catalytic activity results indicated that the doped CeO2 samples show excellent CO oxidation activity and the order of activity was found to be Ce0.7Mn0.3O2−δ > Ce0.7Fe0.3O2−δ > Ce0.7Co0.3O2−δ > CeO2. The superior CO oxidation performance of CeO2-MnOx has been attributed to a unique Ce-Mn synergistic interaction, which facilitates materials with promoted redox properties and improved oxidation activity.

The presence of structural oxygen vacancies, low temperature reducibility and synergetic interaction between Ce−O and Mn−O oxides were responsible for superior CO oxidation performance of Ce−Mn−O nano oxide compared to pure CeO2, Ce−Fe−O and Ce−Co−O samples.









Similar content being viewed by others
References
Vecchietti J, Collins S, Delgado J J, Małecka M, Rio E D, Chen X, Bernal S and Bonivardi A 2011 Top. Catal. 54 201
Xiaodong Z, Zhenping Q, Fangli Y and Yi W 2013 Chin. J. Catal. 34 1277
Royer S and Duprez D 2011 Chem. Cat. Chem. 3 24
Biabani-Ravandi A and Rezaei M 2012 Chem. Eng. J. 184 141
Manasilp A and Gulari E 2002 Appl. Catal., B 37 17
Falcón H, Martinez-Lope M J, Alonso J A and Fierro J L G 2000 Appl. Catal., B 26 131
Rao K N, Bharali P, Thrimurthulu G and Reddy B M 2010 Catal. Commun. 11 863
KasÏpar J, Fornasiero P and Graziani M 1999 Catal. Today 50 285
Katta L, Sudarsanam P, Thrimurthulu G and Reddy B M 2010 Appl. Catal. B 101 101
Rao G R and Mishra B G 2003 Bull. Catal. Soc. India 2 122
Mai H -X, Sun L -D, Zhang Y -W, Si R, Feng W, Zhang H -P, Liu H -C and Yan C -H 2005 J. Phys. Chem. B 109 24380
Meher S K and Ranga Rao G 2012 J. Colloid Interface Sci. 373 46
Kehoe A B, Scanlon D O and Watson G W 2011 Chem. Mater. 23 4464
Saqer S M, Kondarides D I and Verykios X E 2011 Appl. Catal. B 103 275
Yeqin S, Ying Z, Hanfeng L, Zekai Z and Yinfei C 2013 Chin. J. Catal. 34 567
Mousavi S M, Niaei A, Gómez M J, Salari D, Panahi P N and Abaladejo-Fuentes V 2014 Mater. Chem. Phys. 143 921
Tang C -W, Wang C -B and Chien S -H 2009 Catal. Lett. 131 76
Yu D, Liu Y and Wu Z 2010 Catal. Commun. 11 788
Bharali P, Saikia P, Katta L and Reddy B M 2013 J. Ind. Eng. Chem. 19 327
Wang J, Shen M, Wang J, Gao J, Ma J and Liu S 2011 Catal. Today 175 65
Tang Y, Qiao H, Wang H and Tao P 2013 J. Mater. Chem. A 1 12512
Luo J -Y, Meng M, Zha Y -Q and Guo L -H 2008 J. Phys. Chem. C 112 8694
Kongzhai L, Hua W, Yonggang W and Mingchun L 2008 J. Rare Earths 26 245
Zhou G, Shah P R and Gorte R J 2008 Catal. Lett. 120 191
Choudhury B and Choudhury A 2012 Mater. Chem. Phys. 131 666
Sudarsanam P, Mallesham B, Durgasri D N and Reddy B M 2014 RSC Adv. 4 11322
Zou Z -Q, Meng M and Zha Y -Q 2010 J. Phys. Chem. C 114 468
Ertl G, Knoezinger H, Schuth F and Weitkmp J 2008 In Hand book of heterogeneous catalyst 2 nd ed. (Weinheim: Wiley-VcH) Vol. 2 p.728
Channei D, Inceesungvorn B, Wetchakun N, Phanichphant S, Nakaruk A, Koshy P and Sorrell C C 2013 Ceram. Int. 39 3129
Yu Y, Zhong L, Ding J, Cai W and Zhong Q 2015 RSC Adv. 5 23193
Zhang X, Wei J, Yang H, Liu X, Liu W, Zhang C and Yang Y 2013 Eur. J. Inorg. Chem. 4443
Li K, Wang H, Wei Y and Yan D 2011 Int. J. Hydrogen Energy 36 3471
Qiao D, Lu G, Liu X, Guo Y, Wang Y and Guo Y 2011 J. Mater. Sci. 46 3500
Fazio B, Spadaro L, Trunfio G, Negro J and Arena F 2011 J. Raman Spectrosc. 42 1583
Zhu X, Wei Y, Wang H and Li K 4492 Int. J. Hydrogen Energy 38 2103
Lou Y, Wang L, Zhang Y, Zhao Z, Zhang Z, Lu G and Guo Y 2011 Catal. Today 175 610
Yao X, Tang C, Ji Z, Dai Y, Cao Y, Gao F, Dong L and Chen Y 2013 Catal. Sci. Technol. 3 668
Zhang Y -W, Si R, Liao C -S and Yan C -H 2003 J. Phys. Chem. B 107 10159
Wu Z, Jin R, Liu Y and Wang H 2008 Catal. Commun. 9 2217
Zhang F, Wang P, Koberstein J, Khalid S and Chan S -W 2004 Surf. Sci. 563 74
Santra C, Rahman S, Bojja S, James O O, Sen D, Maity S, Mohanty A K, Mazumder S and Chowdhury B 2013 Catal. Sci. Technol. 3 360
Machida M, Uto M, Kurogi D and Kijima T 2000 Chem. Mater. 12 3158
Murugan B and Ramaswamy A V 2008 J. Phys. Chem. C 112 20429
Nesbitt H W and Banerjee D 1998 Am. Mineral. 83 305
Zhang Z, Han D, Wei S and Zhang Y 2010 J. Catal. 276 16
Venkataswamy P, Jampaiah D, Rao K N and Reddy B M 2014 Appl. Catal., A 488 1
Yamashita T and Hayes P 2008 Appl. Surf. Sci. 254 2441
Li J -G, Büchel R, Isobe M, Mori T and Ishigaki T 2009 J. Phys. Chem. C 113 8009
Norman C and Leach C 2011 J. Membr. Sci. 382 158
Li K, Wang H, Wei Y and Yan D 2011 Chem. Eng. J. 173 574
Ma C, Mu Z, He C, Li P, Li J and Hao Z 2011 J. Environ. Sci. 23 2078
Chen L, Li J and Ge M 2009 J. Phys. Chem. C 113 21177
Durgasri D N, Vinodkumar T and Reddy B M 2014 J. Chem. Sci. 126 429
Fraccari E P, D’Alessandro O, Sambeth J, Baronetti G and Mariño F 2014 Fuel Process. Technol. 119 67
Li K, Wang H, Wei Y and Yan D 2010 Chem. Eng. J. 156 512
Laguna O H, Sarria F R, Centeno M A and Odriozola J A 2010 J. Catal. 276 360
Ayastuy J L, Iglesias-González A and Gutiérrez-Ortiz M A 2014 Chem. Eng. J. 244 372
Reddy B M, Bharali P, Saikia P, Park S -E, van den Berg M W E, Muhler M and Grünert W 2008 J. Phys. Chem. C 112 11729
Acknowledgements
We greatly acknowledge Prof. Dr. W. Grünert, Ruhr University Bochum, Germany for providing CO oxidation results. PV thanks Council of Scientific and Industrial Research (CSIR), New Delhi for the Senior Research Fellowship. DJ thanks IICT-RMIT Joint Research Centre for the award of Junior Research Fellowship. Financial support was received from the Department of Science and Technology, New Delhi, under SERB Scheme (SB/S1/PC-106/2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Detailed information about the Williamson-Holl (We-H) plots, CO oxidation profile, calculation of crystallite size using Debye-Scherrer equation, chemical composition by ICP-OES analysis, surface reduction temperatures, and characteristic temperatures of CO oxidation of all the investigated catalysts calcined at 773 K are available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
VENKATASWAMY, P., JAMPAIAH, D., ANIZ, C.U. et al. Investigation of physicochemical properties and catalytic activity of nanostructured Ce0.7M0.3O2−δ (M = Mn, Fe, Co) solid solutions for CO oxidation. J Chem Sci 127, 1347–1360 (2015). https://doi.org/10.1007/s12039-015-0897-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0897-8