Abstract
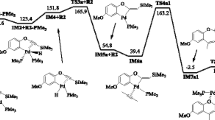
Density functional studies are performed to understand the role of chelating bi-phosphine ligands [(Ph 2P(CH 2) m PPh 2); m = 1–4] in modulating the regio-selectivity of benzoic acid addition to 1-hexyne, in presence of ruthenium(II) catalyst [(Ph 2P(CH 2)mPPh 2)Ru(methallyl) 2]. The Markovnikov addition to 1-hexyne is observed when catalyst 1 a [(Ph 2P(CH 2)PPh 2)Ru(methallyl) 2] is employed, whereas a reverse regio-selectivity is witnessed in presence of 1 d [(Ph 2P(CH 2)4PPh 2)Ru(methallyl) 2]. Anti-Markovnikov addition occurs via the neutral vinylidene intermediates (5 a / d ) formed after 1,2-hydrogen shift in hexyne coordinated ruthenium(II) complexes 3 a / d . The energy profile shows clear preference for Markovnikov addition by 15.0 kcal/mol (\({\Delta } G_{\mathrm {L}}^{S})\) in case of catalyst system 1 a . In contrast, anti-Markovnikov pathway following neutral vinylidenes are more favourable by 9.1 kcal/mol (\({\Delta } G_{\mathrm {L}}^{S})\) for catalyst system 1 d . The Z-enol ester formation is more predominant in the anti-Markovnikov pathway since the activation barrier for this step requires less energy (5.9 kcal/mol, \({\Delta } G_{\mathrm {L}}^{S})\) than the one furnishing the E-product. The calculated results are in good agreement with the reported experimental findings.

Preliminary DFT studies are performed to explore the mechanistic pathway of the benzoic acid addition to 1-hexyne in presence of Ru(II) catalyst. Our calculations emphasized on the understanding of the regio-selectivity controlled by the chelating bi-phosphine ligand present in catalysts. Markovnikov product formation is the lower energy pathway in case of least spacer group (CH2) whereas it is anti-Markovnikov product for the highest spacer group, {(CH2)4} in bi-phosphine ligands.













Similar content being viewed by others
References
(a) Rozen S and Lerman O 1979 J. Am. Chem. Soc. 101 2782; (b) Wexler A, Balchunis R J and Swenton J S 1975 J. Chem. Soc., Chem. Commun. 15 601; (c) House H O 1972 Modern synthetic reactions, 2 nd edn Menlo Park, CA: W A Benjamin 313. (d) Tsuji J, Minami I and Shimizu I 1983 Tetrahedron Lett. 24 4713; (e) Schmitt G Warwel S, Homminga E and Meltzow W 1972 Justus Liebigs Ann. Chem. 763 75
(a) Monthéard J P, Camps M, Seytre G, Guillet J and Dubois J C 1978 Angew. Makromol. Chem. 72 45; (b) Seidel A, Jägers E and Bylsma F 1993 Eur. Pat. 0574 725 A1
Rotem M and Shvo Y 1983 Organometallics 2 1689
(a) Mitsudo T, Hori Y and Watanabe Y 1985 J. Org. Chem. 50 1566 (b) Mitsudo T, Hori Y, Yamakawa Y and Watanabe Y 1987 J. Org. Chem. 52 2230
(a) Doucet H, Hofer J, Bruneau C and Dixneuf P H 1993 J. Chem. Soc., Chem. Commun. 10 850 (b) Doucet H, Martin-Vanca B, Bruneau C and Dixneuf P H 1995 J. Org. Chem. 60 7247 (c) Doucet P, Derrien N, Kabouche Z, Bruneau C and Dixneuf P H 1997 J. Organomet. Chem. 551 151 (d) Bruneau C and Dixneuf P H 1999 Acc. Chem. Res. 32 311 and references therein
Bruneau C, Neveux-Duflos M and Dixneuf P H 1999 Green Chem. 1 183
Goossen L J, Paetzold J and Koley D 2003 Chem. Commun. 6 706
(a) Bruneau C and Dixneuf P H 2008 Angew. Chem. Int. Ed. 47 8492 (b) Rigaut S, Touchard D and Dixneuf P H 2004 Coord. Chem. Rev. 248 1585
Gaussian 09, Revision C.01, Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji H, Caricato M, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G, Sonnenberg J L, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven, T, Montgomery Jr J A, Peralta J E, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J C, Iyengar S S, Tomasi J, Cossi M, Rega N, Millam J M, Klene M, Knox J E, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador P, Dannenberg J J, Dapprich, S, Daniels A D, Farkas Ö, Foresman J B, Ortiz J V, Cioslowski J and Fox D J Gaussian, Inc., Wallingford CT, 2010
(a) Maseras F and Morokuma K 1995 J. Comput. Chem. 16 1170 (b) Vrevenand T and Morokuma K 2000 J. Comput.Chem. 21 1419
(a) Hay P J and Wadt W 1985 J. Chem. Phys. 82 270 (b) Hay P J, and Wadt W 1985 J. Chem. Phys. 82 284 (c) Hay P J and Wadt W 1985 J. Chem. Phys. 82 299
Hehre W J, Radom L, Schleyer P v R and Pople J A 1986 Ab initio molecular orbital theory (New York: Wiley)
Helgren T A and Lipscomb W N 1977 Chem. Phys. Lett. 49 225
Marenich A V, Cramer C J and Truhlar D 2009 J. Phys. Chem. B 113 6378
Reed A E, Curtiss L A and Weinhold F 1998 Chem. Rev. 88 899
(a) Oliván M, Eisenstein O and Caulton K G 1997 Organometallics 16 2227 (b) Oliván M, Clot E, Eisenstein O and Caulton K G 1998 Organometallics 17 3091
Kostic N M and Fenske R F 1982 Organometallics 1 974
Bruneau C and Dixneuf P H 2006 Angew. Chem. 118 2232; Angew. Chem. Int. Ed. 45 2176
Varela-Fernández A, González-Rodríguez C, Varela J A, Castedo L and Saá C 2009 Org. Lett. 11 5350
Wakatsuki Y, Koga N, Yamazaki H and Morokuma K 1994 J. Am. Chem. Soc. 116 8105
Tokunaga M, Suzuki T, Koga N, Fukushima T, Horiuchi A and Wakatsuki Y 2001 J. Am. Chem. Soc. 123 11917
(a) Höhn A, Otto H, Dziallas M and Werner H 1987 J. Chem. Soc., Chem. Commun. 852 (b) Alonso F J G, Höhn A, Wolf J, Otto H and Werner H 1985 Angew. Chem. Int. Ed. 24 406
Cadierno V, Gamasa M P and Gimeno J 1999 Organometallics 18 2821
Silvestre J and Hoffmann R 1985 Helv. Chim. Acta. 68 1461
Arndt M, Salih K S M, Fromm A, Goossen L J, Menges F and Schatteburg G N 2011 J. Am. Chem. Soc. 133 7428
(a) Gooßen L J, Koley D, Hermann H L and Thiel W 2004 Chem. Commun. 19 2141 (b) Goossen L J, Koley D, Hermann H L and Thiel W 2005 J. Am. Chem. Soc. 127, 11102
Consiglio G, Morandini F, Ciani G F and Sironi A 1986 Organometallics 5 1976
Acknowledgement
The authors are thankful to IISER-Kolkata for providing computational facility. BM and TM are thankful to CSIR for their respective SRF and JRF fellowships. KD and SB gratefully acknowledge INSPIRE, DST. DK acknowledges IISER-Kolkata for start-up grant and SERB for DST fast track fellowship (SR/FT/SC-72/2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Steric effect in ligand exchange step (1 x → 2 x ) and related optimized geometries are given in scheme S1 and figure S1. Figure S2 represents the oxidative addition in 3 d intermediate. KS-LUMO of 5 d and nucleophilic approach of that intermediate are shown in figure S2. Absolute total energy (in Hartrees) and Cartesian coordinates of all intermediates and transition states are shown in tables S1 and S2, respectively. For details see www.ias.ac.in/chemsci website.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
MAITY, B., MONDAL, T., DEY, K. et al. Role of ligands in controlling the regioselectivity in ruthenium-catalysed addition of carboxylic acids to terminal alkynes: A DFT study. J Chem Sci 127, 281–293 (2015). https://doi.org/10.1007/s12039-015-0775-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0775-4