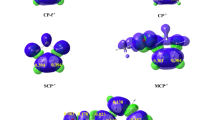
Abstract
A computational study for the [2 + 1] addition of the lithium carbenoids LiCH2X (X = Cl, Br, I) with ketene have been investigated by means of the B3LYP hybrid density functional method. All the reactions examined displayed similar concerted mechanisms for the cyclopropanation of these reagents. The lithium carbenoids react with ketene via an asynchronous attack on one CH2 or C group of ketene with relatively low barrier to reaction in the range of 25·34–33·74 kJ/mol in THF solvent. The trend of the lithium carbenoids reaction barrier with ketene is LiCH2Cl < LiCH2Br < LiCH2I. The results show that the reactions could be highly chemical reactivity with low barriers and could be favoured in experiment. The reactions could proceed easily at lower temperature. The computational results are briefly compared to other carbenoid reactions and related species.
Similar content being viewed by others
References
Rappoport Z (ed) 1987 The chemistry of the cyclopropyl group (Chichester, UK: Wiley)
Fritschi H, Leutenegger U and Pfaltz A 1986 Angew. Chem. Int. Ed. Engl. 25 1005
Evans D A, Woerpel K A, Hinman M M and Faul M M 1991 J. Am. Chem. Soc. 113 726
Rodriguez J B, Marquez V E, Nicklaus M C and Barchi J 1993 Tetrahedron Lett. 34 6233
Zhao Y, Yang T F, Lee M, Chun B K, Du J, Schinazi RF, Lee D, Newton M G and Chu C K 1994 Tetrahedron Lett. 35 5405
Nishiyama H, Itoh Y, Matsumoto H, Park S B and Itoh K 1994 J. Am. Chem. Soc. 116 2223
Doyle M P 1995 In Comprehensive Organometallic Chemistry II (ed.) L S Hegedus (Oxford, UK: Pergamon) Vol. 12
Nishiyama H, Itoh Y, Sugawara Y, Matsumoto H, Aoki K and Itoh K 1995 Bull. Chem. Soc. Jpn. 68 1247
Nishiyama H, Aoki K, Itoh H, Iwamura T, Sakata N, Kurihara O and Motoyama Y 1996 Chem. Lett. 25 1071
Doyle M P, McKervey M A and Ye T 1998 Modern catalytic methods for organic synthesis with Diazo compounds (New York: Wiley)
Boger D L, Ledeboer M W, Kume M and Jin Q 1999 Angew. Chem. Int. Ed. 38 2424
Salaun J 2000 In Small ring compounds in organic synthesis, VI (ed.) A Meijere (Berlin: Springer), Vol. 207, pp. 1–67
Che C M, Huang J S, Lee F W, Li Y, Lai T S, Kwong H L, Teng P F, Lee W S, Lo W C, Peng S M and Zhou Z Y 2001 J. Am. Chem. Soc. 123 4119
Rodriguez-Garcia C, Oliva A, Ortuno R M and Branchadell V 2001 J. Am. Chem. Soc. 123 6157
Simmons H E and Smith R D 1959 J. Am. Chem. Soc. 81 4256
Simmons H E and Smith R D 1958 J. Am. Chem. Soc. 80 5323
Closs G L and Moss R A 1964 J. Am. Chem. Soc. 86 4042
Maruoka K, Fukutani Y and Yamamoto H 1985 J. Org. Chem. 50 4412
Molander G A, Etter J B and Zinke P W 1987 J. Am. Chem. Soc. 109 453
Molander G A and Harring L S 1989 J. Org. Chem. 54 3525
Charette A B and Beauchemin A 2001 J. Organochem. 617 702
Bernardi F, Bottoni A and Miscione P 1997 J. Am. Chem. Soc. 119 12300
Dargel T K and Koch W 1996 J. Chem. Soc. Perkin-Trans. 2 877
Nakamura M, Hirai A and Nakamura E 2003 J. Am. Chem. Soc. 125 2341
Hermann H, Lohrenz J C W, Kuhn A and Boche G 2000 Tetrahedron. 56 4109
Fang W H, Phillips D L, Wang D and Li Y L 2002 J. Org. Chem. 67 154
Li Z H, Ke Z F and Zhao C Y 2006 Organometallics 25 3735
Zhao C Y, Wang D and Phillips D L 2002 J. Am. Chem. Soc. 124 12903
Stiasny H C and Hoffman R W 1995 Chem. Eur. J. 1 619
Zhao C Y, Wang D and Phillips D L 2003 J. Am. Chem. Soc. 125 15200
Wang D, Zhao C Y and Phillips D L 2004 J. Org. Chem. 69 5512
Becke A D 1993 J. Chem. Phys. 98 5648
Lee C, Yang W and Parr R G 1988 Phys. Rev. B37 785
Gonzalez C and Schlegel H B 1990 J. Phys. Chem. 94 5523
Miertus S, Scrocco E and Tomasi J 1981 J. Chem. Phys. 55 117
Miertus S and Tomasi J 1982 J. Chem. Phys. 65 239
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Zakrzewski V G, Montgomery J A, Stratmann R E, Burant J C, Dapprich S, Millam J M, Daniels A D, Kudin K N, Strain M C, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson G A, Ayala P Y, Cui Q, Morokuma K, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Cioslowski J, Ortiz J V, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Gonzalez C, Challacombe M, Gill P M W., Johnson B G, Chen W, Wong M W, Andres J L, Gonzalez C, Head-Gordon M, Replogle E S and Pople J A 1998 GAUSSIAN98, Revision A.7; Gaussian Inc. Pittsburgh, PA
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, X.H., Zhang, F.L. & Geng, Z.Y. A theoretical study of the carbenoids LiCH2X (X = Cl, Br, I) cyclopropanation reaction with ketene. J Chem Sci 122, 363–369 (2010). https://doi.org/10.1007/s12039-010-0041-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-010-0041-8