Abstract
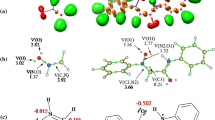
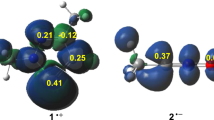
The mechanism and the regioselectivity of the non-polar [3 + 2] cycloaddition reactions between nitrile oxide and two cyclopentenes were theoretically investigated within the molecular electron density theory (MEDT) using DFT methods, namely the B3LYP, MPWB1K and ωB97XD functionals together with the standard 6-31G(d) basis set. The activation energies of these 32CA reactions are relatively high, due to the low nucleophilic power of both cycloalkenes and the relatively moderate electrophilic nature of the nitrile oxide. The B3LYP and MPWB1K functionals reproduced high relative energies values, while the ωB97XD one yields better values in the kinetic and thermodynamic energies. The completely 4-regioselectivity that observed experimentally has been explained by the analysis of the relative energies, in which the formation of 4-regioismeric cycloadducts is always the more kinetically favored one. The electron localization function (ELF) topological analysis showed that the studied 32CA reactions proceed through two-stage one-step nonconcerted mechanism. The effects of the hydrogen bonds on the regioselectivity determination have been investigated by mean both noncovalent interactions (NCI) and quantum theory of atoms in molecule (QTAIM) analyses, which confirm the presence of high strength N − H···O hydrogen bond and a supplementary weak stabilized H…F hydrogen bonds.












Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the supplementary material of this article.
Code availability
All calculations have been performed using the Gaussian 09 program and Multiwfn.
References
N. Nagatoshi, Methods and Applications of Cycloaddition Reactions in Organic Syntheses, Wiley, 2014.
Padwa A (1984) 1,3-Dipolar Cycloaddition Chemistry, Wiley. Interscience, New York 1:2
R. Huisgen, Int. Angew. Chem. Ed. Engl.1963, 2,565–598; O. Tsuge, S. Kanemasa , Advances in Heterocyclic Chemistry.1989, 45, 323–349; Hu. Yunfeng, K. N. Houk, Tetrahedron. 2000, 56, 8239–8243.
Cordero FM, Giomi D, Lascialfari L (2014) Prog Heterocycl Chem 26:319–348
Ríos-Gutiérrez M, Domingo LR, Esseffar M, Oubella A, Itto YA (2020) Molecules 25:1085
a) Nacereddine, AK., Layeb, H., Chafaa, F., Yahia, W., Djerourou, A., & Domingo, L. R. (2015). RSC Advances, 5(79), 64098–64105.; b) Hellel, D., Chafaa, F., Nacereddine, A. K., Djerourou A., & Vrancken, E. (2017).; RSC advances, 7(48), 30128–30141., c) Chafaa, F., Hellel, D., Nacereddine, AK., & Djerourou, A. (2016). Molecular Physics, 114(5), 663–670. ; d) Nacereddine, A. K., Yahia, W., Sobhi, C., Layeb, H., Lechtar, Z and Djerourou, A. (2013). Journal of Applied Biopharmaceutics and Pharmacokinetics, 1, 18–23., e) Barama, L., Bayoud, B., Chafaa, F., Nacereddine, A. K and Djerourou, A. (2018). Structural Chemistry, 29(6), 1709–1721. ; f) Jasiński, R and Dresler, E. (2020). Organics, 1(1), 49–69.
Huisgen R (1967) Cycloadditions-definition, classification, and characterization. Int Angew Chem Ed Engl 6:16
Firestone RA (1968) Mechanism of 1, 3-dipolar cycloadditions. J Org Chem 33:2285–2290
Huisgen R, Mlostoń G, Langhals E (1986) J Org Chem 51:4085
a) T. Hashimoto, K. Maruoka, Chem. Rev. 115 (2015) 5366–5412.; b) S. Roscales, J. Plumet, Org. Biomol. Chem. 16 (2018) 8446–8461., c) Woliński, P., Kącka-Zych, A., Demchuk, O. M., Łapczuk-Krygier, A., Mirosław, B and Jasiński, R. (2020). Journal of Cleaner Production, 275, 122086.
Chien-Liang C, Tzu-Wei C, Yung-Wen C, Jim-Min F (2019) Tetrahedron 75:4458–4470
a) Khorief Nacereddine, A., Merzoud, L., Morell, C., & Chermette, H. (2021). Journal of Computational Chemistry, 42(18), 1296–1311.; b) Chafaa, F., Nacereddine, A. K., & Djerourou, A. (2019). Theoretical Chemistry Accounts, 138(12), 1–11. ; c) Chafaa, F., Hellel, D., Nacereddine, A. K., & Djerourou, A. (2016). Tetrahedron Letters, 57(1), 67–70.
Domingo LR (2016) Molecules 21:1319
Fukui, K. Molecular Orbitals in Chemistry Physics and Biology; Academic Press: New York, NY, USA, 1964.
MJ. Frisch, GW. Trucks, HB. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani,V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian,A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J . Hasegawa, M.Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery, J. E. Peralta, F. Ogliaro Jr, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell J. C. Burant, S. S. Iyengar, J.Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J .J . Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J.Cioslowski, Fox D , Gaussian 09, Revision A.02. Gaussian, Wallingford. 2009.
a) C. Lee, W. Yang, RG. Parr, Phys. Rev.1988 37,785; b) A. D. Becke, J. Chem. Phys. 1993, 98, 1372–1377.
Lynch BJ, Fast PL, Harris M, Truhlar DG (2000) J Phys Chem A 104:4811
Zhao Y, Truhlar DG (2004) J Phys Chem A 108:6908
WJ. Hehre, L. Radom, PVR. Schleyer, J. AB Initio orbital molecular theory A. Pople, Wiley, New York. 1986.
Fukui K (1981) Acc Chem Res 14:363
(a) E. Cances, B. Mennucci and J. Tomasi, J. Chem. Phys., 1997, 107, 3032; (b) M. Cossi, V. Barone, R. Cammi and J. Tomasi, Chem. Phys. Lett., 1996, 255, 327; (c) V. Barone, M. Cossi and J. Tomasi, J. Comput. Chem., 1998, 19, 404.
J. Tomasi, M. Persico, Chem. Rev.1994, 94, 2027–2094; B. Y. Simkin, I. Sheikhet, Quantum chemical and statistical theory of solutions: a computational approach Ellis. Horwood.1995, London.
Becke AD (1993) J Chem Phys 98:5648
Domingo LR (2014) RSC Adv 4:32415–32428
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Tetrahedron 72:1524–1532
A. E. Reed, RB. Weinstock, F . Weinhold, J. Chem. Phys. 1985, 735–746.
a) Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen J, Yang AW (2010) J Am Chem Soc 132, 6498; b) Lane JR, Contreras-Garcia J, Piquemal JP, Miller BJ, Kjaergaard HGJ (2013) Chem Theory Comput 9:3263.
Contreras-Garcia JE, Johnson R, Keinan S, Chaudret R, Piquemal JP, Beratan DN, Yang W (2011) J Chem Theory Comput 7:625
Bader RFW (1990) Atoms in molecules. Claredon press, A quantum theory
Lu T, Chen F (2012) J Comput Chem 33:580–592
Parr RG, Von Szentpaly L, Liu S (1999) J Am Chem Soc 121:1922–1924
(a) RG. Parr, LV. Szentpaly and S. Liu, J. Am. Chem. Soc., 1983, 105, 7512–7516; (b) R. G. Parr and W. Yang, Density Functional Theory of Atoms and Molecules, Oxford University Press, New York, 1989.
(a) LR. Domingo, E. Chamorro and P. Pérez, J. Org. Chem., 2008, 73, 4615–4624; (b) L. R. Domingo and P. Pérez, Org. Biomol. Chem., 2011, 9, 7168–7175.
Kohn W, Sham LJ (1965) Phys Rev 140:1133–1138
Domingo LR, Pérez P, Sáez JA (2013) RSC Adv 3:1486
a) P. Geerlings, F. De Proft, W. Langenaeker, Chem. Rev. 2003, 103, 1793. ; b) L. R. Domingo, M. Ríos-Gutiérrez, P. Pérez, Molecules 2016, 21, 748., c) H. Chermette, J. Comput. Chem. 1999, 20, 129.
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Tetrahedron 58:4417–4423
P. Jaramillo, LR. Domingo, E. Chamorro and PP´erez, J. Mol. Struct.: THEOCHEM, 2008, 865, 68–76.
Domingo LR, Sáez JA (2009) Org Biomol Chem 7:3576
Yahia W, Khorief Nacereddine A, Liacha M, Djerourou A (2018) Int J Quantum Chem 118(11):e25540
Domingo LR, Pérez P, Sáez JA (2012) Org Biomol Chem 10:3841
Benchouk W, Mekelleche SM, Silvi B, Aurell MJ, Domingo LR (2011) J Phys Org Chem 24:611–618
Domingo LR, Aurell MJ, Pérez P (2014) Tetrahedron 70:4519
a) F. Chafaa, A. K. Nacereddine, A. Djerourou, Letter in Organic. 2020, 17, 260–267.; b) A. K. Nacereddine, J. Mol. Mod. 2020, 26, 328.
a) C. Sobhi, A. Khorief Nacereddine, A. Djerourou, M. Ríos-Gutiérrez, L. R. Domingo, J. Phys. Org. Chem. 2016,30,6.; b) Ríos-Gutiérrez M, Nasri L, Khorief Nacereddine A, Djerourou A,Domingo LR (2018) J Phys Org Chem 31:e3830; c) Nasri L, Ríos-Gutiérrez M, Nacereddine AK, Djerourou A, Domingo LR (2017) Theor Chem Accounts 136:104.; d) Nacereddine, A. K., Sobhi, C., Djerourou, A., Ríos-Gutiérrez, M., & Domingo, L. R. (2015). RSC advances, 5(120), 99299–99311.
Grabowski SJ, Sokalski WA, Dyguda E, Leszczynski J (2006) J Phys Chem B 110:6444
Rozas I, Alkorta I, Elguero J (2000) J Am Chem Soc 122:11154
a) Jasiński, R. (2018). Journal of Fluorine Chemistry, 206, 1–7.; b) Sobhi, C., Nacereddine, A. K., Djerourou, A., Aurell, M. J and Domingo, L. R. (2012). Tetrahedron, 68(40), 8457–8462.
Funding
This work was supported by the Ministry of Higher Education and Scientific Research of the Algerian Government [project PRFU Code: A6N0UN202090005].
Author information
Authors and Affiliations
Contributions
Computational calculations were performed by Miss. Hanifa Chouit, conceptualization, methodology and writing original draft of the article were done by Dr. Chafia Sobhi, data curation was done by Dr. Bouasla Souad, the visualization was performed by Miss. Samia Messikh, formal analysis was done by Mr. Azeddine Kheribeche, and reviewing of the article draft and submitting the article were performed by Prof. Abdelmalek Khorief Nacereddine.
Corresponding authors
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chouit, H., Sobhi, C., Bouasla, S. et al. Effect of hydrogen bonds and CF3 group on the regioselectivity and mechanism of [3 + 2] cycloaddition reactions between nitrile oxide and 2,4-disubstituted cyclopentenes. A MEDT study. J Mol Model 28, 104 (2022). https://doi.org/10.1007/s00894-022-05086-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05086-y