Abstract
Spinal cord injury (SCI) is a severe neurological disorder that can lead to paralysis or death. Oxidative stress during SCI is a critical phase causing extensive nerve cell damage and apoptosis, thereby impairing spinal cord healing. Thus, a primary goal of SCI drug therapy is to mitigate oxidative stress. Curculigoside (CUR), a phenolic glucoside extracted from the dried root and rhizome of Curculigo orchioides Gaertn, possesses neuroprotective and antioxidant properties. This study aimed to investigate whether CUR effectively promotes the recovery of spinal cord tissue following SCI and elucidate its mechanism. We employed a hydrogen peroxide (H2O2)-induced PC12 cell model and an SCI rat model to observe the effects of CUR on oxidation and apoptosis. The results demonstrated that CUR significantly reduced the expression of apoptosis-related proteins (Bax and Caspase-3), Annexin V/propidium iodide (PI), and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), while increasing the expression of the anti-apoptotic protein Bcl-2. Moreover, CUR effectively enhanced levels of antioxidants (glutathione [GSH)] and decreased reactive oxygen species (ROS) in vitro. Furthermore, CUR facilitated functional recovery through its anti-apoptotic and anti-oxidative stress effects on spinal cord tissues in SCI rats. These effects were mediated via the Nrf2/NQO1 signaling pathway. Therefore, our study showed that CUR acted as an anti-apoptotic and anti-oxidative stress agent, inhibiting astrocyte activation and promoting neuronal reconstruction and functional recovery. These findings may contribute significantly to the development of SCI treatments and advance the field of SCI drug therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) is a severe neurological condition caused by various factors like accidents, injuries, tuberculosis, and tumors. It typically presents with impairments in limb sensation and movement, loss of reflexes, paralysis, and in severe cases, death [1]. With the advancement of global economy, an increasing number of individuals are affected by SCI, profoundly impacting patients’ physical and mental well-being and imposing significant financial burdens on their families [2]. SCI progresses through two phases: the primary injury phase and the secondary injury phase. Primary SCI results from traumatic events that directly or indirectly impact the spinal cord, causing various irreversible damages that persistently harm spinal cord tissue [3]. Secondary SCI can develop within minutes to days after the primary injury and may continue for about one month, making it more complex than the initial injury. Pathophysiological changes such as oxidative stress, electrolyte disturbances, tissue edema, and cell apoptosis exacerbate spinal tissue damage under stressful microenvironmental conditions [4, 5]. Secondary injuries during SCI are critical because irreversible changes often occur at this stage, significantly affecting the patient’s prognosis. Consequently, recent research on SCI has focused on effective interventions targeting secondary injury [6, 7].
Oxidative stress response is an important pathological process in secondary SCI. Following SCI, there is a reduction in the expression of antioxidant enzymes and the activation of abnormal enzymes, leading to mitochondrial dysfunction, lipid peroxidation, DNA damage, and apoptosis [8, 9]. Moreover, peroxidation and apoptosis by-products exacerbate the production of reactive oxygen species (ROS), accelerating the progression of secondary injury and hindering neuronal regeneration [10]. Therefore, addressing oxidative stress remains a significant focus in neuroscience research, aiming to develop effective solutions to mitigate its impact.
Curculigo orchioides Gaertn is a herbaceous plant belonging to the Asphodelaceae family, widely distributed in tropical regions across Asia, Africa, Oceania, and South America [11]. In traditional Chinese medicine, its dried rhizome, known as "Xian Mao," is renowned for its efficacy in treating various conditions such as joint pain, osteoporosis, and convulsions. Curculigoside (CUR), one of the active components of Curculigo orchioides Gaertn, has demonstrated diverse pharmacological activities in several studies [12,13,14]. Research by Tian et al. highlights the antioxidant and neuroprotective properties of CUR, which play a role in reducing neuronal apoptosis and ROS production [15]. Therefore, CUR holds promise for SCI treatment, although no relevant research has yet been reported on this topic. In this study, we utilized PC12 cell models induced by hydrogen peroxide (H2O2) and SCI rat models to investigate whether CUR can mitigate oxidative stress and prevent neuronal apoptosis. Additionally, we aimed to elucidate the molecular mechanisms underlying these effects. These findings could potentially contribute to the development of pharmaceuticals for the prevention and treatment of SCI.
Materials and Methods
Materials
The following reagents and materials were used in this study: Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum (FBS), and trypsin from Gibco-BRL (New York, USA); penicillin and streptomycin from Hyclone (Logan, USA); CUR sourced from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China); MTT assay kit purchased from Abcam (Cambridge, UK); apoptosis, Western blotting, and real-time quantitative polymerase chain reaction (RT-qPCR) assay kits, as well as H2O2 and Brusatol, obtained from Sigma-Aldrich (Saint Louis, USA); Malondialdehyde (MDA), superoxide dismutase (SOD), ROS, and glutathione (GSH) assay kits acquired from Nanjing Jiancheng Bioengineering Institute (Nanjing, China); and Bcl-2, Caspase-3, Bax, Nrf-2, and NQO-1 antibodies sourced from Affinity Biosciences CO (Beijing, China).
Cell culture
PC12 cells were cultured following a previously published protocol [16]. The cells were maintained in DMEM medium from Gibco-BRL (New York, USA), supplemented with 10% FBS and 1% penicillin/streptomycin from Hyclone (Logan, USA). The cell culture was incubated at 37 °C with 5% CO2. Media were refreshed every 48 h, and subculturing was performed when the cell density reached 90%.
Cell treatment
The PC12 cells were divided into five groups: control group, CUR group, H2O2 group, H2O2 + CUR group, and H2O2 + CUR + Brusatol group. CUR was used at a concentration of 3 μM, H2O2 at a concentration of 300 μM, and Brusatol at a concentration of 100 nM for the treatments.
Cell Viability Assay
According to the previously described method, cell viability was assessed using the MTT assay [17]. The cells were divided into 5 groups and seeded at an initial density of 1 × 104 cells per well for 24 h. Two groups were exposed to varying concentrations of H2O2 (100, 200, 300, 400, 500 μM) and CUR (1, 3, 10 μM). One group was pre-treated with different concentrations of CUR for 2 h, followed by 24 h of co-incubation with 300 μM H2O2. The last group was treated with 300 μM H2O2 and incubated for varying durations. After incubation, 10 μL of MTT solution (5 mg/mL) was added to each well and incubated for 3 h at 37 °C. The formazan crystals formed were dissolved in 100 μL of Dimethylsulfoxide (DMSO) per well [18]. The mixture was shaken at room temperature for 15 min, and absorbance was measured at 570 nm using a microplate reader (Bio-Rad, USA) to determine cell viability.
Apoptosis Assay
The apoptosis rate of cells was determined using the Annexin V/propidium iodide (PI) double-staining method and Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, following established protocols [19, 20]. For Annexin V/PI staining, treated cells were collected, washed, and resuspended in binding buffer containing Annexin V-FITC and PI. After incubating the cells for 15 min, flow cytometry analysis was performed.
The TUNEL assay was performed utilizing the TUNEL apoptosis detection kit, where 4’,6-diamidino-2-phenylindole (DAPI) was used to stain the nuclei. The resulting observations were recorded using fluorescence microscopy (Olympus, Japan).
RT-qPCR Analysis
Total RNA extraction was conducted using the TRIzol reagent following treatment. RT-PCR analysis was carried out using the First-Strand cDNA Synthesis Kit in combination with the PrimeScript RT reagent kit on a SYBR green system (Toyobo, Japan Ltd., Japan). The primer sequences used are detailed in Table 1.
Western Blotting Analysis
Protein concentrations were determined using the BCA protein assay kit. The proteins were separated on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and subsequently transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, USA). The membrane was blocked overnight at 4 °C with the respective primary antibodies (Bcl-2, Caspase-3, Bax, Nrf-2, NQO-1). Following this, the membrane was washed with tris buffered saline + 0.5% [v/v] Tween-20 (TBST) and incubated for 2 h at room temperature with HRP-conjugated secondary antibodies (1:1000 dilution). Glyceraldehyde phosphate dehydrogenase (GAPDH) at a 1:1000 dilution served as the internal standard. Quantitative densitometric analysis was conducted using the ImageQuant LAS 4000 mini detection system (GE Healthcare, Buckinghamshire, UK), and the results were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Oxidative Stress Assay
For the in vitro test, intracellular ROS levels were assessed using DCFH-DA reagent following the manufacturer’s instructions. Fluorescence images were captured using a fluorescence microscope and analyzed with ImageJ software. Additionally, GSH concentration was determined using a GSH assay reagent.
In the in vivo test, oxidative stress markers including MDA, SOD, and GSH were measured using specific assay kits. Spinal tissue samples were processed according to the manufacturer’s instructions to extract the liquid supernatant for analysis. MDA levels were measured at 532 nm, he activity of SOD was assessed at 550 nm, and GSH levels were quantified at 420 nm using a microplate reader.
Animals
All animal experiments were conducted following approval from the Ethics Committee of Experimental Animal Management at Guangzhou University of Chinese Medicine (approval number: 20220803007). Sprague–Dawley (SD) male rats weighing 200-250 g were obtained from the Experimental Animal Center of Guangzhou University of Chinese Medicine and housed in the animal laboratory. The rats underwent a one-week adaptation period under the following conditions: five rats per cage, ambient temperature maintained at 22–26 °C, a 12-h light–dark cycle, and humidity ranging from 55 to 68%.
Establishment of the Rat Model of SCI
The SCI animal model was induced using Allen’s method [21]. Rats were anesthetized, secured for shaving, and a median incision was made on the back to expose the T9-11 spinous processes and lamina, using T10 as a reference point. The spinous process and lamina of T10 were removed to expose the intact spinal cord underneath. A PinPoint™ precision SCI impactor was used to strike the T10 spinal cord with a velocity of 1.2 m/s, a depth of 1.0 mm, and a contact time of 85 ms. This caused rapid congestion and tissue redness in the T10 region. Transient spasms and convulsions in the tails and hind limbs confirmed successful establishment of the SCI model. Subsequently, hemostasis was achieved and the wound was sutured, followed by iodine application to the sutured skin. In sham-operated rats, the spinal cords were exposed similarly but not subjected to the impact procedure..
Grouping and Treatment of SCI Model
Fifty mature male Sprague–Dawley (SD) rats weighing 200-250 g were randomly divided into four groups, each comprising 30 rats: (1) sham group, (2) SCI model group, (3) SCI + NS group, and (4) SCI + CUR group. One day after successful SCI modeling, the rats received oral treatments once daily for 14 days at a dose of 50 mg/kg. The SCI + NS group was administered 10 mL/kg of normal saline, while the SCI + CUR group received 50 mg/kg of CUR. On day 14 post-treatment, the rats were euthanized for analysis.
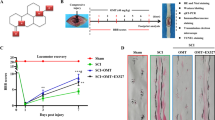
Locomotion Recovery Assay
To evaluate the mobility of rats following SCI, inked footprint analysis and the Basso-Beattie-Bresnahan (BBB) locomotion rating scale were used [22, 23]. Rats were allowed 15 min of free activity in an open field on days 3, 7, and 14 post-surgery. The BBB scale, ranging from 0 to 21 points, was used to assess hindlimb locomotion ability (with 0 indicating paralysis and 21 indicating normal mobility). Inked footprint analysis was conducted on day 14 after injury: the rats’ hind paws were coated with dye and they were guided in a straight line across white paper to capture representative images of their gait and coordination.
Tissue Preparation And Preservation
After euthanizing the rats, tissue samples were collected from the lesion site in the spinal cord. One portion of the tissue was promptly frozen in liquid nitrogen for subsequent oxidative stress assays and western blotting analysis. The remaining tissue was decalcified in 10% ethylenediamine tetraacetic acid (EDTA), dried, embedded in paraffin wax, and then sectioned at a thickness of 5 μm. These sections were used for histological staining and immunohistochemical analysis.
Histopathological Staining
The tissue sections were stained with hematoxylin and eosin (HE) for general histology and with cresyl violet for Nissl staining to assess neuronal morphology and density. Histopathological changes and lesions were observed using an inverted microscope (Olympus, Japan).
Immunofluorescence Staining and Analysis
Before antigen retrieval with 0.01 M citric acid (pH 6.0) at 95 °C for 10 min, tissue sections underwent deparaffinization with xylene and hydration through varying alcohol concentrations. Subsequently, the sections were rinsed with 0.01 mol/L phosphate-buffered saline (PBS) and incubated in a 10% goat serum antibody blocking solution for 2 h at room temperature. Following blocking, the tissues were incubated overnight at 4 °C with primary antibodies against MAP2 (1:200, Boster Biological Engineering Co.), GFAP (1:200, Boster Biological Engineering Co.), and NeuN (1:200, Boster Biological Engineering Co.). The next day, the tissue sections were incubated with Alexa Fluor 594-conjugated goat anti-rat IgG secondary antibody (1:300, Invitrogen) diluted in PBS. Nuclei were counterstained with DAPI. Finally, fluorescent images were captured using a fluorescence microscope to visualize the labeled proteins and cellular structures.
Statistical Analysis
The data were presented as mean ± standard deviation. Statistical analyses were conducted using SPSS version 16.0 software (SPSS Inc., Chicago, IL, USA). Differences among groups were assessed using either one-way analysis of variance (ANOVA) or Student’s t-test. A P value less than 0.05 was considered statistically significant.
Results
Establishment of PC12 Cell Injury Model using H2O2
The PC12 cell line was originally derived from rat pheochromocytoma. Due to its morphological, structural, and functional similarity to neuronal cells, it is widely used in in vitro studies as a model for neuronal cells [24]. PC12 cells exhibit oxidative stress, mitochondrial damage, and apoptosis when stimulated with H2O2 similar to the conditions observed in spinal cord cells of individuals with SCI [25]. To determine the optimal concentrations of H2O2 and CUR, cell viability was assessed using the MTT assay. Following exposure to varying concentrations of H2O2 for 24 h, a significant reduction in cell viability was observed (P < 0.05), with viability dropping to approximately 50% at an H2O2 concentration of 300 μM (Fig. 1-b). Additionally, treatment of PC12 cells with 300 μM H2O2 for different durations revealed a significant decline in cell viability after 24 h of exposure (Fig. 1-c). Therefore, a 24-h treatment with 300 μM H2O2 proved most effective in eliciting cellular responses in PC12 cells [19].
Effects of CUR on the viability of H2O2-induced PC12 cells. a Molecular structure of CUR. b Viability of PC12 cells treated with different concentrations of H2O2(100, 200, 300, 400, 500 μM) for 24 h. (c) Viability of PC12 cells cultured with 300 μM H2O2 for different durations (12, 24, 48 h). (d) Viability of PC12 cells treated with different concentrations of CUR (1, 3, 10 μM) for 24 h. (e) Viability of PC12 cells incubated with 300 μM H2O2 for 24 h after treatment with different concentrations of CUR (1, 3, 10 μM). Data are presented as mean ± standard error of the mean (SEM). ⋆P < 0.05 compared to the control group. # P < 0.05 compared to the H2O2-induced alone group. CUR, Curculigoside
Effect of CUR on PC12 Cell Viability Induced by H2O2
To investigate the impact of CUR (molecular structure in Fig. 1-a) on PC12 cell activity following H2O2 treatment, we initially assessed its effects on normal PC12 cells. Our results clearly demonstrated that CUR had no observable effect on normal PC12 cells, regardless of concentration (Fig. 1-d). However, when PC12 cells were exposed to H2O2, their viability decreased significantly following treatment with CUR concentrations of 1, 3, and 10 μM (P < 0.05). It indicated that the protective effect of curcumin on PC12 cells was evident only at specific concentrations, showing a dose-dependent response (Fig. 1-e). Consequently, a concentration of 3 μM was selected for subsequent experiments.
Protective Effect of CUR on H2O2-induced Apoptosis in PC12 cells
Apoptosis-related tests indicated no statistically significant differences between the control group (4.46% ± 0.22) and the group treated with CUR (4.60% ± 0.23) in terms of the percentage of double-stained positive cells (P > 0.05). However, following treatment with H2O2, there was a significant increase in the proportion of double-stained positive cells (P < 0.05), which was reversed upon addition of CUR (P < 0.05). Subsequently, the proportion of positive cells increased significantly after pre-treatment with Brusatol in the H2O2 + CUR + Brusatol group, demonstrating a significant difference (P < 0.05) (Fig. 2-a, b). These findings were consistent with results obtained from the TUNEL assay (Fig. 2-c, d). Furthermore, analysis of apoptosis-related proteins in PC12 cells induced by H2O2 revealed upregulation of pro-apoptotic proteins Bax and Caspase-3, alongside downregulation of the anti-apoptotic protein Bcl-2 at both the protein and mRNA levels. However, pre-treatment with CUR effectively reversed this expression pattern (Fig. 2-e, f, g). Lastly, to explore the relationship between CUR’s anti-apoptotic and anti-oxidative properties, we utilized Brusatol, an inhibitor of Nrf-2 signaling. Interestingly, Brusatol was found to attenuate the inhibitory effect of CUR against apoptosis.
Effect of CUR on H2O2-induced apoptosis in PC12 cells. (a-b) Annexin V/PI double-staining method used to evaluate apoptosis. (c-d) Quantification of apoptosis using the TUNEL assay. (e) Gene expression levels of Bax, Caspase-3, and Bcl-2 in PC12 cells measured by RT-qPCR. (f-g) Protein expression levels of Bax, Caspase-3, and Bcl-2 in PC12 cells measured by Western blot. Data are presented as mean ± standard error of the mean (SEM). ⋆P < 0.05 compared to the control group, # P < 0.05 compared to the H2O2-induced alone group, and & P < 0.05 compared to the CUR + H2O2-induced group
In Vitro Antioxidative Effects of CUR
The results obtained using the DCFH-DA probe revealed that H2O2 stimulation significantly increased the fluorescence intensity of intracellular ROS compared to the control group (P < 0.05). However, pre-treatment with CUR resulted in a significant reduction in ROS fluorescence intensity following H2O2 stimulation (P < 0.05). Conversely, when PC12 cells pre-treated with CUR were co-treated with Brusatol, the fluorescence intensity of ROS increased significantly (P < 0.05) (Fig. 3-a, b). Similarly, the level of GSH was significantly elevated after H2O2 stimulation (P < 0.05), but it decreased significantly with CUR treatment (P < 0.05). Importantly, intervention with Brusatol reversed this decrease (P < 0.05) (Fig. 3-c). These findings provide compelling evidence of CUR’s protective activity against oxidative stress in PC12 cells.
Inhibition of H2O2-induced oxidative stress by CUR in PC12 cells. (a-b) ROS levels were measured using H2DCF-DA and analyzed for fluorescence intensity. (c) GSH levels were measured using a GSH assay kit. Data are presented as mean ± standard error of the mean (SEM). ⋆P < 0.05 compared to the control group, # P < 0.05 compared to the H2O2-induced alone group, and & P < 0.05 compared to the CUR + H2O2-induced group. CUR, Curculigoside; ROS, reactive oxygen species; GSH, glutathione
CUR Inhibited H2O2-induced Apoptosis of PC12 Cells through the Nrf-2/NQO-1 Signaling Pathway
To investigate the involvement of the Nrf-2/NQO-1 signaling pathway in the antioxidative stress effects of CUR, Western blotting was employed to assess the levels of Nrf-2 and NQO-1 proteins. As depicted in Fig. 4, Nrf-2 and NQO-1 protein expression significantly decreased in H2O2-stimulated cells (P < 0.05), but this reduction was considerably reversed following CUR treatment(P < 0.05). Furthermore, there was a significant decrease in the protein levels of Nrf-2 and NQO-1 in the H2O2 + CUR + Brusatol group (P < 0.05). In conclusion, CUR’s antioxidant and anti-apoptotic effects are mediated through the Nrf-2/NQO-1 signaling pathway.
Protective effect of CUR on PC12 cells against apoptosis and oxidative stress induced by H2O2 via the Nrf-2/NQO-1 signaling pathway. Data are presented as mean ± standard error of the mean (SEM). ⋆P < 0.05 compared to the control group, # P < 0.05 compared to the H2O2-induced alone group, and & P < 0.05 compared to the CUR + H2O2-induced group. CUR, Curculigoside
CUR Promoted Functional Recovery in SCI rats
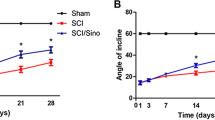
A rat model of SCI (Fig. 5-a) was used to assess the impact of CUR on hind limb movement using the BBB locomotion rating scale and inked footprint analysis. Footprint analysis revealed that rats in the sham group exhibited normal gait, while those in the SCI and SCI + NS groups displayed impaired gait. However, following 14 days of CUR intervention, SCI rats demonstrated partial improvement in the gait of their right hind limb (Fig. 5-b). These findings were further confirmed by the results of the BBB scale (Fig. 5-f). No significant differences were observed between the SCI and SCI + NS groups in terms of functional scores. Nevertheless, after CUR intervention, the BBB scores exhibited an upward trend, with significant differences observed on the 7th and 14th days. These results suggest that CUR could promote the recovery of motor function in the rat model of SCI.
Effect of CUR on tissue repair and motor function recovery following spinal cord injury in rats within 14 days. (a) Hindlimb status of SCI rats. (b) Changes in hindlimb motor function evaluated by inked footprint analysis in SCI rats. (c) Results of HE staining of spinal cord tissue from SCI rats. (d) Results of Nissl staining of spinal cord tissue from SCI rats. (e) Quantification of surviving neurons in spinal cord tissue. (f) Motor function scores of rats in each group. Data are presented as mean ± standard error of the mean (SEM). ⋆P < 0.05 compared to the control group, # P < 0.05 compared to the SCI + NS group (n = 5). CUR, Curculigoside; SCI, spinal cord injury
CUR Inhibited Pathological Changes in Spinal Cord Tissue of SCI Rats
This study aimed to investigate the effect of CUR on the histopathology of SCI in rodents. Results from HE staining indicated that spinal cord tissue from rats in the sham group exhibited normal characteristics, including abundant cells, normal morphology, and dense distribution. In contrast, the SCI group and SCI + NS group showed abnormal spinal cord tissue morphology, characterized by a significant decrease in cell numbers and the presence of multiple large cavities. Following CUR intervention, most areas of the spinal cord tissue returned to normal, with only small vacancies remaining in some regions. Moreover, the number of neural cells with normal morphology increased significantly (Fig. 5-c, e). Nissl staining further demonstrated an increase in the number of Nissl bodies and a reduction in the number of cavities in the CUR-treated group compared to the untreated group (Fig. 5-d). Immunofluorescence staining revealed a significant decrease in MAP2 and NeuN-positive cells, along with a significant increase in GFAP-positive cells in the SCI group and SCI + NS group compared to the sham group. Importantly, these changes were reversed upon administration of CUR (Fig. 6).
Effect of CUR on astrocyte activation and neuronal cell apoptosis. (a-c) Immunofluorescence staining of MAP2, GFAP, and NeuN in spinal tissue samples. Data are presented as mean ± standard error of the mean (SEM). ⋆P < 0.05 compared to the SCI group, # P < 0.05 compared to the SCI + NS group (n = 5). CUR, Curculigoside; SCI, spinal cord injury
Effects of CUR on Apoptosis-Related Proteins Bcl-2, Caspase-3, and Bax in Spinal Cord Tissues of SCI Rats
To investigate the effect of CUR on apoptotic cells in the spinal cord tissue of rats with SCI, the expression of apoptosis-related proteins Bax, Caspase-3, and Bcl-2 in spinal tissue samples was evaluated. Compared to the sham operation group, the expression of Bax and Caspase-3 proteins was found to be upregulated (P < 0.05), while the expression of Bcl-2 protein was downregulated (P < 0.05) in both the SCI and SCI + NS groups. However, intervention with CUR significantly reversed these alterations (Fig. 7). These findings indicated that CUR effectively mitigated apoptosis in SCI.
Effect of CUR on apoptosis in a rat SCI model. (a-d) Western blot analysis of protein expression levels of Bax, Bcl-2, and Caspase-3 in spinal tissue samples. Data are presented as mean ± standard error of the mean (SEM). ⋆P < 0.05 compared to the SCI group, # P < 0.05 compared to the SCI + NS group (n = 5). CUR, Curculigoside; SCI, spinal cord injury
Effect of CUR on Oxidative Stress in Spinal Cord Tissues of SCI Rats
To evaluate the effect of CUR on oxidative stress in spinal cord tissues of rats with SCI, SOD, MDA, and GSH assay kits were used to analyze the supernatant of spinal cord tissue samples. Compared to the sham operation group, the levels of SOD and GSH were significantly reduced (P < 0.05), while the level of MDA was elevated (P < 0.05) in both the SCI and SCI + NS groups. However, following intervention with CUR, the levels of SOD, MDA, and GSH were significantly restored (P < 0.05). These findings highlighted that CUR effectively mitigated oxidative stress in SCI (Fig. 8).
CUR decreased oxidative stress in a rat SCI model. (a) The level of SOD was measured by a SOD assay kit. (b) The level of MDA was measured by an MDA assay kit. (c) The level of GSH was measured by a GSH assay kit. Data are presented as mean ± standard error of the mean (SEM). ⋆P < 0.05 compared with the SCI group, # P < 0.05 compared with the SCI + NS group (n = 5). CUR, Curculigoside; MDA, Malondialdehyde; SOD; superoxide dismutase; ROS, reactive oxygen species; GSH, glutathione
CUR Protected the Spinal Cord of SCI Rats via the Nrf-2/NQO-1 Signaling Pathway
To investigate whether the protective effect of CUR is mediated through the Nrf-2/NQO-1 signaling pathway, the levels of Nrf-2 and NQO-1 proteins in spinal tissue samples were analyzed. As depicted in Fig. 9, the protein levels of Nrf-2 and NQO-1 were significantly reduced in both the SCI and SCI + NS groups (P < 0.05). However, following intervention with CUR, there was a significant increase in the protein levels of Nrf-2 and NQO-1 (P < 0.05). These results strongly suggest that the Nrf-2/NQO-1 signaling pathway might play a crucial role in mediating the protective effects of CUR.
The therapeutic role of CUR in SCI involves modulation of the Nrf-2/NQO-1 signaling pathway. Protein expression levels of Nrf-2 and NQO-1 in spinal tissue samples were analyzed using Western blotting. Data are presented as mean ± standard error of the mean (SEM). ⋆P < 0.05 compared to the SCI group, # P < 0.05 compared to the SCI + NS group (n = 5). CUR, Curculigoside; SCI, spinal cord injury
Discussion
Since ancient times, SCI has been recognized as a severe neurological condition [26, 27]. According to data from the Global Burden of Disease (GBD) released in 2019, approximately 0.93 million new cases of SCI are reported annually, resulting in a total of 27 million individuals living with SCI [28]. Despite significant advancements in SCI research, there remains a lack of universally recognized and fully effective treatments [1]. Pharmacological treatment is among the strategies aimed at protecting neurons, inhibiting nerve cell apoptosis, and promoting nerve cell regeneration [29]. Numerous studies have highlighted the importance of drugs in countering oxidative stress and inhibiting apoptosis [30,31,32,33,34]. CUR is known for its antioxidant properties. For instance, Liu et al. [35] demonstrated that CUR promoted osteoclastogenesis through its antioxidant effects, while Xu et al. [36] showed that CUR protected against cerebral ischemia by combating oxidative stress. However, its effects specifically on SCI have remained obscure.
In this study, we utilized H2O2-induced PC12 cells to investigate the anti-apoptotic and antioxidant properties of CUR, consistent with findings from previous research [37]. Initially, in vitro treatment with CUR significantly enhanced the viability of PC12 cells and reduced the incidence of apoptosis. Analysis of apoptosis-related proteins revealed decreased expression of the key apoptotic proteins Bax and Caspase-3, alongside increased expression of the anti-apoptotic protein Bcl-2. Markers of oxidative stress indicated elevated levels of GSH and reduced levels of ROS expression. Subsequently, we established an SCI rat model and conducted in vivo experiments to confirm the effects of CUR. Additionally, in an in vitro model using H2O2-induced PC12 cells, treatment with Brusatol increased the protein levels of Nrf-2 and NQO-1, suggesting that the molecular mechanisms underlying the antioxidative and anti-apoptotic effects of CUR may involve the Nrf-2/NQO-1 signaling pathway.
Apoptosis, a programmed cell death process mediated by cysteine aspartate-specific proteases (caspases), is prominently observed in SCI [38]. There are two classical apoptotic pathways, and Caspase-3, a pivotal protease in apoptosis, initiates both pathways [33, 39]. Additionally, the Bcl-2/Bax family of proteins plays a critical role, where Bcl-2 inhibits apoptosis while Bax promotes it [40]. The interaction between these proteins forms heterodimers that reduce the release of cytochrome c, effectively inhibiting Caspase-mediated cell apoptosis [41]. Consequently, the ratio of Bcl-2 to Bax proteins has been explored as an effective measure to halt the apoptotic process in neuronal cells [42]. In this study, we assessed apoptosis-related proteins and employed the Annexin V/PI and TUNEL methods to confirm that H2O2 induces cell apoptosis, consistent with prior research [43,44,45,46]. Treatment with CUR reversed these outcomes both in vitro and in vivo, underscoring its anti-apoptotic effects. Previous studies have indicated that the neuroprotective effect of CUR may involve concentration-dependent reductions in apoptosis and necrosis [15], which was consistent with the findings of this study.
Oxidative stress, characterized by an imbalance between elevated levels of ROS and antioxidant defenses, represents a significant pathological process in SCI [47]. Normally, ROS are present in spinal cord tissue and are regulated by antioxidant enzymes. However, disruption of this balance, due to the spinal cord’s high metabolic rate and relatively weak antioxidant capacity, can lead to damage to organelles and cytoskeletal structures caused by excessive oxygen free radicals [48]. Furthermore, polyunsaturated fatty acids in cell membranes can bind ROS, forming highly reactive peroxyl radicals that initiate a chain reaction with other polyunsaturated fatty acids within the cell or in neighboring cells, leading to a progressive and continuous oxidative damage. Unfortunately, spinal cord tissue contains a high concentration of fatty acids, making it particularly susceptible to oxidative stress[2]. A key aspect in evaluating oxidative stress is the assessment of essential indicators such as GSH (an initial antioxidant defense), SOD (an enzyme that catalyzes the dismutation of superoxide radicals), and MDA (a final product of lipid peroxidation) [49, 50]. In this study, CUR treatment demonstrated enhanced antioxidative stress capacity in both in vivo and in vitro models. It suggests that CUR’s antioxidative stress properties may play a beneficial role in neurological disorders. There has been emerging research indicating that CUR reduces and alleviates oxidative stress in brain tissue of mice with Alzheimer’s disease [51], which supports the findings of the current study.
To investigate the molecular mechanisms underlying the antioxidant and anti-apoptotic effects of CUR, we conducted an in vitro study using Brusatol to inhibit the Nrf-2/NQO-1 signaling pathway. Our findings revealed that Brusatol attenuated the efficacy of CUR. Brusatol is a specific inhibitor of the Nrf-2/NQO-1 pathway, which is crucial for the effectiveness of CUR [52]. Among the various pathways involved in oxidative stress, the Nrf-2/NQO-1 signaling pathway is particularly pivotal [53, 54]. Under normal physiological conditions, Nrf-2, a redox-sensitive transcription factor, remains inhibited in the cytoplasm through its interaction with Kelch-like ECH-associated protein-1 (Keap1). However, during oxidative stress, Nrf-2 dissociates from Keap1 and translocates into the nucleus, where it binds to the antioxidant response element (ARE) and activates the expression of downstream antioxidant enzymes such as HO-1 and NQO1, thereby exerting antioxidant effects [55, 56]. Our study demonstrated a significant decrease in the protein levels of Nrf-2 and NQO-1 in PC12 cells upon the induction of H2O2. However, treatment with CUR led to a significant increase in the protein levels of Nrf-2 and NQO-1. This effect was consistent in both in vivo and in vitro models. These findings strongly suggest that CUR exerts its antioxidant and anti-apoptotic effects through modulation of the Nrf-2/NQO-1 signaling pathway, consistent with previous findings by Du et al. [57].
Following SCI, the glial scar formation contributes significantly to the progression of injury [58]. During the secondary injury phase, activated astrocytes in spinal cord tissues transform into reactive astrocytes, which release chondroitin sulfate and chondroitin sulfate proteoglycans, forming a barrier that impedes neuronal reconstruction [26, 59]. GFAP serves as a marker protein for astrocytes and correlates with cellular reactivity, while NeuN is a marker for mature and stable neuronal cells, and MAP2 is crucial for neuronal development, maturation, plasticity, and memory formation [60,61,62]. In the SCI group, there was a significant decrease in NeuN- and MAP2-positive cells, alongside an increase in GFAP-positive cells. In contrast, CUR intervention showed promising results in inhibiting glial scar formation and promoting neuronal reconstruction.
In this study, we also examined the spinal tissue and hind limb motor function in rats with SCI. In the SCI animal model, severe damage to the spinal tissue was evident, accompanied by nearly complete loss of motor function, consistent with established standards [63]. Following treatment with CUR, a notable improvement in hind limb motor function was observed within 14 days, along with evidence of partial repair in the spinal tissue. These findings highlight the therapeutic potential of CUR in animal models of SCI.
Conclusion
In summary, CUR was demonstrated to play a crucial role in inhibiting apoptosis and oxidative stress induced by H2O2 in PC12 cells in vitro. Moreover, it enhanced spinal cord tissue and hind limb function through its antioxidative properties in vivo. This therapeutic effect was mediated by CUR’s suppression of oxidative stress via the Nrf2/NQO1 signaling pathway, which ultimately reduced neuronal cell apoptosis. These findings present promising implications for the development of drugs targeting SCI and advancing SCI treatment strategies.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Courtine G, Sofroniew MV (2019) Spinal cord repair: advances in biology and technology. Nat Med 25:898–908. https://doi.org/10.1038/s41591-019-0475-6
Quadri SA, Farooqui M, Ikram A, Zafar A, Khan MA, Suriya SS et al (2020) Recent update on basic mechanisms of spinal cord injury. Neurosurg Rev 43:425–441. https://doi.org/10.1007/s10143-018-1008-3
Orr MB, Gensel JC (2018) Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics 15:541–553. https://doi.org/10.1007/s13311-018-0631-6
Anjum A, Yazid MD, FauziDaud M, Idris J, Ng AMH, SelviNaicker A et al (2020) Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. IJMS 21:7533. https://doi.org/10.3390/ijms21207533
Oyinbo CA (2011) Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 71:281–299
Sutor TW, Kura J, Mattingly AJ, Otzel DM, Yarrow JF (2022) The Effects of Exercise and Activity-Based Physical Therapy on Bone after Spinal Cord Injury. IJMS 23:608. https://doi.org/10.3390/ijms23020608
Karsy M, Hawryluk G (2019) Modern Medical Management of Spinal Cord Injury. Curr Neurol Neurosci Rep 19:65. https://doi.org/10.1007/s11910-019-0984-1
Berghe TV, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N et al (2010) Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ 17:922–930. https://doi.org/10.1038/cdd.2009.184
Papastefanaki F, Matsas R (2015) From demyelination to remyelination: The road toward therapies for spinal cord injury: Myelin Repair in Spinal Cord Injury. Glia 63:1101–1125. https://doi.org/10.1002/glia.22809
Squair JW, Bélanger LM, Tsang A, Ritchie L, Mac-Thiong J-M, Parent S et al (2017) Spinal cord perfusion pressure predicts neurologic recovery in acute spinal cord injury. Neurology 89:1660–1667. https://doi.org/10.1212/WNL.0000000000004519
Wang S, Liu W, Wang J, Bai X (2020) Curculigoside inhibits ferroptosis in ulcerative colitis through the induction of GPX4. Life Sci 259:118356. https://doi.org/10.1016/j.lfs.2020.118356
Wu Q, Fu D-X, Hou A-J, Lei G-Q, Liu Z-J, Chen J-K et al (2005) Antioxidative Phenols and Phenolic Glycosides from Curculigo orchioides. Chem Pharm Bull 53:1065–1067. https://doi.org/10.1248/cpb.53.1065
Ding H, Gao G, Zhang L, Shen G, Sun W, Gu Z et al (2016) The protective effects of curculigoside A on adjuvant-induced arthritis by inhibiting NF-кB/NLRP3 activation in rats. Int Immunopharmacol 30:43–49. https://doi.org/10.1016/j.intimp.2015.11.026
Wang L, He Y, Han T, Zhao L, Lv L, He Y et al (2017) Metabolites of curculigoside in rats and their antiosteoporotic activities in osteoblastic MC3T3-E1 cells. Fitoterapia 117:109–117. https://doi.org/10.1016/j.fitote.2017.01.009
Tian Z, Yu W, Liu H, Zhang N, Li X, Zhao M et al (2012) Neuroprotective effects of curculigoside against NMDA-induced neuronal excitoxicity in vitro. Food Chem Toxicol 50:4010–4015. https://doi.org/10.1016/j.fct.2012.08.006
Xie D, Deng T, Zhai Z, Qin T, Song C, Xu Y et al (2023) Moschus exerted protective activity against H2O2-induced cell injury in PC12 cells through regulating Nrf-2/ARE signaling pathways. Biomed Pharmacother 159:114290. https://doi.org/10.1016/j.biopha.2023.114290
Khan A, Shal B, Khan AU, Ullah R, Baig MW, UlHaq I et al (2021) Suppression of TRPV1/TRPM8/P2Y Nociceptors by Withametelin via Downregulating MAPK Signaling in Mouse Model of Vincristine-Induced Neuropathic Pain. IJMS 22:6084. https://doi.org/10.3390/ijms22116084
Li X, Zhan J, Hou Y, Hou Y, Chen S, Luo D et al (2019) Coenzyme Q10 Regulation of Apoptosis and Oxidative Stress in H 2 O 2 Induced BMSC Death by Modulating the Nrf-2/NQO-1 Signaling Pathway and Its Application in a Model of Spinal Cord Injury. Oxid Med Cell Longev 2019:1–15. https://doi.org/10.1155/2019/6493081
Wang N, Yang Y, Pang M, Du C, Chen Y, Li S et al (2020) MicroRNA-135a-5p Promotes the Functional Recovery of Spinal Cord Injury by Targeting SP1 and ROCK. Molecular Therapy - Nucleic Acids 22:1063–1077. https://doi.org/10.1016/j.omtn.2020.08.035
Zhou K, Zheng Z, Li Y, Han W, Zhang J, Mao Y et al (2020) TFE3, a potential therapeutic target for Spinal Cord Injury via augmenting autophagy flux and alleviating ER stress. Theranostics 10:9280–9302. https://doi.org/10.7150/thno.46566
Liu J, Zhang S, Gu B, Li H, Wang S, Zhang S (2017) Methotrexate combined with methylprednisolone for the recovery of motor function and differential gene expression in rats with spinal cord injury. Neural Regen Res 12:1507. https://doi.org/10.4103/1673-5374.215263
Basso DM, Beattie MS, Bresnahan JC (1995) A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J Neurotrauma 12:1–21. https://doi.org/10.1089/neu.1995.12.1
Rivlin AS, Tator CH (1977) Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg 47:577–581. https://doi.org/10.3171/jns.1977.47.4.0577
Tan W, Zheng Q, Feng K, Feng X, Zhong W, Liao C et al (2022) Neuroprotection of Gastrodia elata polyphenols against H2O2-induced PC12 cell cytotoxicity by reducing oxidative stress. Front Pharmacol 13:1050775. https://doi.org/10.3389/fphar.2022.1050775
Zhong L, Fang S, Wang A-Q, Zhang Z-H, Wang T, Huang W et al (2022) Identification of the Fosl1/AMPK/autophagy axis involved in apoptotic and inflammatory effects following spinal cord injury. Int Immunopharmacol 103:108492. https://doi.org/10.1016/j.intimp.2021.108492
Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A et al (2017) Traumatic spinal cord injury. Nat Rev Dis Primers 3:17018. https://doi.org/10.1038/nrdp.2017.18
Hughes JT (1988) The Edwin Smith Surgical Papyrus: an analysis of the first case reports of spinal cord injuries. Spinal Cord 26:71–82. https://doi.org/10.1038/sc.1988.15
James SL, Theadom A, Ellenbogen RG, Bannick MS, Montjoy-Venning W, Lucchesi LR et al (2019) Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology 18:56–87. https://doi.org/10.1016/S1474-4422(18)30415-0
Venkatesh K, Ghosh SK, Mullick M, Manivasagam G, Sen D (2019) Spinal cord injury: pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res 377:125–151. https://doi.org/10.1007/s00441-019-03039-1
Jiang D, Yang X, Ge M, Hu H, Xu C, Wen S, et al. Zinc defends against Parthanatos and promotes functional recovery after spinal cord injury through SIRT3 ‐mediated anti‐oxidative stress and mitophagy. CNS Neurosci Ther 2023:cns.14222. https://doi.org/10.1111/cns.14222.
Chio JCT, Punjani N, Hejrati N, Zavvarian M-M, Hong J, Fehlings MG (2022) Extracellular Matrix and Oxidative Stress Following Traumatic Spinal Cord Injury: Physiological and Pathophysiological Roles and Opportunities for Therapeutic Intervention. Antioxid Redox Signal 37:184–207. https://doi.org/10.1089/ars.2021.0120
Zrzavy T, Schwaiger C, Wimmer I, Berger T, Bauer J, Butovsky O et al (2021) Acute and non-resolving inflammation associate with oxidative injury after human spinal cord injury. Brain 144:144–161. https://doi.org/10.1093/brain/awaa360
Abbaszadeh F, Fakhri S, Khan H (2020) Targeting apoptosis and autophagy following spinal cord injury: Therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacol Res 160:105069. https://doi.org/10.1016/j.phrs.2020.105069
Zou P, Zhang X, Zhang R, Chai X, Zhao Y, Li E et al (2022) Blockage of ERCC6 Alleviates Spinal Cord Injury Through Weakening Apoptosis, Inflammation, Senescence, and Oxidative Stress. Front Mol Biosci 9:853654. https://doi.org/10.3389/fmolb.2022.853654
Liu M, Liu S, Zhang Q, Fang Y, Yu Y, Zhu L et al (2021) Curculigoside attenuates oxidative stress and osteoclastogenesis via modulating Nrf2/NF-κB signaling pathway in RAW2647 cells. J Ethnopharmacol 275:114129. https://doi.org/10.1016/j.jep.2021.114129
Xu Y, Wen K, Liu A, Wang X, Xu H, Wen H (2023) Efficacy of curculigoside in protecting against ischemic brain injury through regulation of oxidative stress and NF-κB and PI3K/Akt expression. J Ethnopharmacol 301:115804. https://doi.org/10.1016/j.jep.2022.115804
Wang YK, Hong YJ, Wei M, Wu Y, Huang ZQ, Chen RZ et al (2010) Curculigoside attenuates human umbilical vein endothelial cell injury induced by H2O2. J Ethnopharmacol 132:233–239. https://doi.org/10.1016/j.jep.2010.08.008
Shi Z, Yuan S, Shi L, Li J, Ning G, Kong X et al (2021) Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif 54:e12992. https://doi.org/10.1111/cpr.12992
Shi Y (2002) Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell 9:459–470. https://doi.org/10.1016/s1097-2765(02)00482-3
Gaumer S, Guénal I, Brun S, Théodore L, Mignotte B (2000) Bcl-2 and Bax mammalian regulators of apoptosis are functional in Drosophila. Cell Death Differ 7:804–814. https://doi.org/10.1038/sj.cdd.4400714
Tarantino G (2011) Serum Bcl-2 concentrations in overweight-obese subjects with nonalcoholic fatty liver disease. WJG 17:5280. https://doi.org/10.3748/wjg.v17.i48.5280
Jia Z-Q, Li S-Q, Qiao W-Q, Xu W-Z, Xing J-W, Liu J-T et al (2018) Ebselen protects mitochondrial function and oxidative stress while inhibiting the mitochondrial apoptosis pathway after acute spinal cord injury. Neurosci Lett 678:110–117. https://doi.org/10.1016/j.neulet.2018.05.007
NigdeliogluDolanbay S, Kocanci FG, Aslim B (2021) Neuroprotective effects of allocryptopine-rich alkaloid extracts against oxidative stress-induced neuronal damage. Biomed Pharmacother 140:111690. https://doi.org/10.1016/j.biopha.2021.111690
Luo Z, Wu F, Xue E, Huang L, Yan P, Pan X et al (2019) Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival by inducing HIF-1α in injured neuronal cells derived exosomes culture system. Cell Death Dis 10:134. https://doi.org/10.1038/s41419-019-1410-y
Zhong Z, Yao X, Luo M, Li M, Dong L, Zhang Z et al (2020) Protocatechuic aldehyde mitigates hydrogen peroxide-triggered PC12 cell damage by down-regulating MEG3. Artif Cells, Nanomed Biotechnol 48:602–609. https://doi.org/10.1080/21691401.2020.1725535
Xu M, Wang W, Lu W, Ling X, Rui Q, Ni H (2022) Evodiamine prevents traumatic brain injury through inhibiting oxidative stress via PGK1/NRF2 pathway. Biomed Pharmacother 153:113435. https://doi.org/10.1016/j.biopha.2022.113435
Teleanu DM, Niculescu A-G, Lungu II, Radu CI, Vladâcenco O, Roza E et al (2022) An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. IJMS 23:5938. https://doi.org/10.3390/ijms23115938
Eli I, Lerner DP, Ghogawala Z (2021) Acute Traumatic Spinal Cord Injury. Neurol Clin 39:471–488. https://doi.org/10.1016/j.ncl.2021.02.004
Martemucci G, Portincasa P, Di Ciaula A, Mariano M, Centonze V, D’Alessandro AG (2022) Oxidative stress, aging, antioxidant supplementation and their impact on human health: An overview. Mech Ageing Dev 206:111707. https://doi.org/10.1016/j.mad.2022.111707
He R, Cui M, Lin H, Zhao L, Wang J, Chen S et al (2018) Melatonin resists oxidative stress-induced apoptosis in nucleus pulposus cells. Life Sci 199:122–130. https://doi.org/10.1016/j.lfs.2018.03.020
Gong Y, Wang Y, Li Y, Weng F, Chen T, He L (2024) Curculigoside, a traditional Chinese medicine monomer, ameliorates oxidative stress in Alzheimer’s disease mouse model via suppressing ferroptosis. Phytother Res 38:2462–2481. https://doi.org/10.1002/ptr.8152
Sun Q, Wu Y, Zhao F, Wang J (2017) Maresin 1 Ameliorates Lung Ischemia/Reperfusion Injury by Suppressing Oxidative Stress via Activation of the Nrf-2-Mediated HO-1 Signaling Pathway. Oxid Med Cell Longev 2017:1–12. https://doi.org/10.1155/2017/9634803
Zhao L, Qi Y, Xu L, Tao X, Han X, Yin L et al (2018) MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol 15:284–296. https://doi.org/10.1016/j.redox.2017.12.013
Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J (2016) Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci 73:3221–3247. https://doi.org/10.1007/s00018-016-2223-0
Lv R, Du L, Zhang L, Zhang Z (2019) Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway. Life Sci 217:119–127. https://doi.org/10.1016/j.lfs.2018.11.053
Zhang Q, Liu J, Duan H, Li R, Peng W, Wu C (2021) Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J Adv Res 34:43–63. https://doi.org/10.1016/j.jare.2021.06.023
Du P, Zhang X, Luo K, Li Y, Fu C, Xiao J et al (2022) Curculigoside mitigates hepatic ischemia/reperfusion-induced oxidative stress, inflammation, and apoptosis via activation of the Nrf-2/HO-1 pathway. Hum Exp Toxicol 41:9603271221087146. https://doi.org/10.1177/09603271221087146
Tran AP, Warren PM, Silver J (2018) The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol Rev 98:881–917. https://doi.org/10.1152/physrev.00017.2017
Bradbury EJ, Burnside ER (2019) Moving beyond the glial scar for spinal cord repair. Nat Commun 10:3879. https://doi.org/10.1038/s41467-019-11707-7
Khetani S, Salahandish R, Tabor JB, Chilvers M, Dukelow S, Ho C et al (2023) Nanoporous Carbon Immunosensor for Highly Accurate and Sensitive Clinical Detection of Glial Fibrillary Acidic Protein in Traumatic Brain Injury, Stroke, and Spinal Cord Injury. ACS Biomater Sci Eng 9:3556–3569. https://doi.org/10.1021/acsbiomaterials.3c00048
González SL, López-Costa JJ, Labombarda F, Deniselle MCG, Guennoun R, Schumacher M et al (2009) Progesterone Effects on Neuronal Ultrastructure and Expression of Microtubule-associated Protein 2 (MAP2) in Rats with Acute Spinal Cord Injury. Cell Mol Neurobiol 29:27–39. https://doi.org/10.1007/s10571-008-9291-0
Fan H, Chen Z, Tang H, Shan L, Chen Z, Wang X, et al. Exosomes derived from olfactory ensheathing cells provided neuroprotection for spinal cord injury by switching the phenotype of macrophages/microglia. Bioeng Transla Med 2022;7. https://doi.org/10.1002/btm2.10287.
Wang C, Wang Q, Lou Y, Xu J, Feng Z, Chen Y et al (2017) Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J Cell Mol Med. https://doi.org/10.1111/jcmm.13368
Funding
This study was supported the National Natural Science Foundation of China (No. 82074451, No.82102583 and No. 82004384); The Natural Science Foundation of Guangdong province (No. 2022A1515010793 and No.2024A1515030293); the Science and Technology Program of Guangzhou, China (No.2023A03J0239 and No.2024A04J9907). Guangdong Provincial Department of Finance on the Arrangement of 2022 Healthcare Service and Protection Capacity Enhancement Subsidy Funds (Traditional Chinese Medicine Inheritance and Development Part) (No. Z155080000004). Research Fund for Bajian/Qingmiao Talents of Guangdong Provincial Hospital of Chinese Medicine (No.BJ2022KY07, No.SZ2022QN05).
Author information
Authors and Affiliations
Contributions
Yu Hou: Methodology, Investigation, Writing-review & editing,Funding; Chaolun Liang: Writing-original draft, Investigation; Lili Sui and Yang Li: Investigation; Kai Wang and Xing Li: Investigation; Kunrui Zheng and Haitao Su: Validation; Dianweng Xie: Investigation; Dingkun Lin and Da Guo: Conceptualization, Resources; Le Wang: Conceptualization.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The animal care procedures were reviewed and approved by the Animal Ethics Committee of Guangzhou University of Chinese Medicine (approval number: 20220803007).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
We obtained permissions from the participants to publish their data. All participants gave written consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hou, Y., Liang, C., Sui, L. et al. Curculigoside Regulates Apoptosis and Oxidative Stress Against Spinal Cord Injury by Modulating the Nrf-2/NQO-1 Signaling Pathway In Vitro and In Vivo. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04409-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04409-9