Abstract
The cell cycle is the sequence of events orchestrated by a complex network of cell cycle proteins. Unlike normal cells, mature neurons subsist in a quiescent state of the cell cycle, and aberrant cell cycle activation triggers neuronal death accompanied by neurodegeneration. The periodicity of cell cycle events is choreographed by various mechanisms, including DNA damage repair, oxidative stress, neurotrophin activity, and ubiquitin-mediated degradation. Given the relevance of cell cycle processes in cancer and neurodegeneration, this review delineates the overlapping cell cycle events, signaling pathways, and mechanisms associated with cell cycle aberrations in cancer and the major neurodegenerative disorders. We suggest that dysregulation of some common fundamental signaling processes triggers anomalous cell cycle activation in cancer cells and neurons. We discussed the possible use of cell cycle inhibitors for neurodegenerative disorders and described the associated challenges. We propose that a greater understanding of the common mechanisms driving cell cycle aberrations in cancer and neurodegenerative disorders will open a new avenue for the development of repurposed drugs.
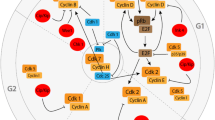
Graphical Abstract
Cell cycle activation and cell cycle re-entry are the two critical cellular phenomena associated with cancer and neurodegeneration, respectively. Various regulatory proteins, such as cyclin-dependent kinases (CDKs), cyclins, and checkpoint kinases, control the cell cycle dynamics at different phases. Further, the integrity of cell cycle is controlled by different mechanisms like DNA damage response, oxidative stress, neurotrophin activity, and the ubiquitin–proteasome system (UPS), the alterations which play an essential role in the pathogenesis of both cancer and neurodegenerative disorders. Pharmacological targeting of important cell cycle regulatory molecules like the application of CDK inhibitors, PARP inhibitors, checkpoint kinase inhibitors, and microtubule inhibitors holds great promise for treating various human cancers and can be repurposed for different neurodegenerative disorders.






Similar content being viewed by others
Data Availability
No datasets were generated or analyzed during the current study.
Abbreviations
- 6-OHDA:
-
6-Hydroxydopamine
- AML:
-
Acute myeloid leukemia
- ALS:
-
Amyotrophic lateral sclerosis
- APC/C:
-
Anaphase-promoting complex
- APP-BP1:
-
Amyloid precursor protein-binding protein 1
- ATM:
-
Ataxia telangiectasia mutated
- ATR:
-
Ataxia telangiectasia and Rad3-related
- BDNF:
-
Brain-derived growth factor
- CDKs:
-
Cyclin-dependent kinase inhibitors
- CHK:
-
Checkpoint kinase
- CNS:
-
Central nervous system
- CREB:
-
C-AMP response element-binding protein
- CRND:
-
Cell cycle–related neuronal cell death
- CRL:
-
Cullin-RING E3 ligases
- DG:
-
Dentate gyrus
- DSBs:
-
Double-strand breaks
- DSBR:
-
DNA double-strand break repair
- EC:
-
Entorhinal cortex
- FALS:
-
Familial, autosomal dominant disorder
- GAK:
-
Cyclin G–associated protein kinase
- GDC:
-
Genomic Data Commons
- GSK-3β:
-
Glycogen synthase kinase-3 beta
- HD:
-
Huntington’s disease
- HIF-1:
-
Hypoxia-inducible factor-1
- HNSCC:
-
Head and neck squamous cell carcinoma
- HR:
-
Homologous recombination
- HTT:
-
Huntingtin
- JNK:
-
C-Jun amino-terminal kinases
- LRRK2:
-
Leucine-rich repeat kinase 2
- MAPK:
-
Mitogen-activated protein kinase
- MCD:
-
Mitotic cell death
- MCL:
-
Mantle cell lymphoma
- MDM2:
-
Murine double minute 2
- MnSOD:
-
Manganese superoxide dismutase
- NDDs:
-
Neurodegenerative disorders
- NER:
-
Nucleotide excision repair
- NHEJ:
-
Non-homologous end-joining
- NGF:
-
Nerve growth factor
- NSCLC:
-
Non-small cell lung cancers
- NT:
-
Neurotrophin
- ORC:
-
Origin recognition complex
- PARP:
-
Poly(ADP-ribose) polymerase
- PHD:
-
Pyrolyl hydroxylase
- PI3K-PKB/Akt/mTOR:
-
Phosphoinositide-3-kinase–protein kinase B and the mammalian target of rapamycin
- PIP3:
-
Phosphatidylinositol-3,4,5-triphosphate
- PTEN:
-
Phosphatase and tensin homolog
- Rb:
-
Retinoblastoma
- ROS:
-
Reactive oxygen species
- RTK:
-
Receptor tyrosine kinases
- SAC:
-
Spindle assembly checkpoint
- SAPKs:
-
Stress-activated MAP kinases
- SCF:
-
SKP1-cullin-1-F-box
- Shh:
-
Sonic hedgehog
- SOD1:
-
Superoxide dismutase
- SSBR:
-
Single-strand break repair
- TS:
-
Template-switch
- TLS:
-
Translesion synthesis
- TRIM:
-
Tripartite motif
- UPS:
-
Ubiquitin-proteasome system
References
Vermeulen K, Van Bockstaele DR, Berneman ZN (2003) The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 36:131. https://doi.org/10.1046/J.1365-2184.2003.00266.X
Lui A, Vanleuven J, Perekopskiy D, et al (2022) FDA-approved kinase inhibitors in preclinical and clinical trials for neurological disorders. Pharmaceuticals 15:. https://doi.org/10.3390/PH15121546
Nandakumar S, Rozich E, Buttitta L (2021) Cell cycle re-entry in the nervous system: from polyploidy to neurodegeneration. Front. Cell Dev, Biol
Sarsour EH, Kumar MG, Chaudhuri L et al (2009) Redox control of the cell cycle in health and disease. Antioxidants Redox Signal 11:2985–3011
Branzei D (2008) Foiani M (2008) Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol 94(9):297–308. https://doi.org/10.1038/nrm2351
López-Sánchez N, Frade JM (2002) Control of the cell cycle by neurotrophins: lessons from the p75 neurotrophin receptor. Histol Histopathol 17:1227–1237. https://doi.org/10.14670/HH-17.1227
Bassermann F, Eichner R, Pagano M (2014) The ubiquitin proteasome system — implications for cell cycle control and the targeted treatment of cancer. Biochim Biophys Acta - Mol Cell Res 1843:150–162. https://doi.org/10.1016/J.BBAMCR.2013.02.028
Zhivotovsky B, Orrenius S (2010) Cell cycle and cell death in disease: past, present and future. J Intern Med 268:395–409. https://doi.org/10.1111/j.1365-2796.2010.02282.x
Scorah J, McGowan CH (2010) Regulation of cell cycle progression. In: Handbook of cell signaling. Academic Press, pp 2545-2553. https://doi.org/10.1016/B978-0-12-374145-5.00302-8
Coudreuse D, Nurse P (2010) Driving the cell cycle with a minimal CDK control network. Nature 468:1074–1080. https://doi.org/10.1038/nature09543
Barnum KJ, O’Connell MJ (2014) Cell cycle regulation by checkpoints. Methods Mol, Biol, p 1170
Chen X, Stauffer S, Chen Y, Dong J (2016) Ajuba phosphorylation by CDK1 promotes cell proliferation and tumorigenesis *. J Biol Chem 291:14761–14772. https://doi.org/10.1074/JBC.M116.722751
Bonni AHK & Azad (2008) Cdk1-FOXO1: a mitotic signal takes center stage in post-mitotic neurons. Cell Cycle 7:3819–3822. https://doi.org/10.4161/CC.7.24.7215
Vincent I, Jicha G, Rosado M, Dickson DW (1997) Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer’s disease brain. J Neurosci 17:3588–3598. https://doi.org/10.1523/jneurosci.17-10-03588.1997
Gopinathan L, Tan SLW, Padmakumar VC et al (2014) Loss of Cdk2 and cyclin A2 impairs cell proliferation and tumorigenesis. Cancer Res 74:3870–3879. https://doi.org/10.1158/0008-5472.CAN-13-3440
Lee KH, Lee S-J, Lee HJ et al (2017) Amyloid β1–42 (Aβ1–42) induces the CDK2-mediated phosphorylation of Tau through the activation of the mTORC1 signaling pathway while promoting neuronal cell death. Front Mol Neurosci 0:229. https://doi.org/10.3389/FNMOL.2017.00229
Gao X, Leone GW, Wang H (2020) Cyclin D-CDK4/6 functions in cancer. Adv Cancer Res 148:147–169. https://doi.org/10.1016/BS.ACR.2020.02.002
Tsujioka Y, Takahashi M, Tsuboi Y et al (1999) Localization and expression of cdc2 and cdk4 in Alzheimer brain tissue. Dement Geriatr Cogn Disord 10:192–198. https://doi.org/10.1159/000017119
Rao HV, Thirumangalakudi L, Desmond P, Grammas P (2007) Cyclin D1, cdk4, and Bim are involved in thrombin-induced apoptosis in cultured cortical neurons. J Neurochem 101:498–505. https://doi.org/10.1111/j.1471-4159.2006.04389.x
Pozo K, Bibb JA (2016) The emerging role of Cdk5 in cancer. Trends in Cancer 2(10):606–618
Gupta KK, Singh SK (2019) Cdk5: A main culprit in neurodegeneration. Int J Neurosci 129:1192–1197. https://doi.org/10.1080/00207454.2019.1645142
Avraham E, Rott R, Liani E et al (2007) Phosphorylation of Parkin by the cyclin-dependent kinase 5 at the linker region modulates its ubiquitin-ligase activity and aggregation. J Biol Chem 282:12842–12850. https://doi.org/10.1074/jbc.M608243200
Bk B, Skuntz S, Prochazkova M et al (2019) Overexpression of the Cdk5 inhibitory peptide in motor neurons rescue of amyotrophic lateral sclerosis phenotype in a mouse model. Hum Mol Genet 28:3175–3187. https://doi.org/10.1093/hmg/ddz118
Luo S, Vacher C, Davies JE, Rubinsztein DC (2005) Cdk5 phosphorylation of huntingtin reduces its cleavage by caspases: implications for mutant huntingtin toxicity. J Cell Biol 169:647–656. https://doi.org/10.1083/jcb.200412071
Yam CH, Fung TK, Poon RYC (2002) Cyclin A in cell cycle control and cancer. Cell Mol Life Sci 59:1317–1326. https://doi.org/10.1007/S00018-002-8510-Y
Oka T, Kubo T, Enokido Y, Hatanaka H (1996) Expression of cyclin A decreases during neuronal apoptosis in cultured rat cerebellar granule neurons. Dev Brain Res 97:96–106. https://doi.org/10.1016/S0165-3806(96)00138-1
Ye C, Wang J, Wu P et al (2017) Prognostic role of cyclin B1 in solid tumors: a meta-analysis. Oncotarget 8:2224. https://doi.org/10.18632/ONCOTARGET.13653
Lee SS, Kim YM, Junn E et al (2003) Cell cycle aberrations by α-synuclein over-expression and cyclin B immunoreactivity in Lewy bodies. Neurobiol Aging 24:687–696. https://doi.org/10.1016/S0197-4580(02)00196-3
Stamatakos M, Palla V, Karaiskos I, et al (2010) Cell cyclins: triggering elements of cancer or not? World J Surg Oncol 8:. https://doi.org/10.1186/1477-7819-8-111
Hoozemans JJM, Brückner MK, Rozemuller AJM et al (2002) Cyclin D1 and cyclin E are co-localized with cyclo-oxygenase 2 (COX-2) in pyramidal neurons in Alzheimer disease temporal cortex. J Neuropathol Exp Neurol 61:678–688. https://doi.org/10.1093/JNEN/61.8.678
Atabay KD, Karabay A (2012) Pin1 inhibition activates cyclin D and produces neurodegenerative pathology. J Neurochem. https://doi.org/10.1111/j.1471-4159.2011.07259.x
Keyomarsi K, Herliczek TW (1997) The role of cyclin E in cell proliferation, development and cancer. Prog Cell Cycle Res 3:171–191
Galper J, Rayner SL, Hogan AL et al (2017) Cyclin F: A component of an E3 ubiquitin ligase complex with roles in neurodegeneration and cancer. Int J Biochem Cell Biol 89:216–220. https://doi.org/10.1016/J.BIOCEL.2017.06.011
Lee A, Rayner SL, Gwee SSL et al (2018) Pathogenic mutation in the ALS/FTD gene, CCNF, causes elevated Lys48-linked ubiquitylation and defective autophagy. Cell Mol Life Sci 75:335–354. https://doi.org/10.1007/S00018-017-2632-8
Zhang Y, Hunter T (2014) Roles of Chk1 in cell biology and cancer therapy. Int J Cancer 134:1013–1023. https://doi.org/10.1002/IJC.28226
Ye W, Blain SW (2011) Chk1 has an essential role in the survival of differentiated cortical neurons in the absence of DNA damage. Apoptosis 16:449–459. https://doi.org/10.1007/S10495-011-0579-Z
Mendoza J, Sekiya M, Taniguchi T et al (2013) Global analysis of phosphorylation of tau by the checkpoint kinases Chk1 and Chk2 in vitro. J Proteome Res 12:2654–2665. https://doi.org/10.1021/pr400008f
Iijima-Ando K, Zhao L, Gatt A et al (2010) A DNA damage-activated checkpoint kinase phosphorylates tau and enhances tau-induced neurodegeneration. Hum Mol Genet 19:1930–1938. https://doi.org/10.1093/HMG/DDQ068
Stolz A, Ertych N, Bastians H (2011) Tumor suppressor CHK2: regulator of DNA damage response and mediator of chromosomal stability. Clin Cancer Res 17:401–405. https://doi.org/10.1158/1078-0432.CCR-10-1215
Liu Z, Sun Q, Wang X (2017) PLK1, A potential target for cancer therapy. Transl Oncol 10:22. https://doi.org/10.1016/J.TRANON.2016.10.003
Song B, Davis K, Liu XS et al (2011) Inhibition of Polo-like kinase 1 reduces beta-amyloid-induced neuronal cell death in Alzheimer’s disease. Aging (Albany NY) 3:846–851. https://doi.org/10.18632/aging.100382
di Rorà AGL, Cerchione C, Martinelli G (2020) Simonetti G (2020) A WEE1 family business: regulation of mitosis, cancer progression, and therapeutic target. J Hematol Oncol 131(13):1–17. https://doi.org/10.1186/S13045-020-00959-2
Tomashevski A, Husseman J, Jin LW et al (2001) Constitutive wee1 activity in adult brain neurons with M phase-type alterations in Alzheimer neurodegeneration. J Alzheimer’s Dis 3:195–207. https://doi.org/10.3233/JAD-2001-3205
Alvarado-Ortiz E, de la Cruz-López KG, Becerril-Rico J et al (2020) Mutant p53 gain-of-function: role in cancer development, progression, and therapeutic approaches. Front Cell Dev Biol 8:607670. https://doi.org/10.3389/FCELL.2020.607670
Thakur A, Siedlak SL, James SL et al (2008) Retinoblastoma protein phosphorylation at multiple sites is associated with neurofibrillary pathology in Alzheimer disease. Int J Clin Exp Pathol 1:134
Chen HZ, Tsai SY, Leone G (2009) Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer 9:785–797. https://doi.org/10.1038/NRC2696
Verdaguer E, de Arriba SG, Clemens A et al (2007) Implication of the transcription factor E2F–1 in the modulation of neuronal apoptosis. Biomed Pharmacother 61:390–399. https://doi.org/10.1016/J.BIOPHA.2006.11.001
Ranganathan S, Bowser R (2010) p53 and cell cycle proteins participate in spinal motor neuron cell death in ALS. Open Pathol J 4:11–22. https://doi.org/10.2174/1874375701004010011
Hou H, Sun D (2019) Zhang X (2019) The role of MDM2 amplification and overexpression in therapeutic resistance of malignant tumors. Cancer Cell Int 191(19):1–8. https://doi.org/10.1186/S12935-019-0937-4
Esteras N, Alquézar C, Bermejo-Pareja F et al (2013) Downregulation of extracellular signal-regulated kinase 1/2 activity by calmodulin KII modulates p21Cip1 levels and survival of immortalized lymphocytes from Alzheimer’s disease patients. Neurobiol Aging 34:1090–1100. https://doi.org/10.1016/J.NEUROBIOLAGING.2012.10.014
Lee SJ, Kim DC, Choi BH et al (2006) Regulation of p53 by activated protein kinase C-delta during nitric oxide-induced dopaminergic cell death. J Biol Chem 281:2215–2224. https://doi.org/10.1074/JBC.M509509200
Sadeghi H, Golalipour M, Yamchi A et al (2018) CDC25A pathway toward tumorigenesis: Molecular targets of CDC25A in cell-cycle regulation. J Cell Biochem 120:2919–2928. https://doi.org/10.1002/JCB.26838
Ding X-L, Husseman J, Tomashevski A et al (2000) The cell cycle Cdc25A tyrosine phosphatase is activated in degenerating postmitotic neurons in Alzheimer’s disease. Am J Pathol 157:1983–1990. https://doi.org/10.1016/S0002-9440(10)64837-7
Chen Y-C, Hsieh H-H, Chang H-C et al (2021) CDC25B induces cellular senescence and correlates with tumor suppression in a p53-dependent manner. J Biol Chem 296:100564–100565. https://doi.org/10.1016/J.JBC.2021.100564
Vincent I, Bu B, Hudson K et al (2001) Constitutive Cdc25B tyrosine phosphatase activity in adult brain neurons with M phase-type alterations in Alzheimer’s disease. Neuroscience 105:639–650. https://doi.org/10.1016/S0306-4522(01)00219-6
Liggett WH, Sidransky D (1998) Role of the p16 tumor suppressor gene in cancer. J Clin Oncol 16:1197–1206. https://doi.org/10.1200/JCO.1998.16.3.1197
Arendt T, Rödel L, Gärtner U, Holzer M (1996) Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer’s disease. NeuroReport 7:3047–3049. https://doi.org/10.1097/00001756-199611250-00050
Feddersen RM, Ehlenfeldt R, Yunis WS et al (1992) Disrupted cerebellar cortical development and progressive degeneration of Purkinje cells in SV40 T antigen transgenic mice. Neuron 9:955–966. https://doi.org/10.1016/0896-6273(92)90247-B
Tsai KY, Hu Y, Macleod KF et al (1998) Mutation of E2f–1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-deficient mouse embryos. Mol Cell 2:293–304. https://doi.org/10.1016/S1097-2765(00)80274-9
Freeman RS, Estus S, Johnson EM (1994) Analysis of cell cycle-related gene expression in postmitotic neurons: selective induction of cyclin D1 during programmed cell death. Neuron 12:343–355. https://doi.org/10.1016/0896-6273(94)90276-3
Frade JM (2000) Unscheduled re-entry into the cell cycle induced by NGF precedes cell death in nascent retinal neurones. J Cell Sci 113:1139–1148. https://doi.org/10.1242/jcs.113.7.1139
Lombardi D, Lasagni L (2016) Cell-cycle alterations in post-mitotic cells and cell death by mitotic catastrophe. Cell Biol - New Insights. https://doi.org/10.5772/61783
Thome KC, Dhar SK, Quintana DG et al (2000) Subsets of human origin recognition complex (ORC) subunits are expressed in non-proliferating cells and associate with non-ORC proteins. J Biol Chem 275:35233–35241. https://doi.org/10.1074/jbc.M005765200
Frank CL, Tsai LH (2009) Alternative functions of core cell cycle regulators in neuronal migration, neuronal maturation, and synaptic plasticity. Neuron 62:312–326
López-Sánchez N, Frade JM (2013) Genetic evidence for p75NTR-dependent tetraploidy in cortical projection neurons from adult mice. J Neurosci 33:7488–7500. https://doi.org/10.1523/JNEUROSCI.3849-12.2013
Yuan Z, Becker EBE, Merlo P et al (2008) Activation of FOXO1 by Cdk1 in cycling cells and postmitotic neurons. Science 319:1665–1668. https://doi.org/10.1126/science.1152337
Konishi Y, Lehtinen M, Donovan N, Bonni A (2002) Cdc2 phosphorylation of BAD links the cell cycle to the cell death machinery. Mol Cell 9:1005–1016. https://doi.org/10.1016/S1097-2765(02)00524-5
Marlier Q, D’aes T, Verteneuil S et al (2020) Core cell cycle machinery is crucially involved in both life and death of post-mitotic neurons. Cell Mol Life Sci 77:4553–4571
Nandakumar S, Rozich E, Buttitta L (2021) Cell cycle re-entry in the nervous system: from polyploidy to neurodegeneration. Front Cell Dev Biol 9:1511. https://doi.org/10.3389/FCELL.2021.698661/BIBTEX
Hammond EM, Dorie MJ, Giaccia AJ (2003) ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J Biol Chem 278:12207–12213. https://doi.org/10.1074/jbc.M212360200
Patterson JC, Joughin BA, van de Kooij B et al (2019) ROS and oxidative stress are elevated in mitosis during asynchronous cell cycle progression and are exacerbated by mitotic arrest. Cell Syst 8:163-167.e2. https://doi.org/10.1016/j.cels.2019.01.005
Langley B, Ratan RR (2004) Oxidative stress-induced death in the nervous system: Cell cycle dependent or independent? J Neurosci Res 77:621–629
Schwartz EI, Smilenov LB, Price MA et al (2007) Cell cycle activation in postmitotic neurons is essential for DNA repair. Cell Cycle 6:318–329. https://doi.org/10.4161/cc.6.3.3752
Gómez-Crisóstomo NP, Rodríguez Martínez E, Rivas-Arancibia S (2014) Oxidative stress activates the transcription factors FoxO 1a and FoxO 3a in the hippocampus of rats exposed to low doses of ozone. Oxid Med Cell Longev 2014:. https://doi.org/10.1155/2014/805764
Bianco MR, Berbenni M, Amara F et al (2011) Cross-talk between cell cycle induction and mitochondrial dysfunction during oxidative stress and nerve growth factor withdrawal in differentiated PC12 cells. J Neurosci Res 89:1302–1315
Ortmann B, Druker J, Rocha S (2014) Cell cycle progression in response to oxygen levels. Cell Mol Life Sci 71:3569–3582
Chao HX, Poovey CE, Privette AA et al (2017) DNA damage checkpoint dynamics drive cell cycle phase transitions. bioRxiv 8:14728
Takata M, Sasaki MS, Sonoda E et al (1998) Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J 17:5497–5508. https://doi.org/10.1093/emboj/17.18.5497
Mills KD, Ferguson DO, Alt FW (2003) The role of DNA breaks in genomic instability and tumorigenesis. Immunol Rev 194:77–95. https://doi.org/10.1034/j.1600-065X.2003.00060.x
Suberbielle E, Djukic B, Evans M, et al (2015) DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice. Nat Commun 6:. https://doi.org/10.1038/ncomms9897
Konopka A, Atkin JD (2022) DNA damage, defective dna repair, and neurodegeneration in amyotrophic lateral sclerosis. Front Aging Neurosci 14:279
Descamps S, Toillon RA, Adriaenssens E et al (2001) Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. J Biol Chem 276:17864–17870. https://doi.org/10.1074/jbc.M010499200
Greene LA, Tischler AS (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A 73:2424–2428. https://doi.org/10.1073/pnas.73.7.2424
Khwaja F, Tabassum A, Allen J, Djakiew D (2006) The p75NTR tumor suppressor induces cell cycle arrest facilitating caspase mediated apoptosis in prostate tumor cells. Biochem Biophys Res Commun 341:1184–1192. https://doi.org/10.1016/j.bbrc.2006.01.073
Ikeda T, Puro DG (1994) Nerve growth factor: a mitogenic signal for retinal müller glial cells. Brain Res 649:260–264. https://doi.org/10.1016/0006-8993(94)91072-3
Boutahar N, Reynaud E, Lassabliere F, Borg J (2010) Brain-derived neurotrophic factor inhibits cell cycle reentry but not endoplasmic reticulum stress in cultured neurons following oxidative or excitotoxic stress. J Neurosci Res 88:2263–2271. https://doi.org/10.1002/jnr.22384
Fasanaro P, Capogrossi MC, Martelli F (2010) Regulation of the endothelial cell cycle by the ubiquitin-proteasome system. Cardiovasc Res 85:272–280
Jin J, Shirogane T, Xu L et al (2003) SCFβ-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev 17:3062–3074. https://doi.org/10.1101/gad.1157503
Barford D (2011) Structure, function and mechanism of the anaphase promoting complex (APC/C). Q Rev Biophys 44:153–190
Venuto S, Merla G (2019) E3 Ubiquitin Ligase TRIM Proteins. Cell Cycle and Mitosis Cells 8:510. https://doi.org/10.3390/cells8050510
Mulvaney KM, Matson JP, Siesser PF et al (2016) Identification and characterization of MCM3 as a kelch-like ECH-associated protein 1 (KEAP1) substrate. J Biol Chem 291:23719–23733. https://doi.org/10.1074/jbc.M116.729418
Uruno A, Yamamoto M (2023) The KEAP1-NRF2 System and Neurodegenerative Diseases. https://home.liebertpub.com/ars 38:974–988. https://doi.org/10.1089/ARS.2023.0234
Kishi T, Ikeda A, Koyama N et al (2008) A refined two-hybrid system reveals that SCFCdc4-dependent degradation of Swi5 contributes to the regulatory mechanism of S-phase entry. Proc Natl Acad Sci U S A 105:14497–14502. https://doi.org/10.1073/pnas.0806253105
Bornstein G, Bloom J, Sitry-Shevah D et al (2003) Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem 278:25752–25757. https://doi.org/10.1074/jbc.M301774200
Alessandrini A, Chiaur DS, Pagano M (1997) Regulation of the cyclin-dependent kinase inhibitor p27 by degradation and phosphorylation. Leukemia 11:342–345
Kamura T, Hara T, Kotoshiba S et al (2003) Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci U S A 100:10231–10236. https://doi.org/10.1073/pnas.1831009100
Boix-Perales H, Horan I, Wise H, et al (2007) The E3 ubiquitin ligase skp2 regulates neural differentiation independent from the cell cycle. Neural Dev 2:. https://doi.org/10.1186/1749-8104-2-27
Mimura S, Komata M, Kishi T et al (2009) SCFDia2 regulates DNA replication forks during S-phase in budding yeast. EMBO J 28:3693–3705. https://doi.org/10.1038/emboj.2009.320
Strohmaier H, Spruck CH, Kaiser P et al (2001) Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413:316–322. https://doi.org/10.1038/35095076
Ang XL, Seeburg DP, Sheng M, Harper JW (2008) Regulation of Postsynaptic RapGAP SPAR by Polo-like Kinase 2 and the SCFβ-TRCP Ubiquitin Ligase in Hippocampal Neurons. J Biol Chem 283:29424. https://doi.org/10.1074/JBC.M802475200
Thomas Y, Coux O, Baldin V (2010) βTrCP-dependent degradation of CDC25B phosphatase at the metaphase-anaphase transition is a pre-requisite for correct mitotic exit. Cell Cycle 9:4338–4350. https://doi.org/10.4161/cc.9.21.13593
Liu Q, Tang Y, Chen L et al (2016) E3 Ligase SCFβTrCP-induced DYRK1A Protein Degradation Is Essential for Cell Cycle Progression in HEK293 Cells*. J Biol Chem 291:26399–26409. https://doi.org/10.1074/JBC.M116.717553
Li DZ, Liu SF, Zhu L et al (2017) FBXW8-dependent degradation of MRFAP1 in anaphase controls mitotic cell death. Oncotarget 8:97178–97186. https://doi.org/10.18632/oncotarget.21843
Bassermann F, Von Klitzing C, Münch S et al (2005) NIPA defines an SCF-type mammalian E3 ligase that regulates mitotic entry. Cell. https://doi.org/10.1016/j.cell.2005.04.034
Koepp DM, Schaefer LK, Ye X et al (2001) Phosphorylation-dependent ubiquitination of cyctin E by the SCFFbw7 ubiquitin ligase. Science 294:173–177. https://doi.org/10.1126/science.1065203
Gomez-Pastor R, Burchfiel ET, Neef DW et al (2017) (2017) Abnormal degradation of the neuronal stress-protective transcription factor HSF1 in Huntington’s disease. Nat Commun 81(8):1–17. https://doi.org/10.1038/ncomms14405
Li M, Zhang P (2009) The function of APC/CCdh1 in cell cycle and beyond. Cell Div 4:2. https://doi.org/10.1186/1747-1028-4-2
Park HJ, Costa RH, Lau LF et al (2008) Anaphase-Promoting Complex/Cyclosome-Cdh1-Mediated Proteolysis of the Forkhead Box M1 Transcription Factor Is Critical for Regulated Entry into S Phase. Mol Cell Biol 28:5162–5171. https://doi.org/10.1128/mcb.00387-08
Almeida A (2012) Regulation of APC/C-Cdh1 and Its Function in Neuronal Survival. Mol Neurobiol 46:547–554. https://doi.org/10.1007/s12035-012-8309-2
Beck J, Maerki S, Posch M et al (2013) Ubiquitylation-dependent localization of PLK1 in mitosis. Nat Cell Biol 15:430–439. https://doi.org/10.1038/ncb2695
Kong X, Shu X, Wang J, et al (2022) Fine-tuning of mTOR signaling by the UBE4B-KLHL22 E3 ubiquitin ligase cascade in brain development. Dev 149:. https://doi.org/10.1242/DEV.201286/VIDEO-1
Fang G, Hongtao Y, Kirschner MW (1998) Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell 2:163–171. https://doi.org/10.1016/S1097-2765(00)80126-4
Senga T, Sivaprasad U, Zhu W et al (2006) PCNA is a cofactor for CDT1 degradation by CUL4/DDB1-mediated N-terminal ubiquitination. J Biol Chem 281:6246–6252. https://doi.org/10.1074/jbc.M512705200
Patrón LA, Nagatomo K, Eves DT et al (2019) Cul4 ubiquitin ligase cofactor DCAF12 promotes neurotransmitter release and homeostatic plasticity. J Cell Biol 218:993–1010. https://doi.org/10.1083/JCB.201805099
Lin DI, Barbash O, Kumar KGS et al (2006) Phosphorylation-Dependent Ubiquitination of Cyclin D1 by the SCFFBX4-αB Crystallin Complex. Mol Cell 24:355–366. https://doi.org/10.1016/j.molcel.2006.09.007
Lehman NL, Verschuren EW, Hsu JY et al (2006) Overexpression of the anaphase promoting complex/cyclosome inhibitor Emi1 leads to tetraploidy and genomic instability of p53-deficient cells. Cell Cycle 5:1569–1573. https://doi.org/10.4161/cc.5.14.2925
Malureanu L, Jeganathan KB, Jin F et al (2010) Cdc20 hypomorphic mice fail to counteract de novo synthesis of cyclin B1 in mitosis. J Cell Biol 191:313–329. https://doi.org/10.1083/jcb.201003090
Huang HC, Shi J, Orth JD, Mitchison TJ (2009) Evidence that Mitotic Exit Is a Better Cancer Therapeutic Target Than Spindle Assembly. Cancer Cell 16:347–358. https://doi.org/10.1016/j.ccr.2009.08.020
Dang F, Nie L, Wei W (2021) Ubiquitin signaling in cell cycle control and tumorigenesis. Cell Death Differ 28:427–438
Staropoli JF, Abeliovich A (2005) The ubiquitin-proteasome pathway is necessary for maintenance of the postmitotic status of neurons. J Mol Neurosci 27:175–183. https://doi.org/10.1385/JMN:27:2:175
Chauhan M, Modi PK, Sharma P (2020) Aberrant activation of neuronal cell cycle caused by dysregulation of ubiquitin ligase Itch results in neurodegeneration. Cell Death Dis 11:1–13. https://doi.org/10.1038/s41419-020-2647-1
Staropoli JF, McDermott C, Martinat C et al (2003) Parkin Is a Component of an SCF-like Ubiquitin Ligase Complex and Protects Postmitotic Neurons from Kainate Excitotoxicity. Neuron 37:735–749. https://doi.org/10.1016/S0896-6273(03)00084-9
Almeida A, Bolaños JP, Moreno S (2005) Cdh1/Hct1-APC is essential for the survival of postmitotic neurons. J Neurosci 25:8115–8121. https://doi.org/10.1523/JNEUROSCI.1143-05.2005
Malumbres M, Barbacid M (2001) To cycle or not to cycle: A critical decision in cancer. Nat Rev Cancer 1:222–231. https://doi.org/10.1038/35106065
Hamilton E, Infante JR (2016) Targeting CDK4/6 in patients with cancer. Cancer Treat Rev 45:129–138
Wölfel T, Hauer M, Schneider J et al (1995) A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science 269:1281–1284. https://doi.org/10.1126/science.7652577
Qie S, Diehl JA (2016) Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med 94:1313–1326
Teixeira LK, Wang X, Li Y et al (2015) Cyclin e deregulation promotes loss of specific genomic regions. Curr Biol 25:1327–1333. https://doi.org/10.1016/j.cub.2015.03.022
Keck JM, Summers MK, Tedesco D et al (2007) Cyclin E overexpression impairs progression through mitosis by inhibiting APCCdh1. J Cell Biol 178:371–385. https://doi.org/10.1083/jcb.200703202
Menon DR, Luo Y, Arcaroli JJ et al (2018) CDK1 interacts with SOX2 and promotes tumor initiation in human melanoma. Cancer Res 78:6561–6574. https://doi.org/10.1158/0008-5472.CAN-18-0330
Otto T, Sicinski P (2017) Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer 17:93–115. https://doi.org/10.1038/NRC.2016.138
Ozono E, Yamaoka S, Ohtani K (2013) To grow, stop or die? – Novel tumor-suppressive mechanism regulated by the transcription factor E2F. In: Future aspects of tumor suppressor gene. InTech. https://doi.org/10.5772/54510
Pandey N, Vinod PK (2022) Model scenarios for cell cycle re-entry in Alzheimer’s disease. iScience 25:. https://doi.org/10.1016/J.ISCI.2022.104543
van Leeuwen LAG, Hoozemans JJM (2015) Physiological and pathophysiological functions of cell cycle proteins in post-mitotic neurons: implications for Alzheimer’s disease. Acta Neuropathol 129:511–525. https://doi.org/10.1007/S00401-015-1382-7
Ueberham U, Arendt T (2005) The expression of cell cycle proteins in neurons and its relevance for Alzheimer’s disease. Curr Drug Targets CNS Neurol Disord 4:293–306
Hernández-Ortega K, Ferrera P, Arias C (2007) Sequential expression of cell-cycle regulators and Alzheimer’s disease-related proteins in entorhinal cortex after hippocampal excitotoxic damage. J Neurosci Res 85:1744–1751. https://doi.org/10.1002/jnr.21301
Der CS, Yang JL, Lin YC et al (2020) Emerging Roles of Inhibitor of Differentiation-1 in Alzheimer’s Disease: Cell Cycle Reentry and Beyond. Cells 9(7):1746
Chen Y, McPhie DL, Hirschberg J, Neve RL (2000) The amyloid precursor protein-binding protein APP-BP1 drives the cell cycle through the S-M checkpoint and causes apoptosis in neurons. J Biol Chem 275:8929–8935. https://doi.org/10.1074/jbc.275.12.8929
Höglinger GU, Breunig JJ, Depboylu C et al (2007) The pRb/E2F cell-cycle pathway mediates cell death in Parkinson’s disease. Proc Natl Acad Sci U S A 104:3585–3590. https://doi.org/10.1073/pnas.0611671104
Smith PD, Crocker SJ, Jackson-Lewis V et al (2003) Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.2232515100
Alves Da Costa C, Paitel E, Vincent B, Checler F (2002) α-synuclein lowers p53-dependent apoptotic response of neuronal cells: Abolishment by 6-hydroxydopamine and implication for Parkinson’s disease. J Biol Chem 277:50980–50984. https://doi.org/10.1074/jbc.M207825200
Wolfrum P, Fietz A, Schnichels S, Hurst J (2022) The function of p53 and its role in Alzheimer’s and Parkinson’s disease compared to age-related macular degeneration. Front, Neurosci
Lee SB, Kim JJ, Nam HJ et al (2015) Parkin Regulates Mitosis and Genomic Stability through Cdc20/Cdh1. Mol Cell 60:21–34. https://doi.org/10.1016/j.molcel.2015.08.011
López-Grueso MJ, Padilla CA, Bárcena JA, Requejo-Aguilar R (2020) Parkinson´s related protein DJ-1 is involved in aberrant cell cyclere-entry through Cdk5. https://doi.org/10.21203/rs.2.22860/v1
Pelegrí C, Duran-Vilaregut J, del Valle J et al (2008) Cell cycle activation in striatal neurons from Huntington’s disease patients and rats treated with 3-nitropropionic acid. Int J Dev Neurosci 26:665–671. https://doi.org/10.1016/j.ijdevneu.2008.07.016
Feng Z, Jin S, Zupnick A et al (2006) p53 tumor suppressor protein regulates the levels of huntingtin gene expression. Oncogene 25:1–7. https://doi.org/10.1038/sj.onc.1209021
Godin JD, Colombo K, Molina-Calavita M et al (2010) Huntingtin Is Required for Mitotic Spindle Orientation and Mammalian Neurogenesis. Neuron 67:392–406. https://doi.org/10.1016/j.neuron.2010.06.027
Trinh E, Boutillier AL, Loeffler JP (2001) Regulation of the retinoblastoma-dependent Mdm2 and E2F–1 signaling pathways during neuronal apoptosis. Mol Cell Neurosci 17:342–353. https://doi.org/10.1006/mcne.2000.0928
Bajaj NP, Al-Sarraj ST, Anderson V, Kibble M, Leigh N, Miller CC (1998) Cyclin-dependent kinase-5 is associated with lipofuscin in motor neurones in amyotrophic lateral sclerosis. Neurosci Lett 245(1):45–48. https://doi.org/10.1016/s0304-3940(98)00176-1
Cova E, Ghiroldi A, Guareschi S et al (2010) G93A SOD1 alters cell cycle in a cellular model of Amyotrophic Lateral Sclerosis. Cell Signal 22:1477–1484. https://doi.org/10.1016/j.cellsig.2010.05.016
Cross DAE, Alessi DR, Cohen P et al (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789. https://doi.org/10.1038/378785a0
Kandel ES, Skeen J, Majewski N et al (2002) Activation of Akt/Protein Kinase B Overcomes a G2/M Cell Cycle Checkpoint Induced by DNA Damage. Mol Cell Biol 22:7831–7841. https://doi.org/10.1128/mcb.22.22.7831-7841.2002
Chang F, Lee JT, Navolanic PM et al (2003) Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia 17:590–603. https://doi.org/10.1038/sj.leu.2402824
Nicholson KM, Anderson NG (2002) The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal 14:381–395
Kurose K, Gilley K, Matsumoto S et al (2002) Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet 32:355–357. https://doi.org/10.1038/ng1013
Pei JJ, Hugon J (2008) mTOR-dependent signalling in Alzheimer’s disease. J Cell Mol Med 12:2525–2532
Norambuena A, Wallrabe H, McMahon L et al (2017) mTOR and neuronal cell cycle reentry: How impaired brain insulin signaling promotes Alzheimer’s disease. Alzheimer’s Dement 13:152–167. https://doi.org/10.1016/j.jalz.2016.08.015
Murata H, Sakaguchi M, Jin Y et al (2011) A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: Activation of AKT via MTORC2. J Biol Chem 286:7182–7189. https://doi.org/10.1074/jbc.M110.179390
Albanese C, Johnson J, Watanabe G et al (1995) Transforming p21(ras) mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem 270:23589–23597. https://doi.org/10.1074/jbc.270.40.23589
Matsushime H, Quelle DE, Shurtleff SA et al (1994) D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol 14:2066–2076. https://doi.org/10.1128/mcb.14.3.2066
Kawada M, Yamagoe S, Murakami Y et al (1997) Induction of p27Kip1 degradation and anchorage independence by Ras through the MAP kinase signaling pathway. Oncogene 15:629–637. https://doi.org/10.1038/sj.onc.1201228
Gutierrez GJ, Tsuji T, Cross JV et al (2010) JNK-mediated phosphorylation of Cdc25C regulates cell cycle entry and G2/M DNA damage checkpoint. J Biol Chem 285:14217–14228. https://doi.org/10.1074/jbc.M110.121848
Gutierrez GJ, Tsuji T, Chen M et al (2010) Interplay between Cdh1 and JNK activity during the cell cycle. Nat Cell Biol 12:686–695. https://doi.org/10.1038/ncb2071
Thoms HC, Dunlop MG, Stark LA (2007) p38-mediated inactivation of cyclin D1/cyclin-dependent kinase 4 stimulates nucleolar translocation of RelA and apoptosis in colorectal cancer cells. Cancer Res 67:1660–1669. https://doi.org/10.1158/0008-5472.CAN-06-1038
Thornton TM, Rincon M (2009) Non-classical p38 map kinase functions: Cell cycle checkpoints and survival. Int J Biol Sci 5:44–52. https://doi.org/10.7150/ijbs.5.44
Guo Y, Pan W, Liu S, et al (2020) ERK/MAPK signalling pathway and tumorigenesis (Review). Exp Ther Med 1997–2007. https://doi.org/10.3892/etm.2020.8454
Torii S, Yamamoto T, Tsuchiya Y, Nishida E (2006) ERK MAP kinase in G1 cell cycle progression and cancer. Cancer Sci 97:697–702. https://doi.org/10.1111/j.1349-7006.2006.00244.x
Hydbring P, Bahram F, Su Y et al (2010) Phosphorylation by Cdk2 is required for Myc to repress Ras-induced senescence in cotransformation. Proc Natl Acad Sci U S A 107:58–63. https://doi.org/10.1073/pnas.0900121106
Urasaki Y, Fiscus RR, Le TT (2018) Detection of the Cell Cycle-Regulated Negative Feedback Phosphorylation of Mitogen-Activated Protein Kinases in Breast Carcinoma using Nanofluidic Proteomics. Sci Rep 8:. https://doi.org/10.1038/s41598-018-28335-8
Modi PK, Komaravelli N, Singh N, Sharma P (2012) Interplay between MEK-ERK signaling, cyclin D1, and cyclin-dependent kinase 5 regulates cell cycle reentry and apoptosis of neurons. Mol Biol Cell 23:3722–3730. https://doi.org/10.1091/mbc.E12-02-0125
Rodriguez-Blanco J, Martín V, Herrera F et al (2008) Intracellular signaling pathways involved in post-mitotic dopaminergic PC12 cell death induced by 6-hydroxydopamine. J Neurochem 107:127–140. https://doi.org/10.1111/j.1471-4159.2008.05588.x
Zhang M, Zhang L, Hei R et al (2021) CDK inhibitors in cancer therapy, an overview of recent development. Am J Cancer Res 11:1913–1935
Monaco E III, Vallano M (2012) Cyclin-Dependent Kinase Inhibitors: Cancer Killers to Neuronal Guardians. Curr Med Chem 10:367–379. https://doi.org/10.2174/0929867033368277
Pallàs M, Canudas AM, Verdaguer E et al (2005) Inhibitors of cyclin-dependent kinases: Potential drugs for the treatment of neurodegenerative disorders? Curr Med Chem - Cent Nerv Syst Agents 5:101–109. https://doi.org/10.2174/1568015054022371
Jorda EG, Verdaguer E, Canudas AM et al (2003) Neuroprotective action of flavopiridol, a cyclin-dependent kinase inhibitor, in colchicine-induced apoptosis. Neuropharmacology 45:672–683. https://doi.org/10.1016/S0028-3908(03)00204-1
Harnor S, Pickles J, Cano C et al (2017) Modulation of the DNA-Damage Response by Inhibitors of the Phosphatidylinositol 3-Kinase Related Kinase (PIKK) Family. Top Med Chem 28:189–189. https://doi.org/10.1007/7355_2017_26
Ronco C, Martin AR, Demange L, Benhida R (2017) ATM, ATR, CHK1, CHK2 and WEE1 inhibitors in cancer and cancer stem cells. Medchemcomm 8:295. https://doi.org/10.1039/C6MD00439C
Chwastek J, Jantas D, Lasoń W (2017) The ATM kinase inhibitor KU-55933 provides neuroprotection against hydrogen peroxide-induced cell damage via a γH2AX/p-p53/caspase-3-independent mechanism: Inhibition of calpain and cathepsin D. Int J Biochem Cell Biol 87:38–53. https://doi.org/10.1016/J.BIOCEL.2017.03.015
Lu XH, Mattis VB, Wang N et al (2014) Targeting ATM ameliorates mutant Huntingtin toxicity in cell and animal models of Huntington’s disease. Sci Transl Med 6:268ra178. https://doi.org/10.1126/SCITRANSLMED.3010523/SUPPL_FILE/6-268RA178_SM.PDF
Bellozi PMQ, Gomes GF, de Oliveira LR et al (2019) NVP-BEZ235 (Dactolisib) Has Protective Effects in a Transgenic Mouse Model of Alzheimer’s Disease. Front Pharmacol 10:1345. https://doi.org/10.3389/fphar.2019.01345
Brunden KR, Yao Y, Potuzak JS et al (2011) The characterization of microtubule-stabilizing drugs as possible therapeutic agents for Alzheimer’s disease and related tauopathies. Pharmacol Res. https://doi.org/10.1016/j.phrs.2010.12.002
Zhang B, Maiti A, Shively S et al (2005) Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc Natl Acad Sci U S A 102:227–231. https://doi.org/10.1073/PNAS.0406361102
Jordan MA (2004) Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 44(4):253–265. https://doi.org/10.1038/nrc1317
Blazejewski SM, Bennison SA, Liu X (2021) Toyo-oka K (2021) High-throughput kinase inhibitor screening reveals roles for Aurora and Nuak kinases in neurite initiation and dendritic branching. Sci Reports 111(11):1–20. https://doi.org/10.1038/s41598-021-87521-3
Lee JS, Lee Y, André EA et al (2019) Inhibition of Polo-like kinase 2 ameliorates pathogenesis in Alzheimer’s disease model mice. PLoS ONE 14:e0219691. https://doi.org/10.1371/JOURNAL.PONE.0219691
Weston LJ, Stackhouse TL, Spinelli KJ et al (2021) Genetic deletion of Polo-like kinase 2 reduces alpha-synuclein serine-129 phosphorylation in presynaptic terminals but not Lewy bodies. J Biol Chem 296:100273. https://doi.org/10.1016/J.JBC.2021.100273
Huszar D, Theoclitou ME, Skolnik J, Herbst R (2009) Kinesin motor proteins as targets for cancer therapy. Cancer Metastasis Rev 28:197–208. https://doi.org/10.1007/S10555-009-9185-8
Hernandez I, Luna G, Rauch JN, et al (2019) A farnesyltransferase inhibitor activates lysosomes and reduces tau pathology in mice with tauopathy. Sci Transl Med 11:. https://doi.org/10.1126/scitranslmed.aat3005
Rose M, Burgess JT, O’Byrne K et al (2020) PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front Cell Dev Biol 8:879. https://doi.org/10.3389/FCELL.2020.564601/BIBTEX
Chen A (2011) PARP inhibitors: its role in treatment of cancer. Chin J Cancer 30:463. https://doi.org/10.5732/CJC.011.10111
Mao K, Zhang G (2021) The role of PARP1 in neurodegenerative diseases and aging. FEBS J. https://doi.org/10.1111/FEBS.15716
Puentes LN, Lengyel-Zhand Z, Reilly SW, Mach RH (2021) Evaluation of a Low-Toxicity PARP Inhibitor as a Neuroprotective Agent for Parkinson’s Disease. Mol Neurobiol 58:3641–3652. https://doi.org/10.1007/S12035-021-02371-4
Salech F, Ponce DP, Paula-Lima AC et al (2020) Nicotinamide, a Poly [ADP-Ribose] Polymerase 1 (PARP-1) Inhibitor, as an Adjunctive Therapy for the Treatment of Alzheimer’s Disease. Front Aging Neurosci 12:255. https://doi.org/10.3389/FNAGI.2020.00255/BIBTEX
Brown DG, Shorter J, Wobst HJ (2019) Emerging small-molecule therapeutic approaches for amyotrophic lateral sclerosis and frontotemporal dementia. Bioorg Med Chem Lett 126942. https://doi.org/10.1016/j.bmcl.2019.126942
Paldino E, Cardinale A, D’Angelo V, et al (2017) Selective Sparing of Striatal Interneurons after Poly (ADP-Ribose) Polymerase 1 Inhibition in the R6/2 Mouse Model of Huntington’s Disease. Front Neuroanat 11:. https://doi.org/10.3389/FNANA.2017.00061
O’Hare MJ, Hou ST, Morris EJ et al (2000) Induction and modulation of cerebellar granule neuron death by E2F–1. J Biol Chem 275:25358–25364. https://doi.org/10.1074/jbc.M001725200
Kabadi SV, Stoica BA, Byrnes KR et al (2011) Selective CDK Inhibitor Limits Neuroinflammation and Progressive Neurodegeneration after Brain Trauma. J Cereb Blood Flow Metab 32:137–149. https://doi.org/10.1038/JCBFM.2011.117
Chen Y, Hou Y, Ge R et al (2018) Protective effect of roscovitine against rotenone-induced parkinsonism. Restor Neurol Neurosci 36:629–638. https://doi.org/10.3233/RNN-180817
Mookherjee P, Johnson GVW (2001) Tau phosphorylation during apoptosis of human SH-SY5Y neuroblastoma cells. Brain Res 921:31–43. https://doi.org/10.1016/S0006-8993(01)03074-8
Wei W, Wang X, Kusiak JW (2002) Signaling events in amyloid β-peptide-induced neuronal death and insulin-like growth factor I protection. J Biol Chem 277:17649–17656. https://doi.org/10.1074/jbc.M111704200
Chung YH, Lin CW, Huang HY et al (2020) Targeting Inflammation, PHA-767491 Shows a Broad Spectrum in Protein Aggregation Diseases. J Mol Neurosci 70:1140–1152. https://doi.org/10.1007/S12031-020-01521-Y
Johnson K, Liu L, Majdzadeh N et al (2005) Inhibition of neuronal apoptosis by the cyclin-dependent kinase inhibitor GW8510: Identification of 3′ substituted indolones as a scaffold for the development of neuroprotective drugs. J Neurochem 93:538–548. https://doi.org/10.1111/J.1471-4159.2004.03004.X
Leost M, Schultz C, Link A et al (2000) Paullones are potent inhibitors of glycogen synthase kinase-3β and cyclin-dependent kinase 5/p25. Eur J Biochem 267:5983–5994. https://doi.org/10.1046/J.1432-1327.2000.01673.X
Wang Y, Hoi P, Chan J, Lee S (2014) New Perspective on the Dual Functions of Indirubins in Cancer Therapy and Neuroprotection. Anticancer Agents Med Chem 14:1213–1219. https://doi.org/10.2174/1871520614666140825112924
Shemesh OA, Spira ME (2011) Rescue of neurons from undergoing hallmark tau-induced Alzheimer’s disease cell pathologies by the antimitotic drug paclitaxel. Neurobiol Dis 43:163–175. https://doi.org/10.1016/J.NBD.2011.03.008
Zheng YL, Kesavapany S, Gravell M et al (2005) A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. EMBO J 24:209–220. https://doi.org/10.1038/SJ.EMBOJ.7600441
Li Y, Yang DQ (2010) The ATM inhibitor KU-55933 suppresses cell proliferation and induces apoptosis by blocking Akt in cancer cells with overactivated Akt. Mol Cancer Ther 9:113–125. https://doi.org/10.1158/1535-7163.MCT-08-1189
Mansour HM, Fathi AM, El-Khatib AS, Khattab MM (2023) Kinases control of regulated cell death revealing druggable targets for Parkinson’s disease. Ageing Res, Rev
Zhu Y, Mao C, Wu J et al (2014) Improved ataxia telangiectasia mutated kinase inhibitor KU60019 provides a promising treatment strategy for non-invasive breast cancer. Oncol Lett 8:2043. https://doi.org/10.3892/OL.2014.2444
Flemming A (2015) (2015) Banking on ATM. Nat Rev Drug Discov 142(14):93–93. https://doi.org/10.1038/nrd4542
Bellozi PMQ, de Lima IV A, Dória JG et al (2016) Neuroprotective effects of the anticancer drug NVP-BEZ235 (dactolisib) on amyloid-β 1–42 induced neurotoxicity and memory impairment. Sci Rep. https://doi.org/10.1038/srep25226
Furukawa K, Mattson MP (1995) Cytochalasins protect hippocampal neurons against amyloid beta-peptide toxicity: evidence that actin depolymerization suppresses Ca2+ influx. J Neurochem 65:1061–1068. https://doi.org/10.1046/J.1471-4159.1995.65031061.X
Zhang B, Carroll J, Trojanowski JQ et al (2012) The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci 32:3601–3611. https://doi.org/10.1523/JNEUROSCI.4922-11.2012
Brunden KR, Gardner NM, James MJ et al (2013) MT-stabilizer, dictyostatin, exhibits prolonged brain retention and activity: Potential therapeutic implications. ACS Med Chem Lett 4:886–889. https://doi.org/10.1021/ML400233E
Das V, Miller JH (2012) Microtubule stabilization by peloruside A and paclitaxel rescues degenerating neurons from okadaic acid‐induced tau phosphorylation. undefined 35:1705–1717. https://doi.org/10.1111/J.1460-9568.2012.08084.X
Tan CC, Zhang XY, Tan L, Yu JT (2018) Tauopathies: Mechanisms and Therapeutic Strategies. J Alzheimer’s Dis 61:487–508. https://doi.org/10.3233/JAD-170187
McGurk L, Mojsilovic-Petrovic J, Van Deerlin VM, et al (2018) Nuclear poly(ADP-ribose) activity is a therapeutic target in amyotrophic lateral sclerosis. Acta Neuropathol Commun 6:. https://doi.org/10.1186/S40478-018-0586-1
Mao K, Chen J, Yu H, et al (2020) Poly (ADP-ribose) polymerase 1 inhibition prevents neurodegeneration and promotes α-synuclein degradation via transcription factor EB-dependent autophagy in mutant α-synucleinA53T model of Parkinson’s disease. Aging Cell 19:. https://doi.org/10.1111/ACEL.13163
Liu DZ (2017) Repurposing cancer drugs to treat neurological diseases – Src inhibitors as examples. Neural Regen Res 12:910. https://doi.org/10.4103/1673-5374.208569
Nagy Z (2007) The dysregulation of the cell cycle and the diagnosis of Alzheimer’s disease. Biochim Biophys Acta - Mol Basis Dis 1772:402–408. https://doi.org/10.1016/J.BBADIS.2006.11.001
Song J, Wang S, Tan M, Jia J (2012) G1/S checkpoint proteins in peripheral blood lymphocytes are potentially diagnostic biomarkers for Alzheimer’s disease. Neurosci Lett 526:. https://doi.org/10.1016/J.NEULET.2012.08.020
Joseph C, Mangani AS, Gupta V et al (2020) Cell cycle deficits in neurodegenerative disorders: Uncovering molecular mechanisms to drive innovative therapeutic development. Aging Dis 11:946–966. https://doi.org/10.14336/AD.2019.0923
Yan VC, Butterfield HE, Poral AH et al (2020) Why Great Mitotic Inhibitors Make Poor Cancer Drugs. Trends in cancer 6:924–941. https://doi.org/10.1016/J.TRECAN.2020.05.010
Juric V, Murphy B (2020) Cyclin-dependent kinase inhibitors in brain cancer: current state and future directions. Cancer Drug Resist 3:48–62. https://doi.org/10.20517/CDR.2019.105
Liu DZ, Ander BP (2012) Cell Cycle Inhibition without Disruption of Neurogenesis Is a Strategy for Treatment of Aberrant Cell Cycle Diseases: An Update. Sci World J 2012:13. https://doi.org/10.1100/2012/491737
Herrup K (2010) The involvement of cell cycle events in the pathogenesis of Alzheimer’s disease. Alzheimer’s Res Ther 2:2–3. https://doi.org/10.1186/alzrt37
Acknowledgements
We would like to thank the senior management of Delhi Technological University (DTU) and the Department of Biotechnology (DBT), Government of India, for providing PhD fellowship to DA.
Author information
Authors and Affiliations
Contributions
This work was conceived by Pravir Kumar. Did Advani and Pravir Kumar contributed to the design and conceptualization of the manuscript. Did Advani collected the data. Critical evaluation was done by Did Advani, and the manuscript was written by Dia Advani. Editing and supervision have been done by Pravir Kumar. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Advani, D., Kumar, P. Uncovering Cell Cycle Dysregulations and Associated Mechanisms in Cancer and Neurodegenerative Disorders: A Glimpse of Hope for Repurposed Drugs. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04130-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04130-7