Abstract
The anaphase promoting complex/cyclosome (APC/C) is a multi-subunit E3 ubiquitin ligase playing essential functions in mitosis. It is conserved from yeast to human and relies on two adaptor proteins, Cdc20 and Cdh1, to bring in substrates. Both APCCdc20 and APCCdh1 are implicated in the control of mitosis through mediating ubiquitination and degradation of important mitotic regulators such as cyclin B1, securin, and Plk1. In addition, APCCdh1 is thought to prevent premature S phase entry by limiting the accumulation of mitotic cyclins in G1 and to regulate processes unrelated to cell cycle. In this review, we will summarize our current understanding of APCCdh1 function in cell cycle and beyond.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
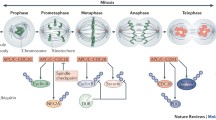
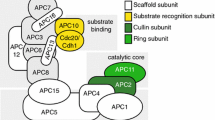
Two ubiquitin E3-ligase complexes, SCF (Skp1/CUL1/F-box protein) and APC/C (anaphase promoting complex/cyclosome), control the timely transitions of cell cycle phases by promoting the degradation of many key cell cycle regulators. SCF complex mainly functions in G1, S and early M phases, whereas APC/C regulates mitosis including metaphase-anaphase transition and mitotic exit and maintains G1 phase [1, 2]. APC/C is a large (1.5 MDa complex) composed of at lease 11 core subunits. It relies on two WD-40 repeat-containing adaptor proteins, Cdc20/fizzy(fzy)/p55CDC and Hct1/srw1/fizzy-related(fzr)/Cdh1, to engage with its substrates. Destruction box (RXX LXXXX N/D/E) and KEN box are motifs frequently found in APC's substrates, but other motifs are also possible for recognition by APCCdc20 or APCCdh1 [3]. The consensus sequence of destruction box can be found in many proteins. However, not all of these proteins are APC's substrates. Thus, there must be other sequence constrains we do not understand yet that define true APC substrates. Moreover, some substrates only have an RxxL motif and yet are recognized by APC, indicating the last amino acid in the consensus is not stringently conserved.
APCCdc20 initiates the metaphase-anaphase transition through mediating the ubiquitination and degradation of cyclin B1 and securin. To prevent premature separation of sister chromatids and mitotic exit, APCCdc20 is inhibited by Mad2 and BubR1 through the spindle assembly checkpoint mechanism [4–9]. Only when the sister chromatids are aligned at the metaphase plate and have established bivalent spindle attachment can the inhibition of APC/Cdc20 be released. In contrast to APCCdc20, APCCdh1 is inactive in early mitosis [3] when it is inhibited by phosphorylation [10] and binding of Nup90/Rae1 complex [11, 12]. APCCdh1 only becomes active from late mitosis to G1. The difference in the timing of activation between APCCdc20 and APCCdh1 suggests a functional division between the two E3 ubiquitin ligases in mitosis. Recent analyses of mice deficient in Cdc20 or Cdh1 strongly support that notion [13–15]. It appears that Cdc20 is required for metaphase to anaphase transition [15], whereas Cdh1 plays a nonessential role in mitotic exit but an essential role in G1/S regulation [13, 14].
Mitotic Function of Cdh1
A large number of mitotic regulators are degraded at the end of mitosis. These include Cdc20, Aurora B, Plk1, etc. and are most likely the substrates of APCCdh1 (Table 1) [16–43]. Although many of these mitotic regulators did accumulate in the absence of Cdh1, they were eventually degraded, probably because of the stabilization of Cdc20 that compensates for the loss of Cdh1 [13, 14]. As a result, Cdh1-deficient cells could still proliferate. However, these cells did accumulate mitotic errors and display difficulties in completing cytokinesis, resulting in the formation of binucleated cells at a high frequency [13, 14].
The lethality associated with the loss of Cdh1 is largely a result of failed development of placenta, an essential organ for embryonic life in mammals [13, 14]. In the placenta, there is a special cell type, giant cells which are polyploid. The polyploidy is acquired through endoreplication. In the absence of Cdh1, the placental giant cells failed to form, suggesting that APCCdh1 is required for DNA endoreplication. To undergo endoreplication, a cell must not enter mitosis after a round of DNA synthesis. One critical factor for mitotic entry is cyclin B1, a substrate of APCCdh1 [33]. Therefore, it is possible that the failed formation of giant cells in Cdh1 mutant embryos was a result of the inability of the would-be giant cells to block the accumulation of cyclin B1. Indeed, cyclin B1 was readily detectable in the mutant placenta in the area where the giant cells should reside [14].
Like an endoreplicating cell, cells with their DNA damaged do not enter mitosis either. A recent study revealed that APCCdh1-medicated degradation of Plk1 played an important role in preventing mitotic entry of DNA-damaged cells [44]. Under normal conditions, Cdh1 is kept inactive from late G1 to early mitosis. When cells suffered DNA damage, Cdc14B is translocated out of nucleoli into nuclearplasm where the phosphatase dephophorylates and activates Cdh1 [44]. It is unknown if a similar mechanism is employed by the placental giant cells to activate Cdh1 in G2 to prevent mitotic entry.
Maintenance of G1 by APCCdh1
In Drosophila, loss of fzr leads to an extra division cycle in the epidermis, a likely result of the accumulation of mitotic cyclins in G1 [45]. In HeLa cells, knocking down the expression of Cdh1 causes stabilization of Skp2 [46, 47], a F-box containing protein responsible for bringing p27Kip1 to the SCF complex for ubiquitination [48, 49]. As a result, p27 is destabilized in the cells with impaired Cdh1 function and the G1 phase is shortened in these cells.
Our recent work identified Ets2 as a new substrate of APCCdh1 [14]. Ets2 is a member of the Ets family of transcription factors which share a unique DNA binding domain, the ETS domain [50, 51]. The first Ets protein was identified as transduced mutant form of Ets1, v-Ets, in a retrovirus, E26 (E T wenty six-s pecific, so the name Ets) avian leukosis virus (ALV), that induces erythroblastosis in avian species [52]. It is well known that Ets2 is activated by Ras-Raf-MAPK signaling and mediates some effects of this important signaling pathway [53–59]. The most prominent effect of Ras signaling is the stimulation of proliferation which relies in part on the induction of cyclin D1 expression by Est2 [60, 61]. Increased expression of Ets2 has been associated with initiation and progression of various cancer types [62–65] and its expression was altered in cervical cancer cell lines due to chromosomal changes in 21q22.1–22.2 where human ETS2 resides [66]. Moreover, Ets2 was found overexpressed in esophageal squamous cell carcinoma [67]. These results suggest that Ets2 is an oncogene. By targeting both Skp2 and Ets2, APCCdh1 maintains G1 by increasing the levels of a Cdk inhibitor p27 through destabilizing Skp2 and by limiting the expression of cyclin D1 through promoting Ets2 degradation (Fig. 1). Since both Skp2 and Ets2 are potential oncogenes, APCCdh1 may possess tumor-suppression activity. Indeed, mice heterozygous for Cdh1 display increased rates of tumorigenesis at old ages [13].
It was shown previously by Nasmyth's group that inactivation of APC/C by deleting its subunit APC2 in adult hepatocytes induced these otherwise quiescent cells to re-enter cell cycle [68]. This is most likely a result of the loss of APCCdh1 activity and subsequent accumulation of Skp2 and Ets2.
To enter S-phase, APCCdh1 must be inactivated. Several different mechanisms are in place to contain APCCdh1 [69, 70]. First, the ubiquitination of APC-specific ubiqutin-conjugating enzyme (E2) UbcH10 by APCCdh1 itself provides a negative feedback mechanism that would eventually destroy APCCdh1 activity [71, 72]. Second, as Cdk activity accumulates, Cdh1 is phosphorylated. The phosphorylation promotes Cdh1 dissociation from APC [10, 73]. Third, phosphorylated Cdh1 is targeted by SCF liagse [74], further limiting the activity of APCCdh1. Finally, in late G1 phase, E2F activates the transcription of early mitotic inhibitor-1 (Emi1)/Rca1, which inhibits the activity of APC/CCdh1 as a pseudo-substrate [75, 76].
Cdh1 and Cellular Senescence
Opposite to the expected high proliferation rates in Cdh1-deficient cells, Cdh1-/- mouse embryonic fibroblasts (MEFs) proliferate poorly and entered senescence after only a few passages [77]. We found that the induction of p16Ink4a in these cells was the cause for the reduced proliferation potential. The reason for the increased levels of p16 in Cdh1-/- MEFs could simply be that p16 is a substrate of APCCdh1. Alternatively, it could be that a transcriptional activator of p16 is a substrate. It turned out that the latter was the case [14]. We demonstrated that Cdh1-deficiency induced upregulation of p16 was a result of Ets2 stabilization. It was shown previously that Est2 could activate p16 expression [78]. The expression of p16 is regulated by a number of factors in response to various stimuli. Under normal conditions, Ets2 levels are not enough to induce p16 expression and senescence because of Id1 and Bim1 (Fig. 2A). Id1 interacts with Ets2 and blocks its transcriptional activation of p16 [78, 79], whereas Bmi1 directly represses p16 promoter [80]. Our identification of the APCCdh1-Ets2-p16 axis shows the delicacy of the balance that determines the level of p16 expression. Loss of Cdh1 leads to increases in the levels of Ets2 to the point that it overcomes Id1's inhibition and results in senescence (Fig. 2B). This would imply that Ets2 activity required for p16 activation and that for other targets are different, which remains to be determined.
Cdh1 and cellular senescence. (A) Under normal conditions, the expression of p16 is tightly controlled by both positive and negative regulators. (B) Deletion of Cdh1 causes accumulation of Est2, leading to overexpression of p16 and senescence. (C) Oncogenic Ras may also induce senescence through Ets2.
Overactivation of Ras signaling could also cause senescence in primary cells [81–83]. It is likely that Ets2 mediates, at least in part, this senescence effect of Ras signaling (Fig. 2C). It remains to be determined however if Ras signaling interacts with APCCdh1. The phosphorylation of Ets2 by Erk may interfere with the recognition of Ets2 by Cdh1, for example, leading to stabilization of Est2. Therefore, when Ras signaling is overactivated, APCCdh1 would only be able to provide limited balancing function against Ets2. By placing p16 under the transcriptional control of Ets2, evolution has setup a failsafe mechanism to prevent unwarranted proliferation.
Cdh1 and Neural Function
Two places where appreciable levels of Cdh1 (and APC subunits) but not Cdc20 are expressed in adult mice are the brain and the liver [84]. In the liver, APCCdh1 is likely required for preventing hepatocytes from reentering the cell cycle spontaneously (see above). What is the function of APCCdh1 in postmitotic neurons? The depletion of Cdh1 expression in primary neuron of the cerebellar cortex promotes significant elongations of axons, indicating a role of APCdh1 in limiting axonal growth [85], possibly through regulating the abundance of SnoN and Id2 [86, 87]. APCCdh1 may also control pattering of axon growth in the mammalian brain [85].
We assessed neural functioning of mice that are heterozygous Cdh1 mutant. Electrophysiology studies of hippocampus revealed a deficit in late phase long-term potentiation (L-LTP), a process that underlies synaptic plasticity and is known to depend on both protein synthesis and degradation [88]. In behavior tests, we found that Cdh1 heterozygous mice performed poorly in contextual fear conditioning [14], a hippocampus-dependent process [89, 90], but no difference was observed in cued fear condition which is less dependent on hippocampus [89, 90]. Similar behavior findings were also reported by Malumbres' group [13]. It is unclear at the moment what are the substrates of APCCdh1 that play a role in memory formation in mammals. However, studies from lower organisms may provide a clue. In C. elegans, the abundance of GLR-1 glutamate receptors in the ventral nerve cord is regulated by anaphase promoting complex, albeit unlikely to be in a direct manner [91]. In Drosophila, Liprin-α, a multidomain scaffolding protein which is localized to synapses and regulates synaptic activities, seems to be a substrate of APCCdh1 [92]. It remains to be determined if mammalian homologues of Liprin-α and GLR-1 are regulated by APCCdh1 and if their misregulation can account for the learning and memory defects displayed by Cdh1 heterozygous mice.
Conclusion
Tremendous progresses have been made towards understanding the regulation and function of APC/C in the last decade. Its role in cell cycle regulation has largely been elucidated. It appears that APCCdc20 is dedicated to mitosis while APCCdh1 has a much broader application not restricted to the cell cycle. The most intriguing question remains to be addressed is the role of APC/C in non-dividing cells. Accumulating evidence points to the involvement of APCCdh1 in many aspects of neural function including axon growth, morphology and plasticity of synapses, and learning and memory. Identification of the relevant substrates will provide significant insights into the functioning of the complex nervous system. Further, in addition to its potential role in maintaining the G0 state of hepatocytes, whether APCCdh1 participates in the physiological function of the liver remains to be elucidated
References
Vodermaier HC: APC/C and SCF: Controlling Each Other and the Cell Cycle. Curr Biol 2004, 14(18):R787-R796. 10.1016/j.cub.2004.09.020
Nakayama KI, Nakayama K: Ubiquitin ligases: cell-cycle control and cancer. Nature Reviews Cancer 2006, 6: 369–381. 10.1038/nrc1881
Harper JW, Burton JL, Solomon MJ: The anaphase-promoting complex: it's not just for mitosis any more. Genes & development 2002, 16(17):2179–2206. 10.1101/gad.1013102
Hwang LH, Lau LF, Smith DL, Mistrot CA, Hardwick KG, Hwang ES, Amon A, Murray AW: Budding yeast Cdc20: a target of the spindle checkpoint. Science (New York, NY) 1998, 279(5353):1041–1044.
Fang G, Yu H, Kirschner MW: The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes & development 1998, 12(12):1871–1883. 10.1101/gad.12.12.1871
Kallio M, Weinstein J, Daum JR, Burke DJ, Gorbsky GJ: Mammalian p55CDC mediates association of the spindle checkpoint protein Mad2 with the cyclosome/anaphase-promoting complex, and is involved in regulating anaphase onset and late mitotic events. J Cell Biol 1998, 141(6):1393–1406. 10.1083/jcb.141.6.1393
Wu H, Lan Z, Li W, Wu S, Weinstein J, Sakamoto KM, Dai W: p55CDC/hCDC20 is associated with BUBR1 and may be a downstream target of the spindle checkpoint kinase. Oncogene 2000, 19(40):4557–4562. 10.1038/sj.onc.1203803
Fang G: Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Molecular biology of the cell 2002, 13(3):755–766. 10.1091/mbc.01-09-0437
Tang Z, Bharadwaj R, Li B, Yu H: Mad2-Independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev Cell 2001, 1(2):227–237. 10.1016/S1534-5807(01)00019-3
Kramer ER, Scheuringer N, Podtelejnikov AV, Mann M, Peters JM: Mitotic regulation of the APC activator proteins CDC20 and CDH1. Molecular biology of the cell 2000, 11(5):1555–1569.
Jeganathan KB, Baker DJ, van Deursen JM: Securin associates with APCCdh1 in prometaphase but its destruction is delayed by Rae1 and Nup98 until the metaphase/anaphase transition. Cell Cycle 2006, 5(4):366–370.
Jeganathan KB, Malureanu L, van Deursen JM: The Rae1-Nup98 complex prevents aneuploidy by inhibiting securin degradation. Nature 2005, 438(7070):1036–1039. 10.1038/nature04221
García-Higuera I, Manchado E, Dubus P, Cañamero M, Méndez J, Moreno S, Malumbres M: Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nature cell biology 2008, 10(7):802–811. 10.1038/ncb1742
Li M, Shin Y-H, Hou L, Huang X, Wei Z, Klann E, Zhang P: The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nature cell biology 2008, 10(9):1083–1089. 10.1038/ncb1768
Li M, York JP, Zhang P: Loss of Cdc20 causes a securin-dependent metaphase arrest in two-cell mouse embryos. Molecular and cellular biology 2007, 27(9):3481–3488. 10.1128/MCB.02088-06
Prinz S, Hwang ES, Visintin R, Amon A: The regulation of Cdc20 proteolysis reveals a role for the APC components Cdc23 and Cdc27 during S phase and early mitosis. Curr Biol 1998, 8(13):750–760. 10.1016/S0960-9822(98)70298-2
Pfleger CM, Kirschner MW: The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes & Development 2000, 14: 655–665.
Zur A, Brandeis M: Securin degradation is mediated by fzy and fzr, and is required for complete chromatid separation but not for cytokinesis. The EMBO journal 2001, 20: 792–801. 10.1093/emboj/20.4.792
Zou H, McGarry TJ, Bernal T, Kirschner MW: Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science (New York, NY) 1999, 285(5426):418–422.
Karamysheva Z, Diaz-Martinez L, Crow SE, Li B, Yu H: Multiple APC/C degrons mediate the degradation of human SGO1. Journal of Biological Chemistry 2008, in press.
Zhao W-m, Coppinger JA, Seki A, Cheng X-l JRY III, Fang G: RCS1, a substrate of APC/C, controls the metaphase to anaphase transition. The Proceedings of the National Academy of Sciences 2008, 105(36):13415–13420. 10.1073/pnas.0709227105
Antonio C, Ferby I, Wilhelm H, Jones M, Karsenti E, Nebreda AR, Vernos I: Xkid, a Chromokinesin Required for Chromosome Alignment on the Metaphase Plate. Cell 2000, 102: 425–435. 10.1016/S0092-8674(00)00048-9
Funabiki H, Murray AW: The Xenopus Chromokinesin Xkid Is Essential for Metaphase Chromosome Alignment and Must Be Degraded to Allow Anaphase Chromosome Movement. Cell 2000, 102: 411–424. 10.1016/S0092-8674(00)00047-7
Stewart S, Fang G: Destruction box-dependent degradation of aurora B is mediated by the anaphase-promoting complex/cyclosome and Cdh1. Cancer research 2005, 65(19):8730–8735. 10.1158/0008-5472.CAN-05-1500
Juang Y-L, Huang J, Peters J-M, McLaughlin ME, Tai C-Y, Pellman D: APC-Mediated Proteolysis of Ase1 and the Morphogenesis of the Mitotic Spindle. Science (New York, NY) 1997, 275: 1311–1314.
Visintin R, Prinz S, Amon A: CDC20 and CDH1: a family of substrate-specific activators of APC- dependent proteolysis. Science 1997, 278(5337):460–463. 10.1126/science.278.5337.460
Castro A, Vigneron S, Bernis C, Labbe J-C, Prigent C, Lorca T: The D-Box-activating domain (DAD) is a new proteolysis signal that stimulates the silent D-Box sequence of Aurora-A. EMBO Reports 2002, 3: 1209–1214. 10.1093/embo-reports/kvf241
Castro A, Arlot-Bonnemains Y, Vigneron S, Labbé J-C, Prigent C, Lorca T: APC/Fizzy-Related targets Aurora-A kinase for proteolysis. EMBO Reports 2002, 3: 457–462. 10.1093/embo-reports/kvf095
Stewart S, Fang G: Anaphase-Promoting Complex/Cyclosome Controls the Stability of TPX2 during Mitotic Exit. Molecular and cellular biology 2005, 25(23):10516–10527. 10.1128/MCB.25.23.10516-10527.2005
Lindon C, Pines J: Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J Cell Biol 2004, 164(2):233–241. 10.1083/jcb.200309035
Zhao W-m, Fang G: Anillin Is a Substrate of Anaphase-promoting Complex/Cyclosome (APC/C) That Controls Spatial Contractility of Myosin during Late Cytokinesis. The Journal of biological chemistry 2005, 280(39):33516–33524. 10.1074/jbc.M504657200
Seki A, Fang G: CKAP2 is a spindle-associated protein degraded by APC/C-CDH1 during mitotic exit. The Journal of biological chemistry 2007, 282(20):15103–15113. 10.1074/jbc.M701688200
King RW, Peters J-M, Tugendreich S, Rolfe M, Hieter P, Kirschner MW: A 20s complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 1995, 81: 279–288. 10.1016/0092-8674(95)90338-0
Geley S, Kramer E, Gieffers C, Gannon J, Peters J-M, Hunt T: Anaphase-promoting Complex/Cyclosome-dependent Proteolysis of Human Cyclin A Starts at the Beginning of Mitosis and Is Not Subject to the Spindle Assembly Checkpoint. The Journal of cell biology 2001, 153: 137–148. 10.1083/jcb.153.1.137
Duursma A, Agami R: p53-Dependent Regulation of Cdc6 Protein Stability Controls Cellular Proliferation. Molecular and cellular biology 2005, 25(16):6937–6947. 10.1128/MCB.25.16.6937-6947.2005
Mailand N, Diffley JFX: CDKs Promote DNA Replication Origin Licensing in Human Cells by Protecting Cdc6 from APC/C-Dependent Proteolysis. Cell 2005, 122: 915–926. 10.1016/j.cell.2005.08.013
Petersen BO, Wagener C, Marinoni F, Kramer ER, Melixetian M, Denchi EL, Gieffers C, Matteucci C, Peters J-M, Helin K: Cell cycle- and cell growth-regulated proteolysis of mammalian CDC6 is dependent on APC-CDH1. Genes & Development 2000, 14: 2330–2343. 10.1101/gad.832500
McGarry TJ, Kirschner MW: Geminin, an Inhibitor of DNA Replication, Is Degraded during Mitosis. Cell 1998, 93(6):1043–1053. 10.1016/S0092-8674(00)81209-X
Zielke N, Querings S, Rottig C, Lehner C, Sprenger F: The anaphase-promoting complex/cyclosome (APC/C) is required for rereplication control in endoreplication cycles. Genes & Development 2008, 22: 1690–1703. 10.1101/gad.469108
Park HJ, Costa RH, Lau LF, Tyner AL, Raychaudhuri P: Anaphase-Promoting Complex/Cyclosome-Cdh1-Mediated Proteolysis of the Forkhead Box M1 Transcription Factor Is Critical for Regulated Entry into S Phase. Molecular and cellular biology 2008, 28(17):5162–5171. 10.1128/MCB.00387-08
Laoukili J, Alvarez-Fernandez M, Stahl M, Medema RH: FoxM1 is degraded at mitotic exit in a Cdh1-dependent manner. Cell Cycle 2008, 7(17):2720–2726.
Wan Y, Liu X, Kirschner MW: The anaphase-promoting complex mediates TGF-beta signaling by targeting SnoN for destruction. Mol Cell 2001, 8(5):1027–1039. 10.1016/S1097-2765(01)00382-3
Stroschein SL, Bonni S, Wrana JL, Luo K: Smad3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes & Development 2001, 15: 2822–2836.
Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M: The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell 2008, 134(2):256–267. 10.1016/j.cell.2008.05.043
Sigrist SJ, Lehner CF: Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 1997, 90(4):671–681. 10.1016/S0092-8674(00)80528-0
Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M: Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 2004, 428(6979):190–193. 10.1038/nature02330
Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG Jr: Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 2004, 428(6979):194–198. 10.1038/nature02381
Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H: p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol 1999, 9(12):661–664. 10.1016/S0960-9822(99)80290-5
Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, et al.: Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. The EMBO journal 2000, 19(9):2069–2081. 10.1093/emboj/19.9.2069
Macleod K, Leprince D, Stehelin D: The ets gene family. Trends in biochemical sciences 1992, 17(7):251–256. 10.1016/0968-0004(92)90404-W
Crepieux P, Coll J, Stehelin D: The Ets family of proteins: weak modulators of gene expression in quest for transcriptional partners. Critical reviews in oncogenesis 1994, 5(6):615–638.
Leprince D, Gegonne A, Coll J, de Taisne C, Schneeberger A, Lagrou C, Stehelin D: A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature 1983, 306(5941):395–397. 10.1038/306395a0
Foulds CE, Nelson ML, Blaszczak AG, Graves BJ: Ras/mitogen-activated protein kinase signaling activates Ets-1 and Ets-2 by CBP/p300 recruitment. Molecular and cellular biology 2004, 24(24):10954–10964. 10.1128/MCB.24.24.10954-10964.2004
Langer SJ, Bortner DM, Roussel MF, Sherr CJ, Ostrowski MC: Mitogenic signaling by colony-stimulating factor 1 and ras is suppressed by the ets-2 DNA-binding domain and restored by myc overexpression. Molecular and cellular biology 1992, 12(12):5355–5362.
Galang CK, Der CJ, Hauser CA: Oncogenic Ras can induce transcriptional activation through a variety of promoter elements, including tandem c-Ets-2 binding sites. Oncogene 1994, 9(10):2913–2921.
Yang BS, Hauser CA, Henkel G, Colman MS, Van Beveren C, Stacey KJ, Hume DA, Maki RA, Ostrowski MC: Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Molecular and cellular biology 1996, 16(2):538–547.
McCarthy SA, Chen D, Yang BS, Garcia Ramirez JJ, Cherwinski H, Chen XR, Klagsbrun M, Hauser CA, Ostrowski MC, McMahon M: Rapid phosphorylation of Ets-2 accompanies mitogen-activated protein kinase activation and the induction of heparin-binding epidermal growth factor gene expression by oncogenic Raf-1. Mol Cell Biol 1997, 17(5):2401–2412.
Wasylyk C, Bradford AP, Gutierrez-Hartmann A, Wasylyk B: Conserved mechanisms of Ras regulation of evolutionary related transcription factors, Ets1 and Pointed P2. Oncogene 1997, 14(8):899–913. 10.1038/sj.onc.1200914
Patton SE, Martin ML, Nelsen LL, Fang X, Mills GB, Bast RC Jr, Ostrowski MC: Activation of the ras-mitogen-activated protein kinase pathway and phosphorylation of ets-2 at position threonine 72 in human ovarian cancer cell lines. Cancer research 1998, 58(10):2253–2259.
Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell RG: Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. The Journal of biological chemistry 1995, 270(40):23589–23597. 10.1074/jbc.270.40.23589
Carbone GM, Napoli S, Valentini A, Cavalli F, Watson DK, Catapano CV: Triplex DNA-mediated downregulation of Ets2 expression results in growth inhibition and apoptosis in human prostate cancer cells. Nucleic Acids Res 2004, 32(14):4358–4367. 10.1093/nar/gkh744
de Nigris F, Mega T, Berger N, Barone MV, Santoro M, Viglietto G, Verde P, Fusco A: Induction of ETS-1 and ETS-2 transcription factors is required for thyroid cell transformation. Cancer research 2001, 61(5):2267–2275.
Neznanov N, Man AK, Yamamoto H, Hauser CA, Cardiff RD, Oshima RG: A single targeted Ets2 allele restricts development of mammary tumors in transgenic mice. Cancer research 1999, 59(17):4242–4246.
Sapi E, Flick MB, Rodov S, Kacinski BM: Ets-2 transdominant mutant abolishes anchorage-independent growth and macrophage colony-stimulating factor-stimulated invasion by BT20 breast carcinoma cells. Cancer research 1998, 58(5):1027–1033.
Sementchenko VI, Schweinfest CW, Papas TS, Watson DK: ETS2 function is required to maintain the transformed state of human prostate cancer cells. Oncogene 1998, 17(22):2883–2888. 10.1038/sj.onc.1202220
Simpson S, Woodworth CD, DiPaolo JA: Altered expression of Erg and Ets-2 transcription factors is associated with genetic changes at 21q22.2–22.3 in immortal and cervical carcinoma cell lines. Oncogene 1997, 14(18):2149–2157. 10.1038/sj.onc.1201058
Li X, Lu JY, Zhao LQ, Wang XQ, Liu GL, Liu Z, Zhou CN, Wu M, Liu ZH: Overexpression of ETS2 in human esophageal squamous cell carcinoma. World J Gastroenterol 2003, 9(2):205–208.
Wirth KG, Ricci R, Giménez-Abián JF, Taghybeeglu S, Kudo NR, Jochum W, Vasseur-Cognet M, Nasmyth K: Loss of the anaphase-promoting complex in quiescent cells causes unscheduled hepatocyte proliferation. Genes & Development 2004, 18: 88–98. 10.1101/gad.285404
Peters J-M: The anaphase promoting complex/cyclosome: a machine designed to destroy. Nature Reviews Molecular Cell Biology 2006, 7: 644–656. 10.1038/nrm1988
Pesin JA, Orr-Weaver TL: Regulation of APC/C Activators in Mitosis and Meiosis. Annual Review of Cell and Developmental Biology 2008, 24: 475–499. 10.1146/annurev.cellbio.041408.115949
Rape M, Kirschner MW: Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature 2004, 432: 588–595. 10.1038/nature03023
Rape M, Reddy SK, Kirschner MW: The Processivity of Multiubiquitination by the APC Determines the Order of Substrate Degradation. Cell 2006, 124(1):89–103. 10.1016/j.cell.2005.10.032
Lukas C, Sørensen CS, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters J-M, Bartek J, Lukas J: Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature 1999, 401: 815–818. 10.1038/44611
Benmaamar R, Pagano M: Involvement of the SCF Complex in the Control of Cdh1 Degradation in S-phase. Cell Cycle 2005, 4(9):1230–1232.
Hsu JY, Reimann JDR, Sørensen CS, Lukas J, Jackson PK: E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APCCdh1. Nature cell biology 2002, 4: 358–366. 10.1038/ncb785
Grosskortenhaus R, Sprenger F: Rca1 Inhibits APC-Cdh1Fzr and Is Required to Prevent Cyclin Degradation in G2. Developmental Cell 2002, 2(1):29–40. 10.1016/S1534-5807(01)00104-6
Li M, Shin YH, Hou L, Huang X, Wei Z, Klann E, Zhang P: The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nature cell biology 2008, 10: 1083–1089. 10.1038/ncb1768
Ohtani N, Zebedee Z, Huot TJ, Stinson JA, Sugimoto M, Ohashi Y, Sharrocks AD, Peters G, Hara E: Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature 2001, 409(6823):1067–1070. 10.1038/35059131
Alani RM, Young AZ, Shifflett CB: Id1 regulation of cellular senescence through transcriptional repression of p16/Ink4a. Proceedings of the National Academy of Sciences of the United States of America 2001, 98(14):7812–7816. 10.1073/pnas.141235398
Jacobs JJ, Scheijen B, Voncken JW, Kieboom K, Berns A, van Lohuizen M: Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev 1999, 13(20):2678–2690. 10.1101/gad.13.20.2678
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW: Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88(5):593–602. 10.1016/S0092-8674(00)81902-9
Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW: Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev 1998, 12(19):3008–3019. 10.1101/gad.12.19.3008
Zhu J, Woods D, McMahon M, Bishop JM: Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev 1998, 12(19):2997–3007. 10.1101/gad.12.19.2997
Gieffers C, Peters BH, Kramer ER, Dotti CG, Peters JM: Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proceedings of the National Academy of Sciences of the United States of America 1999, 96(20):11317–11322. 10.1073/pnas.96.20.11317
Konishi Y, Stegmüller J, Matsuda T, Bonni S, Bonni A: Cdh1-APC Controls Axonal Growth and Patterning in the Mammalian Brain. Science (New York, NY) 2004, 303: 1026–1030.
Lasorella A, Stegmuller J, Guardavaccaro D, Liu G, Carro MS, Rothschild G, de la Torre-Ubieta L, Pagano M, Bonni A, Iavarone A: Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature 2006, 442(7101):471–474. 10.1038/nature04895
Stegmüller J, Konishi Y, Huynh MA, Yuan Z, DiBacco S, Bonni A: Cell-Intrinsic Regulation of Axonal Morphogenesis by the Cdh1-APC Target SnoN. Neuron 2006, 50(3):389–400. 10.1016/j.neuron.2006.03.034
Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nagerl UV: A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron 2006, 52(2):239–245. 10.1016/j.neuron.2006.08.015
Kim JJ, Fanselow MS: Modality-specific retrograde amnesia of fear. Science 1992, 256(5057):675–677. 10.1126/science.1585183
Phillips RG, LeDoux JE: Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral neuroscience 1992, 106(2):274–285. 10.1037/0735-7044.106.2.274
Juo P, Kaplan JM: The Anaphase-Promoting Complex Regulates the Abundance of GLR-1 Glutamate Receptors in the Ventral Nerve Cord of C. elegans. Curr Biol 2004, 14(22):2057–2062. 10.1016/j.cub.2004.11.010
Roessel Pv, Elliott DA, Robinson IM, Prokop A, Brand AH: Independent Regulation of Synaptic Size and Activity by the Anaphase-Promoting Complex. Cell 2004, 119(5):707–718. 10.1016/j.cell.2004.11.028
Acknowledgements
This work was supported by grants from The National Institute of Health to PZ (CA122623-02 and CA116097-04). ML is supported by a NIH postdoctoral training grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ML and PZ wrote the manuscript together.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Li, M., Zhang, P. The function of APC/CCdh1 in cell cycle and beyond. Cell Div 4, 2 (2009). https://doi.org/10.1186/1747-1028-4-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1747-1028-4-2