Abstract
Microglial polarization plays an essential role in the progression and regression of neurodegenerative disorders. Cyanidin-3-O-glucoside (C3G), a dietary anthocyanin found in many fruits and vegetables, has been reported as an antioxidant, anti-inflammatory, and antitumor agent. However, there have been no reports on whether C3G can regulate the M1/M2 shift in an Alzheimer’s disease model. We attempted to investigate the effects of C3G on M1/M2 polarization and the mechanism to regulate anti-inflammation and phagocytosis, both in vitro and in vivo. HMC3 cells were treated with β-amyloid (Aβ42) in the presence or absence of 50 μM C3G for different time intervals, and APPswe/PS1ΔE9 mice were orally administered 30 mg/kg/day of C3G for 38 weeks. The in vitro data revealed that C3G could shift the M1 phenotype of microglia to M2 by reducing the expression of M1-specific markers (CD86 and CD80), inflammatory cytokines (IL-Iβ, IL-6, TNF-α), reactive oxygen species, and enhancing the expression of M2-specific markers (CD206 and CD163). The APPswe/PS1ΔE9 mice results were consistent with the in vitro data, indicating a significant reduction in inflammatory cytokines and higher expression of M2-specific markers such as CD206 and Arg1 in C3G-treated Alzheimer’s disease model mice. Additionally, C3G was found to upregulate PPARγ expression levels both in vitro and in vivo, whereas a PPARγ antagonist (GW9662) was found to block C3G-mediated effects in vitro. In this study, we confirmed that C3G could regulate microglial polarization by activating PPARγ and eliminating accumulated β-amyloid by enhancing Aβ42 phagocytosis through the upregulation of TREM2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative brain disorder that affects memory and cognitive functions and challenges an individual’s ability to think, communicate clearly, and carry out day-to-day tasks. It accounts for two-thirds of all dementia cases, with 50–70% of people with dementia afflicted with AD. AD is characterized by the presence of β-amyloid (Aβ) plaques and neurofibrillary tangles (NFTs) within the brain. Aβ plaques develop as a consequence of the faulty cleavage of amyloid precursor protein (APP), whereas NFTs are produced via the hyper-phosphorylation and disorganization of tau proteins [1].
Recent studies have identified neuroinflammation as the primary cause of AD progression [2]. Microglia, the brain macrophages, play an essential role in countering the extracellular accumulation of Aβ plaques by dividing them into two distinct phenotypes. The M1 phenotype contributes to neurotoxicity, oxidative stress, and neuronal and synaptic damage. In contrast, the M2 phenotype plays an essential role in regulating neuroinflammation by clearing cell debris and misfolded proteins and providing support for neuroregeneration. The M1 phenotype of microglia produces pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6 and expresses the M1-specific markers CD86 and CD80 [3,4,5]. However, cells with the M2 phenotype release anti-inflammatory cytokines such as IL-10 and IL-4 and the M2-specific anti-inflammatory markers CD206 and CD163 [6,7,8]. Additionally, reactive oxygen species (ROS) play a pivotal role in the pathogenesis of AD and are responsible for promoting a number of harmful effects, including damage to cellular DNA, lipids, and proteins [9,10,11].
Anthocyanins are a class of polyphenolic flavonoids found in the fruits, flowers, leaves, and seeds of a range of plants, including cherry, blueberry, blackcurrant, mulberry, black soybean, and honeyberry [12, 13]. These compounds have attracted considerable attention in recent years, owing to their potential therapeutic properties, with beneficial effects such as those associated with neuroinflammation, excitotoxicity, and protein homeostasis [14, 15]. Cyanidin-3-O-glucoside (C3G) is a flavonoid anthocyanin studied as an ant oxidative, anti-inflammatory, and antitumor agent [16,17,18,19]. It has also been found to have several beneficial effects in treating different diseases and disorders, such as non-alcoholic fatty acid liver disease, cardiovascular diseases, and neurological disorders [20,21,22,23].
Peroxisome-proliferator activated receptors (PPARs) are ligand-activated nuclear receptors that regulate different biological pathways, including those associated with cell proliferation, development, metabolism, reproduction, and inflammation. These receptors can be sub-divided into three isoforms, namely, PPAR-α, PPAR-γ, and PPAR-δ, which are distributed in different tissues, in which they perform various biological functions [24]. Although studies have reported that the beneficial effects of C3G are mediated via PPARs [25, 26], there have been no reports on the role of PPARγ in the C3G-mediated neuroprotective effects in Aβ42-treated human microglial HMC3 cells. Additionally, whereas it has been established that the impact of certain flavonoids on M2 polarization is mediated via the activation of PPARγ, no studies have been reported to date that has examined the effects of C3G in mediating microglial phenotypes via PPARγ.
The triggering receptor expressed on myeloid cells (TREM2) is a receptor found on the surface of microglial cells that plays an essential role in the phagocytosis of Aβ. The progression of AD has been reported to be associated with alterations in the expression of TREM2 and poor phagocytosis of Aβ by microglial cells [27,28,29,30]. Moreover, recent studies have revealed that TREM2 functions as an M2-specific microglia marker [31,32,33], and C3G has been reported to clear Aβ25-35 and inhibit Aβ40 fibrillogenesis in vivo [34]. To the best of our knowledge, however, no previous studies have examined the effects of C3G on TREM2 expression and Aβ42 phagocytosis in human microglial cells.
In this study, we investigated the effects of C3G in regulating the M1/M2 shift of microglia and Aβ42 phagocytosis in Aβ42-treated human microglial cells and sought to determine the role of PPARγ and TREM2 in the C3G-mediated effects.
Material and Methods
Materials
The HMC3 (ATCC® CRL-3304™) cell line used in this study was obtained from the American Type Culture Collection (ATCC, Manassas, USA). C3G (Cat. No. CFN99740) was purchased from ChemFaces (Wuhan, Hubei, China). Specific antibodies against CD86 (sc-19617), CD206 (sc-58986), CD163 (sc-20066), and TREM2 (sc-373828) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Phycoerythrin (PE)-conjugated CD80 antibody (12–0809-42) was purchased from eBioscience (San Diego, CA, USA). TREM2 blocking antibody (MAB17291-100) was purchased from Bio-techne (Minneapolis, MN, USA). PE-conjugated secondary antibody (ab97024) and Aβ42 peptide (ab120301) were purchased from Abcam (Cambridge, UK). The PPARγ antagonist (GW9662; Cat. no M6191) was purchased from Sigma (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), trypsin, and fetal bovine serum (FBS), antibiotic–antimycotic solution, were supplied by Thermo Fisher (San Jose, CA, USA).
Cell culture and Treatments
The HMC3 immortalized human microglial cell line was grown optimally in DMEM supplemented with 10% heat-inactivated FBS and 1% antibiotic–antimycotic solution and maintained in a 5% CO2 humidified incubator at 37 °C. Both the β-amyloid and C3G were prepared in DMSO, so DMSO was used as a control in our study.
Cell Viability Assay
HMC3 cells were seeded in 96-well plates (1 × 104 cells/well) and allowed to adhere and grow for 24 h. After stabilization for 24 h, the cells were exposed to different concentrations (0.1, 1, 2, and 4 μM) of Aβ42 for 24 h and co-treated with 1 μM of Aβ42 and C3G (25, 50, 100, 200 μM) for 24 h incubation under a 5% CO2 atmosphere. Cell viability was assessed using a Cell Counting Kit-8 assay (Dojindo Molecular Technologies, Rockville, MD, USA), as recommended by the manufacturer. The absorbance was measured at 450 nm using a microplate reader (BioTek Inc., Winooski, VT, USA).
Annexin V-FITC Apoptosis Detection Assay
Annexin V-FITC apoptosis detection assay kit (APOAF-20TST) purchased from Sigma (St. Louis, MO, USA) allows flow cytometry–based determination of apoptotic cells. The assay involves two significant components: annexin V conjugated with fluorescein isothiocyanate (FITC) to label phosphatidylserine sites on the membrane surface and propidium iodide (PI) to label the cellular DNA in necrotic cells where the cell membrane has been totally compromised. The combination of annexin V and PI allows the differentiation of viable cells (annexin V negative, PI negative), early apoptotic (annexin V positive, PI negative), and necrotic cells (annexin V positive, PI positive). In brief, HMC3 cells were treated with 1 μM of Aβ42 and co-treated with 1 μM of Aβ42 and 50 μM of C3G for 24 h and harvested. Cells were washed with DPBS, and each group, including the control, was divided into triplicates. For each sample, cells were suspended into 500 µL of 1X binding buffer containing 5 µL of annexin V-FITC and 5 µL of PI in the dark for 10 min at room temperature. Flow cytometry analysis was performed to detect apoptotic and viable cells using an FC500 MLP cytometer (Beckman Coulter Inc., Fullerton, CA, USA).
RNA Preparation and Real-Time PCR
Having treated the HMC3 cells with the 1 μM of Aβ42 for different time intervals (1, 3, 6, and 12 h), and co-treated with 1 μM of Aβ42 and 50 μM of C3G for 3 h, total RNA was extracted using an RNA extraction kit (iNtRON Biotechnology, Gyeonggi-do, Korea) according to the manufacturer’s instructions. In total, 50 ng of RNA was reverse transcribed to complementary DNA using a PCR (TaKaRa Bio, Kusatsu, Shiga, Japan). RT-PCR was performed with TB Green (TaKaRa Bio, Kusatsu, Shiga, Japan) using an ABI QuantStudio3 PCR system (Applied Biosystems, Foster City, CA). All reactions were performed in triplicate. PCR amplification of target genes in human microglial cells and mouse cortex tissue was performed using specific primer sets, the sequences of which are listed in Table 1. Gene expression was normalized using human or mouse β-actin genes as endogenous controls. Cells were plated in 96-well plates (1 × 104 cells/well) and allowed to adhere and grow for 24 h. After stabilization for 24 h, the cells were exposed to different concentrations of the desired compounds and incubated for different time intervals under a 5% CO2 atmosphere. Cell viability was assessed using a Cell Counting Kit-8 assay, with absorbance being measured at 450 nm using a microplate reader.
Flow Cytometry
HMC3 cells were treated with 1 μM of Aβ42, and co-treated with 1 μM of Aβ42 and 50 μM of C3G for 24 h. Cell surface markers were stained with primary anti-mouse CD86 MAb, anti-mouse CD206 MAb, anti-mouse CD163 MAb, and anti-mouse TREM2 MAb for 30 min at 4 °C. After washing twice with phosphate-buffered saline (PBS), cells were treated with secondary PE-conjugated goat anti-mouse IgG H&L antibody for 30 min at 4 °C and washed twice with PBS. For observing cell surface expression of CD80, PE-conjugated CD80 antibody was used for staining at 4 °C for 30 min. Following further washes, the cells were resuspended in 0.4 mL of PBS and analyzed to express cell surface proteins using a Cytomics FC500 MLP cytometer (Beckman Coulter Inc., Fullerton, CA, USA).
For observing Aβ42-phagocytosis through flow cytometry, cells were treated with 1 μM of Aβ42, and co-treated with 1 μM of Aβ42 and 50 μM of C3G for 24 h. Cells were harvested and suspended in 4% paraformaldehyde for 10 min, washed and treated with 0.1% Triton-X for 10 min followed by treatment with anti-β-amyloid, 1–42 antibody for 30 min at 4 °C. Intracellular uptake of Aβ42 was observed by treating the cells with PE-conjugated goat anti-mouse IgG H&L antibody for 30 min and analyzed using cytometer.
2′,7′–Dichlorofluorescein Diacetate Assay
Dichloro-dihydro-fluorescein diacetate (DCFH-DA) is a membrane-permeable compound that can be enzymatically converted to the highly fluorescent compound 2′,7′-dichlorofluorescein (DCF) in the presence of ROS. HMC3 cells were grown in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin solution at 37 °C in a 5% CO2 atmosphere, with the medium being changed every other day. Approximately 5 × 104 cells were seeded in black 96-well plates. After incubating for 24 h in a cell incubator, the cell medium was removed, followed by the treatment of 1 μM of Aβ42, and co-treatment of 1 μM of Aβ42 and 50 μM of C3G for 24 h. The medium was washed out, and the cells were treated with 2′,7′-DCFH-DA for 30 min.
Fluorescence was monitored on a microplate reader with excitation and emission wavelengths of 488 nm and 525 nm, respectively, using a microplate reader and also examined under a fluorescence microscope. The results are expressed as a percentage relative to that of DFC fluorescence in the control cells.
Immunofluorescence
HMC3 cells were treated with the 1 μM of Aβ42, and co-treated with 1 μM of Aβ42 and 50 μM of C3G for 24 h and washed with PBS, after which they were treated with 4% paraformaldehyde for 10 min and washed three times with PBS. After that, the cells were permeabilized with 0.1% Triton X-100 for 10 min and washed three times with PBS. After permeabilization, the cells were blocked with 5% bovine serum albumin in PBST (PBS + 0.1% Tween-20). The cells were then stained with the primary anti-β-amyloid, 1–42 antibody by incubating overnight. Having been washed three times, the cells were incubated with fluorescein isothiocyanate–conjugated secondary antibodies for 30 min and washed. The cell nuclei were stained with 1 mg/mL 4′,6-diamidino-2-phenylindole for 5 min, washed three times with PBS, and analyzed using a fluorescence microscope (Nikon, Tokyo, Japan).
Animal Diet and Tissue Sample Preparation
Nine-month-old female AD mice (B6C3-Tg (APPswe/PS1ΔE9 85Dbo/Mmjax)) were purchased from the Jackson Laboratory, and age-matched 9-month-old non-transgenic mice (C57BL/6 J Jms) were obtained from SLC (Hamamatsu-shi, Shizuoka, Japan). The AD and non-transgenic mice were acclimatized and fed normal food pellets.
After acclimating for 2 weeks, the mice were randomized into the following three groups: non-transgenic mice (NC), AD mice treated with PBS (APPswe), and AD mice orally administered 30 mg/kg/day C3G (APPswe_C3G). The mice had ad libitum access to food and water for 38 weeks. Food intake was monitored twice per week, and body weight was measured once a week. All animal experiments were conducted in accordance with the Ministry of Food and Drug Safety (MFDS) Guidelines for Care and Use of Laboratory Animals and were approved by Eulji University (approval no. EUIACUC 20–13). All efforts were made to minimize the number of animals used and their suffering. At the end of the experimental period, the mice were fasted overnight and anesthetized with CO2. Blood was collected by cardiac puncture, and the brains were removed. The cortex was dissected from the right hemisphere, and total RNA was isolated using an RNA extraction kit (iNtRON Biotechnology, Gyeonggi-do, Korea).
Statistical Analysis
The experiments were conducted at least three times, and all data are shown as mean ± standard deviation (SD). Significant differences among groups were analyzed by GraphPad Prism 5.03 (GraphPad Sofware, San Diego, CA, USA). One-way ANOVA and Tukey’s post hoc tests were used to analyze the results. Differences were considered statistically significant at P < 0.05.
Results
To assess the effects of Aβ42 on HMC3 cells, the cells were treated with different concentrations (0.1–4 μM) of Aβ42 for 24 h. Aβ42 was accordingly found to induce cytotoxicity in HMC3 cells in a concentration-dependent manner, with a 1-μM concentration promoting a 20% reduction in cell viability after 24 h. We thus used Aβ42 at this concentration in further experiments (Fig. 1a). Having established a suitable cytotoxic concentration of Aβ42, HMC3 cells were treated with 1 μM of Aβ42 in the presence of different concentrations (25–200 μM) of C3G for 24 h to determine the effects of C3G on Aβ42-induced cytotoxicity. In line with expectations, we found that C3G protected the cells from Aβ42-induced cytotoxicity in a concentration-dependent manner, with the identified optimum concentration of 50 μM being used in further experiments (Fig. 1b). We repeated a similar experiment using the Annexin-V apoptosis assay kit to confirm our results. After giving the desired treatments, the percentage of viable and apoptotic cells was observed. It was found that the number of viable cells was decreased, and dead cells were increased significantly in the Aβ42-treated group compared to the control. However, in the co-treatment group of Aβ42 and C3G, cells retained their viability, and the number of dead cells also decreased significantly (Fig. 1c–d). Having assessed the cytoprotective effects of C3G, we went on to examine the anti-inflammatory effects of C3G. HMC3 cells were initially treated with 1 μM Aβ42 for different time intervals (0, 1, 3, 6, and 12 h) to determine the length of Aβ42 incubation necessary to induce optimal mRNA expression of common pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) for induction of an Alzheimer’s-like pathology. We accordingly found that incubation with 1 μM of Aβ42 for 3 h promoted a significant increase in the expression of pro-inflammatory cytokine mRNAs, with IL-1β, TNF-α, and IL-6 showing 2.45-, 2.6-, and 2.45-fold increases, respectively, compared with the control (Fig. 2a).
Cyanidin-3-O-glucoside (C3G) protects HMC3 cells from β-amyloid (Aβ42)-induced cytotoxicity. a Human microglial HMC3 cells were treated with different concentrations (0.1, 1, 2, and 4 μM) of Aβ42 for 24 h. b HMC3 cells were co-treated with 1 μM Aβ42 and different concentrations (25, 50, 100, and 200 μM) of C3G, and cell viability was observed using CCK assay. c–d HMC3 cells were treated with 1 μM Aβ42 and co-treated with 1 μM Aβ42 and 50 μM C3G and analyzed for apoptotic cells through annexin-V apoptosis assay kit. **p < 0.01, ***p < 0.001, and **** p < 0.0001
C3G downregulates the expression of Aβ42-induced pro-inflammatory cytokines in HMC3 cells. a HMC3 cells were treated with 1 μM Aβ42 for different time intervals (0, 1, 3, 6, and 12 h), and the mRNA expression of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) was analyzed by RT-PCR. b Having established that maximal expression of the cytokines was obtained with a 3-h incubation, we examined the effects of 50 μM C3G in a co-treatment with 1 μM Aβ42 for 3 h on cytokine mRNA expression levels by RT-PCR. a *p < 0.05, **p < 0.01, and ***p < 0.001 with 0 group. b # p < 0.05 with Con; * p < 0.05 and ** p < 0.01 with Aβ42
After determining the optimal incubation time, we repeated the experiment in the presence or absence of 50 μM C3G. Compared with the Aβ42-treated group, the levels of IL-1β and IL-6 mRNA were found to show a significant return to normal levels in the group receiving the combined Aβ42 and C3G treatment. In contrast, those of TNF-α showed a slight non-significant reduction (Fig. 2b). These findings accordingly indicated the potential of C3G to block the production of pro-inflammatory cytokines.
Having determined the effects of C3G in modulating Aβ42-induced pro-inflammatory cytokine production, we examined the effects of C3G for shifting the M1 phenotype of microglia to an M2 phenotype. To this end, HMC3 cells were treated with 1 μM Aβ42 alone or in combination with 50 μM C3G for 24 h. The effects were assessed by determining cell surface protein expression levels of markers specific for M1 (CD86 and CD80) and M2 (CD206 and CD163) using flow cytometry.
We found that the cell surface expression of CD86 and CD80 proteins in the Aβ42-treated group was significantly upregulated, by up to 62% and 15%, respectively, compared with the control, but were downregulated in the Aβ42 and C3G co-treated group. In contrast, cell surface expression of the M2-specific markers CD206 and CD163 was virtually unaffected in the Aβ42-treated group. In contrast, significantly upregulated expression of up to 17% and 17%, respectively, was detected in the Aβ42 and C3G co-treated groups (Fig. 3a–d). These results thus indicate the potential of C3G to shift the M1 phenotype of microglia to an M2 phenotype.
C3G regulates microglial polarization in Aβ42-induced HMC3 cells. Cells were treated with 1 μM Aβ42 and co-treated with 1 μM Aβ42 and 50 μM C3G for 24 h. a–b Relative cell surface protein expression of the M1-specific markers CD86 and CD80 and c–d M2-specific markers CD206 and CD163 was determined using flow cytometry. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001
PPARγ has previously been found to mediate a number of the beneficial effects of flavonoids and has also been reported involved in macrophage polarization. We thus proceeded to examine the effects of C3G on the protein expression of PPARγ in β-amyloid-induced HMC3 cells and determine the role of PPARγ in shifting the phenotype of microglia.
HMC3 cells were initially treated with 1 μM Aβ42 for 24 h in the presence or absence of 50 μM C3G, and cell surface expression of the PPARγ protein was determined using flow cytometry. We observed that Aβ42 had almost no effect on the expression of PPARγ, whereas a significantly upregulated expression (by 23%) was detected in cells co-treated with Aβ42 and C3G, thereby indicating the role of PPARγ in mediating the anti-inflammatory effects of C3G (Fig. 4a).
C3G regulates microglial M1/M2 polarization via PPARγ. a HMC3 cells were treated with 1 μM Aβ42 or co-treated with 1 μM Aβ42 and 50 μM C3G. The cell surface protein expression of PPARγ was observed using flow cytometry. b–c HMC3 cells were pretreated with 10 μM GW9662 for 30 min, followed by co-treatment with 1 μM Aβ42 and 50 μM C3G for 24 h. Expression of the M1-specific marker CD86 and M2-specific marker CD206 was determined using flow cytometry. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001
These experiments were subsequently repeated in the presence of a PPARγ antagonist (GW9662). HMC3 cells were initially pretreated with 10 μM GW9662 for 30 min, followed by post-treatment with Aβ42 and C3G for 24 h. We monitored the protein expression of the classically activated microglia-specific markers CD86 and CD206. We accordingly observed that the C3G-mediated downregulation of CD86 was significantly blocked in the presence of the PPARγ antagonist. Moreover, we detected the upregulated expression of this protein by up to 26%, 56%, and 40% compared with that in cells receiving the Aβ42, control, and Aβ42/C3G treatments, respectively (Fig. 4b).
Contrastingly, the expression of CD206 was found to be either virtually unaffected or non-significantly downregulated (Fig. 4c). Therefore, these results indicate that PPARγ was involved in mediating the display of M1 specific marker CD86 although not M2 marker CD206.
And to determine the anti-oxidative effects of C3G, HMC3 cells were treated with 1 μM Aβ42 in the presence or absence of 50 μM C3G for 24 h. ROS levels were determined using a DCFDA assay with microplate reader/fluorescence microscopy detection. We found that in cells treated with Aβ42 alone, there was a significant increase in ROS levels of up to 23% compared with that in the control, whereas ROS levels in cells receiving the combined Aβ42 and C3G treatment were observed to be decreased significantly (Fig. 5a). Similar results were obtained based on fluorescence microscopy observation, as indicated by the higher intensity of fluorescence signals detected in the Aβ42-treated cells compared with the control and Aβ42 and C3G co-treated cells (Fig. 5b).
C3G attenuates Aβ42-induced reactive oxygen species (ROS) production. HMC3 cells were treated with 1 μM Aβ42 and co-treated with 1 μM Aβ42 and 50 μM C3G for 24 h. Intracellular ROS levels in Aβ42-induced HMC3 cells were determined using DCFDA assay through a microplate reader and b fluorescence microscopy. **p < 0.01 and ***p < 0.001. Scar bar is 100 μm
TREM2 is considered a common phagocytic receptor, and the findings of numerous studies have revealed altered patterns of TREM2 expression in AD pathology. In the present study, HMC3 cells were treated with Aβ42 (1 μM) for 24 h in the presence or absence of 50 μM C3G, and we subsequently examined the levels of cell surface TREM2 protein expression using flow cytometry. Cells co-treated with Aβ42 and C3G were found to be characterized by a significantly upregulated expression of TREM2 (up to 25%). In contrast, expression was unaffected in cells treated with Aβ42 alone (Fig. 6a).
C3G mediates Aβ42-phagocytosis in Aβ42-treated HMC3 cells via triggering receptor expressed on myeloid cells (TREM2). HMC3 cells were treated with 1 μM Aβ42 and co-treated with 1 μM Aβ42 and 50 μM C3G for 24 h. a Cell surface protein expression of TREM2 was determined using flow cytometry. b Intercellular uptake of Aβ42 was determined using flow cytometry. c–d HMC3 cells were pretreated with 1 μM TREM2-blocking antibody for 1 h, followed by co-treatment with 1 μM Aβ42 and 50 μM C3G for 24 h. Intercellular uptake of Aβ42 was determine using flow cytometry and fluorescence microscopy (d). **p < 0.01, ***p < 0.001, and ****p < 0.0001. Scar bar is 50 μm
To confirm our findings, we assessed whether C3G had any effect on the uptake of Aβ42 by HMC3 cells. HMC3 cells were initially treated with Aβ42 (1 μM) for 24 h in the presence or absence of 50 μM C3G, after which the cells were assessed by flow cytometry for intracellular Aβ42-uptake using an anti-Aβ42-antibody. We found that compared with the cells receiving the Aβ42 treatment, the presence of C3G enhanced the phagocytosis of Aβ42 by up to 69% (Fig. 6b).
Furthermore, to determine whether Aβ42 phagocytosis is mediated via TREM2, we used a TREM2 blocking antibody. Cells were initially treated with the TREM2 blocking antibody (1 µg/mL) for 1 h, followed by co-treatment with 1 μM Aβ42 and 50 μM C3G for 24 h. After that, intracellular Aβ42 uptake was examined based on flow cytometry analysis, which revealed that in cells treated with the TREM2 blocking antibody, the intracellular uptake of Aβ42 was significantly reduced compared with that observed in cells co-treated with C3G and Aβ42 and comparable to that observed in cells treated with Aβ42 alone. Similar observations were made using fluorescence microscopy. In cells co-treated with Aβ42 and C3G, the signal’s intensity was higher than that in cells treated with Aβ42 alone and TREM2 blocking antibody (Fig. 6c–d). These findings thus tend to indicate that TREM2 plays a role in the C3G-enhanced phagocytosis of Aβ42.
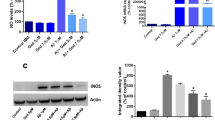
To confirm in vitro observation, we examined C3G-mediated anti-inflammatory effects in an AD (APPswe/PS1ΔE9) mouse model. The results revealed that C3G significantly suppressed IL-1β, TNF-α, and IL-6 gene expression in the cortex compared with that in APPswe mice, in which these cytokines showed 1.8, 1.5-, and 2-fold higher expression, respectively, compared with that in the vehicle control (Fig. 7a–c). Moreover, mRNA expression of the M1 macrophage marker CD86 decreased non-significantly, but M2 macrophage markers Arg-1 and CD206 were found to be significantly increased in the cortex of APPswe + C3G mice compared with that in APPswe mice (Fig. 7d–f). We also established that the mRNA expression of PPARγ, which was identified as a significant target in the regulation of inflammation in the cortex of APPswe + C3G mice, was significantly increased compared with that in VC and APPswe mice. Similarly, levels of TREM2 expression in the cortex of APPswe + C3G mice were also increased, although it was not significant (Fig. 7g–h).
C3G attenuates neuroinflammation in the cortex of APPswe/PS1ΔE9 model mice. Mice were divided into three groups: non-transgenic mice (VC), AD mice administered PBS (APPswe), and AD mice administered 30 mg/kg/day C3G (APPswe + C3G). RNA was extracted from the cortex region of mouse brains and analyzed for mRNA expression of a–c inflammatory cytokines (IL-1β, TNF-α, and IL-6), d M1-specific marker (CD86), e–f M2-specific markers (Arg1 and CD206), and g-h PPARγ and TREM2 using RT-PCR. *p < 0.05, **p < 0.01, and ***p < 0.001
Discussion
AD is one of the significant health concerns of the present century and is recognized as the most common cause of dementia. It is an affliction from which an estimated 50 million people are believed to suffer worldwide [35]. It accounts for some two-thirds (50–70%) of all dementia cases, with those having pre-existing cardiovascular diseases, diabetes, or hypertension considered an exceptionally high risk of developing AD later in life. Although the exact cause of AD is yet to be conclusively identified, two factors that have been established to be closely linked with AD progression are the accumulation of Aβ plaques in the cerebral cortex and the neurofibrillary tangle formation of intra-nerve filamentous neurons associated with tau protein hyperphosphorylation [1]. These altered proteins and their excess accumulation within the brain alter normal brain cell functions. Along with neurons and astrocytes, brain-resident macrophages (microglia) are among the principal cells of the brain that play essential roles in dealing with extracellular stress [36]. Microglia are characterized by two distinct phenotypes; M1 type is the classically activated form associated with neurotoxicity, oxidative stress, synapses, and neuronal damage, and the other alternatively activated M2 or anti-inflammatory phenotypes play an important role in regulating inflammation, removing cell debris and misfolded proteins, and providing neurotrophic support for neuroregeneration [4, 37]. Neuroinflammation attributable to microglial cell activity has been reported as one of the main pathophysiological events involved in the progression of various neurodegenerative disorders, such as AD, Parkinson’s disease (PD), and multiple sclerosis [38]. In this regard, the findings of recent studies have provided evidence to indicate that microglial polarization (shifting the M1 phenotype to M2) is among the promising strategies for the treatment of a range of neurodegenerative disorders [39,40,41,42,43,44].
Dietary anthocyanins have attracted the attention of scientists on account of their established neuroprotective effects in the central nervous system [12]. These anthocyanins are absorbed as glycosides in rodents and humans, after which they cross the blood–brain barrier and localize within different regions of the brain, such as the cortex, cerebellum, hippocampus, and striatum [13]. C3G has been identified as the most abundant anthocyanin present in vegetables and fruits, and numerous studies have demonstrated the neuroprotective effects of C3G for AD, PD, and other neurodegenerative disorders in the recent years [25, 44,45,46,47,48,49,50,51,52,53,54,55]. However, there have been no studies that have examined C3G-mediated effects in Aβ-treated HMC3 cells. In the present study, we evaluated the impact of C3G in attenuating Aβ42-induced toxicity in HMC3 cells.
We initially assessed the concentration dependency of the effect of Aβ42 on HMC3 cells. Based on a 24-h incubation experiment, we found that Aβ42 can significantly induce cytotoxicity in HMC3 cells which was consistent with previous in vitro findings [25, 56]. We established that Aβ42 at a concentration of 1 µM would be ideal for performing further experiments. Moreover, we found that exposing cells to 1 µM Aβ42 in the presence of C3G had no appreciable effect on cell viability. The impact of C3G was found to be concentration-dependent, as has also been determined in previous studies [25, 52, 56]. Based on these observations, we established 50 µM to be a suitable working concentration for C3G, as this contributed to maintaining normal HMC3 viability in the presence of Aβ42.
As reported previously, the release of pro-inflammatory cytokines from microglial cells under pathological conditions is a significant event in AD [57]. In the present study, we examined the mRNA expression of different pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) after treating HMC3 cells with 1 µM Aβ42 for different time intervals. Accordingly, we identified 3 h as a suitable incubation time for the induction of inflammation, as this length of incubation was found to be conducive to the maximal expression of the assessed inflammatory markers. Having established this parameter, we proceeded to repeat the same experiment in the presence and absence of C3G and thus found that C3G can attenuate the Aβ42-induced release of pro-inflammatory cytokines, which, consistent with previous findings [45, 46, 50, 51, 55], provided convincing evidence for the role of C3G as an anti-inflammatory agent.
It has previously been reported that natural flavonoids such as apigenin and chrysin can potentially shift the M1/M2 status of macrophages via activation of PPARγ in mice fed a high-fat diet [58, 59], and the findings of a recent study have revealed that C3G can ameliorate PM10-induced pulmonary injury by modulating M1/M2 macrophage polarization [60]. Also, recent studies have provided the evidences for the anti-inflammatory role of PPARγ in various diseases and in mediating macrophage polarization [61, 62]. In the present study, we found that C3G treatment induced a shift in the phenotype of microglia from M1 to M2 by downregulating the cell surface expression of M1-specific markers (CD80 and CD86), which were found to be increased in the Aβ42-treated group and induced cell surface expression of M2-specific markers (CD206 and CD163).
We also found that C3G induced cell surface expression of PPARγ protein in Aβ42-induced HMC3 cells. Conversely, the presence of the PPARγ antagonist GW9662 was observed to suppress the downregulatory effect of C3G on Aβ42 induction of the classically activated M1 marker CD86. In contrast, upregulation of the alternatively activated M2 marker CD206 was slightly downregulated in the presence of GW9662, although it was not significant. This latter observation, contrasts with the findings of previous studies on flavonoids such as apigenin and chrysin, in which CD206 was found to be significantly downregulated in the presence of GW9662 [58, 59]. These results do, nevertheless, indicate that the C3G-induced microglial shift is mediated via PPARγ.
ROS play a prominent role in the pathogenesis of AD [9,10,11]. In this regard, the anti-oxidative properties of anthocyanins are well established, and C3G has previously been demonstrated to attenuate Aβ25-35-induced ROS production [13, 50]. Consistently, we found that C3G inhibited Aβ42-induced ROS production in HMC3 cells.
Among the factors contributing to the progression of AD is the limited phagocytosis of Aβ plaques by microglial cells [63]. TREM2 is a well-established phagocytic receptor present on the surface of microglial cells, which has been demonstrated to play a role in anti-inflammatory activities [25,26,27,28,29,30,31]. Li et al. recently examined the effects of bilberry anthocyanins in improving neuroinflammation and cognitive impairment in an in vivo model of AD via the CD33/TREM2/TYROBP pathway and found that bilberry could inhibit altered TREM2 expression in the hippocampus [64]. Furthermore, Akhter et al. have described the pivotal role of TREM2 in phagocytosis, inflammation, and apoptosis [30]. In the present study, we found that C3G induced the cell surface expression of TREM2 in Aβ42-treated HMC3 cells and also enhanced the phagocytosis of Aβ42 in these cells, thereby indicating that the upregulated expression of TREM2 is a critical factor contributing to a reduction in Aβ toxicity and enhancement of phagocytosis. Confirmatory evidence in this regard was obtained by pre-treatment with a TREM2-blocking antibody for 1 h prior to the treatment with Aβ and C3G, which abolished the enhanced phagocytosis. Collectively, these findings thus provide compelling evidence to indicate that the enhancement of Aβ42 phagocytosis in HMC3 cells induced by C3G is mediated via TREM2.
Having characterized the effects of C3G in vitro, we went on to examine the activity of this anthocyanin in vivo in an APPswe/PS1ΔE9 mouse model and accordingly obtained consistent results. We found that C3G was able to downregulate mRNA expression of the pro-inflammatory cytokines IL-1β, TNF-α, and IL-6 in the cortex region of the brains of APPswe/PS1ΔE9 mice, which is consistent with the findings of a previous study that established the protective effects of C3G and cyanidin in a colitis mouse model [65]. Similarly, our observations of the enhanced mRNA expression of M2-specific markers CD206 and Arg1 are comparable to those reported previously [60]. Also, similar to the in vitro findings, here also, we found enhanced expression levels of PPARγ and TREM2 in the C3G treated group which were in the close proximity of the previously published studies on flavonoids [58, 64, 66].
To the best of our knowledge, this is the first study to demonstrate the effects of C3G in shifting the M1/M2 polarization of microglia via activation of PPARγ and the TREM2-mediated enhancement of Aβ phagocytosis in Aβ42-treated HMC3 cells. However, further studies, both in vitro and in vivo, are required to establish the precise mechanism underlying the C3G-regulated M1/M2 shift. Furthermore, additional studies are needed to determine the role of phagocytic receptors other than TREM2 present on the microglial cell surface, thereby enabling us elucidate the mechanisms underlying the C3G-medicated phagocytosis of Aβ42.
Conclusions
In conclusion, in this study, we established the potential utility of the anthocyanin C3G as a therapeutic agent in the treatment of AD, based on our observations of its effects in regulating the M1/M2 status of microglia, in addition to controlling different cellular inflammatory pathways, as previously reported. C3G not only has anti-inflammatory properties but also contributes to eliminating accumulated β-amyloid by enhancing phagocytosis.
Data Availability
All the data and results obtained during the current study are available from the corresponding author on reasonable request.
Change history
22 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12035-023-03877-9
References
Srivastava S, Ahmad R, Khare SK (2021) Alzheimer’s disease and its treatment by different approaches: a review. Eur J Med Chem 216:113320. https://doi.org/10.1016/j.ejmech.2021.113320
Leng F, Edison P (2021) Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol 17(3):157–172. https://doi.org/10.1038/s41582-020-00435-y
Tarique AA, Logan J, Thomas E, Holt PG, Sly PD, Fantino E (2015) Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. American j ournal of respiratory cell and molecular biology 53(5):676–688
Park HJ, Oh SH, Kim HN, Jung YJ, Lee PH (2016) Mesenchymal stem cells enhance α-synuclein clearance via M2 microglia polarization in experimental and human parkinsonian disorder. Acta Neuropathol 132(5):685–701
Ma Y, Wang J, Wang Y, Yang GY (2017) The biphasic function of microglia in ischemic stroke. Prog Neurobiol 157:247–272. https://doi.org/10.1016/j.pneurobio.2016.01.005
Tedesco S, Bolego C, Toniolo A, Nassi A, Fadini GP, Locati M, Cignarella A (2015) Phenotypic activation and pharmacological outcomes of spontaneously differentiated human monocyte-derived macrophages. Immunobiology 220(5):545–554
Wang S, Zhang J, Sui L, Xu H, Piao Q, Liu Y, Qu X, Sun Y et al (2017) Antibiotics induce polarization of pleural macrophages to M2-like phenotype in patients with tuberculous pleuritis. Sci Rep 7(1):1–10
Rebelo SP, Pinto C, Martins TR, Harrer N, Estrada MF, Loza-Alvarez P, Cabeçadas J, Alves PM et al (2018) 3D-3-culture: a tool to unveil macrophage plasticity in the tumour microenvironment. Biomaterials 163:185–197
Manoharan S, Guillemin GJ, Abiramasundari RS, Essa MM, Akbar M, Akbar MD (2016) The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: a mini review. Oxidative medicine and cellular longevity 2016
Jiang T, Sun Q, Chen S (2016) Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog Neurobiol 147:1–19
Thapa A, Carroll NJ (2017) Dietary modulation of oxidative stress in Alzheimer’s disease. Int J Mol Sci 18(7):1583
Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D (2007) Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res 51(6):675–683
Rashid K, Wachira FN, Nyabuga JN, Wanyonyi B, Murilla G, Isaac AO (2014) Kenyan purple tea anthocyanins ability to cross the blood brain barrier and reinforce brain antioxidant capacity in mice. Nutr Neurosci 17(4):178–185
Wang Y, Zhao L, Lu F, Yang X, Deng Q, Ji B, Huang F (2015) Retinoprotective effects of bilberry anthocyanins via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms in a visible light-induced retinal degeneration model in pigmented rabbits. Molecules 20(12):22395–22410
Winter AN, Bickford PC (2019) Anthocyanins and their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants 8(9):333
Morais CA, de Rosso VV, Estadella D, Pisani LP (2016) Anthocyanins as inflammatory modulators and the role of the gut microbiota. J Nutr Biochem 33:1–7
Zhao S, Liu W, Wang J, Shi J, Sun Y, Wang W, Ning G, Liu R et al (2017) Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J Mol Endocrinol 58(1):1–14
Sukprasansap M, Chanvorachote P, Tencomnao T (2020) Cyanidin-3-glucoside activates Nrf2-antioxidant response element and protects against glutamate-induced oxidative and endoplasmic reticulum stress in HT22 hippocampal neuronal cells. BMC Complement Med Ther 20(1):46. https://doi.org/10.1186/s12906-020-2819-7
Liu M, Du Y, Li H, Wang L, Ponikwicka-Tyszko D, Lebiedzinska W, Pilaszewicz-Puza A, Liu H et al (2019) Cyanidin-3-o-glucoside pharmacologically inhibits tumorigenesis via estrogen receptor β in melanoma mice. Front Oncol 9:1110
Sivasinprasasn S, Pantan R, Thummayot S, Tocharus J, Suksamrarn A, Tocharus C (2016) Cyanidin-3-glucoside attenuates angiotensin II-induced oxidative stress and inflammation in vascular endothelial cells. Chem Biol Interact 260:67–74
Zhang J, Wu J, Liu F, Tong L, Chen Z, Chen J, He H, Xu R et al (2019) Neuroprotective effects of anthocyanins and its major component cyanidin-3-O-glucoside (C3G) in the central nervous system: an outlined review. Eur J Pharmacol 858
Li X, Shi Z, Zhu Y, Shen T, Wang H, Shui G, Loor JJ, Fang Z et al (2020) Cyanidin-3-O-glucoside improves non-alcoholic fatty liver disease by promoting PINK1-mediated mitophagy in mice. Br J Pharmacol 177(15):3591–3607
Park M, Yoo JH, Lee YS, Lee HJ (2019) Lonicera caerulea extract attenuates non-alcoholic fatty liver disease in free fatty acid-induced HepG2 hepatocytes and in high fat diet-fed mice. Nutrients 11 (3). https://doi.org/10.3390/nu11030494
Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S (2011) The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Adv Pharm Technol Res 2(4):236–240. https://doi.org/10.4103/2231-4040.90879
Song N, Zhang L, Chen W, Zhu H, Deng W, Han Y, Guo J, Qin C (2016) Cyanidin 3-O-β-glucopyranoside activates peroxisome proliferator-activated receptor-γ and alleviates cognitive impairment in the APP(swe)/PS1(ΔE9) mouse model. Biochim Biophys Acta 9:1786–1800. https://doi.org/10.1016/j.bbadis.2016.05.016
Jia Y, Wu C, Kim YS, Yang SO, Kim Y, Kim JS, Jeong MY, Lee JH et al (2020) A dietary anthocyanin cyanidin-3-O-glucoside binds to PPARs to regulate glucose metabolism and insulin sensitivity in mice. Commun Biol 3(1):514. https://doi.org/10.1038/s42003-020-01231-6
Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O'Loughlin E et al (2017) The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47 (3):566–581 e569. https://doi.org/10.1016/j.immuni.2017.08.008
Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E et al (2017) The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47 (3):566–581. e569
Wolfe CM, Fitz NF, Nam KN, Lefterov I, Koldamova R (2019) The role of APOE and TREM2 in Alzheimer’s disease—current understanding and perspectives. Int J Mol Sci 20(1):81
Akhter R, Shao Y, Formica S, Khrestian M, Bekris LM (2021) TREM2 alters the phagocytic, apoptotic and inflammatory response to Aβ42 in HMC3 cells. Mol Immunol 131:171–179
Zhang Y, Feng S, Nie K, Li Y, Gao Y, Gan R, Wang L, Li B et al (2018) TREM2 modulates microglia phenotypes in the neuroinflammation of Parkinson’s disease. Biochem Biophys Res Commun 499(4):797–802
Zhang J, Zheng Y, Luo Y, Du Y, Zhang X, Fu J (2019) Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-κB pathways in BV2 cells. Mol Immunol 116:29–37
Ruganzu JB, Zheng Q, Wu X, He Y, Peng X, Jin H, Zhou J, Ma R et al (2021) TREM2 overexpression rescues cognitive deficits in APP/PS1 transgenic mice by reducing neuroinflammation via the JAK/STAT/SOCS signaling pathway. Exp Neurol 336
Liu F, Zhao F, Wang W, Sang J, Jia L, Li L, Lu F (2020) Cyanidin-3-O-glucoside inhibits Aβ40 fibrillogenesis, disintegrates preformed fibrils, and reduces amyloid cytotoxicity. Food Funct 11(3):2573–2587
Patterson C (2018) The state of the art of dementia research: New frontiers. World Alzheimer Report 2018
Vasic V, Barth K, Schmidt MH (2019) Neurodegeneration and neuro-regeneration—Alzheimer’s disease and stem cell therapy. Int J Mol Sci 20(17):4272
Cherry JD, Olschowka JA, O’Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11(1):1–15
Kwon HS, Koh S-H (2020) Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Translational neurodegeneration 9(1):1–12
Yang X, Xu S, Qian Y, Xiao Q (2017) Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav Immun 64:162–172
Askari VR, Shafiee-Nick R (2019) The protective effects of β-caryophyllene on LPS-induced primary microglia M1/M2 imbalance: a mechanistic evaluation. Life Sci 219:40–73
Zhang B, Wei Y-Z, Wang G-Q, Li D-D, Shi J-S, Zhang F (2019) Targeting MAPK pathways by naringenin modulates microglia M1/M2 polarization in lipopolysaccharide-stimulated cultures. Front Cell Neurosci 12:531
Li L, Li L, Zhang J, Huang S, Liu W, Wang Z, Liang S, Tao J et al (2020) Disease stage-associated alterations in learning and memory through the electroacupuncture modulation of the cortical microglial M1/M2 Polarization in Mice with Alzheimer’s Disease. Neural Plasticity 2020
Qiu Z, Lu P, Wang K, Zhao X, Li Q, Wen J, Zhang H, Li R et al (2020) Dexmedetomidine inhibits neuroinflammation by altering microglial M1/M2 polarization through MAPK/ERK pathway. Neurochem Res 45(2):345–353
Jin X, Liu M-Y, Zhang D-F, Zhong X, Du K, Qian P, Gao H, Wei M-J (2019) Natural products as a potential modulator of microglial polarization in neurodegenerative diseases. Pharmacol Res 145:104253
Shin W-H, Park S-J, Kim E-J (2006) Protective effect of anthocyanins in middle cerebral artery occlusion and reperfusion model of cerebral ischemia in rats. Life Sci 79(2):130–137
Min J, Yu S-W, Baek S-H, Nair KM, Bae O-N, Bhatt A, Kassab M, Nair MG et al (2011) Neuroprotective effect of cyanidin-3-O-glucoside anthocyanin in mice with focal cerebral ischemia. Neurosci Lett 500(3):157–161
Qin L, Zhang J, Qin M (2013) Protective effect of cyanidin 3-O-glucoside on beta-amyloid peptide-induced cognitive impairment in rats. Neurosci Lett 534:285–288
Strathearn KE, Yousef GG, Grace MH, Roy SL, Tambe MA, Ferruzzi MG, Wu Q-L, Simon JE et al (2014) Neuroprotective effects of anthocyanin-and proanthocyanidin-rich extracts in cellular models of Parkinson’s disease. Brain Res 1555:60–77
Ereminas G, Majiene D, Sidlauskas K, Jakstas V, Ivanauskas L, Vaitiekaitis G, Liobikas J (2017) Neuroprotective properties of anthocyanidin glycosides against H2O2-induced glial cell death are modulated by their different stability and antioxidant activity in vitro. Biomed Pharmacother 94:188–196
Ali T, Kim T, Rehman SU, Khan MS, Amin FU, Khan M, Ikram M, Kim MO (2018) Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer’s disease. Mol Neurobiol 55(7):6076–6093
Pacheco SM, Soares MSP, Gutierres JM, Gerzson MFB, Carvalho FB, Azambuja JH, Schetinger MRC, Stefanello FM et al (2018) Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer’s type. J Nutr Biochem 56:193–204
Wang S, Tan Y, Lu H, Pang W, Li Y, Zhang A, Jiang Y (2018) Protective effect of cyanidin-3-glucoside against injury induced by amyloid β protein 25–35 in primary cultured hippocampal neurons. J Hyg Res 47 (3):413–418
Skemiene K, Pampuscenko K, Rekuviene E, Borutaite V (2020) Protective effects of anthocyanins against brain ischemic damage. J Bioenerg Biomembr 52(2):71–82
Kaewmool C, Udomruk S, Phitak T, Pothacharoen P, Kongtawelert P (2020) Cyanidin-3-O-Glucoside protects PC12 cells against neuronal apoptosis mediated by LPS-stimulated BV2 microglial activation. Neurotox Res 37(1):111–125
Fan D, Alamri Y, Liu K, MacAskill M, Harris P, Brimble M, Dalrymple-Alford J, Prickett T et al (2018) Supplementation of blackcurrant anthocyanins increased cyclic glycine-proline in the cerebrospinal fluid of parkinson patients: potential treatment to improve insulin-like growth factor-1 function. Nutrients 10(6):714
Belkacemi A, Ramassamy C (2016) Innovative anthocyanin/anthocyanidin formulation protects SK-N-SH cells against the amyloid-β peptide-induced toxicity: relevance to Alzheimer’s disease. Cent Nerv Syst Agents Med Chem 16 (1):37–49
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–934
Feng X, Qin H, Shi Q, Zhang Y, Zhou F, Wu H, Ding S, Niu Z (2014) Chrysin attenuates inflammation by regulating M1/M2 status via activating PPARγ. Biochem Pharmacol 89(4):503–514
Feng X, Weng D, Zhou F, Owen YD, Qin H, Zhao J, WenYu HY, Chen J et al (2016) Activation of PPARγ by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization. EBioMedicine 9:61–76. https://doi.org/10.1016/j.ebiom.2016.06.017
Cui Y, Lin Y, Meng X, Ma J, Deng H, Liu X, He X, Zhao J (2021) Cyanidin-3-galactoside from Aronia melanocarpa ameliorates PM10 induced pulmonary injury by modulating M1/M2 macrophage polarization and NRF2/Sirt1 MAPK signaling. Journal of Functional Foods 78:104363. https://doi.org/10.1016/j.jff.2021.104363
Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM (2013) PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 19(5):557–566
Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B et al (2007) PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6(2):137–143
Galloway DA, Phillips AE, Owen DR, Moore CS (2019) Phagocytosis in the brain: homeostasis and disease. Front Immunol 10:790
Li J, Zhao R, Jiang Y, Xu Y, Zhao H, Lyu X, Wu T (2020) Bilberry anthocyanins improve neuroinflammation and cognitive dysfunction in APP/PSEN1 mice via the CD33/TREM2/TYROBP signaling pathway in microglia. Food Funct 11(2):1572–1584
Gan Y, Fu Y, Yang L, Chen J, Lei H, Liu Q (2020) Cyanidin-3-O-glucoside and cyanidin protect against intestinal barrier damage and 2, 4, 6-Trinitrobenzenesulfonic acid-induced colitis. J Med Food 23(1):90–99
Feng X, Weng D, Zhou F, Owen YD, Qin H, Zhao J, Huang Y, Yang N et al (2016) Activation of PPARγ by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization. EBioMedicine 9:61–76
Funding
This study was supported by the Cooperative Research Program of the Center for Companion Animal Research (Project No. PJ01476703), funded by the Rural Development Administration, Republic of Korea and Gachon University research fund of 2020 (GCU-202008490009).
Author information
Authors and Affiliations
Contributions
Investigation and data organization, S. and M.P.; original draft preparation and visualization, S., J-H.S., and M.P.; conceptualization and supervision, H.-J.L. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The authors declare that they have given their consent to the scientific contents and authorship to this manuscript. The study was conducted in accordance with the guidelines stipulated by the Ministry of Food and Drug Safety for the Care and Use of Laboratory Animals. This study was approved by the Institutional Animal Care and Use Committee of the Eulji University School of Medicine (EUIACUC 2013).
Consent for Publication
Yes, all the authors have given their consent for the publication of this manuscript in its present form.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanjay, Shin, JH., Park, M. et al. Cyanidin-3-O-Glucoside Regulates the M1/M2 Polarization of Microglia via PPARγ and Aβ42 Phagocytosis Through TREM2 in an Alzheimer’s Disease Model. Mol Neurobiol 59, 5135–5148 (2022). https://doi.org/10.1007/s12035-022-02873-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02873-9