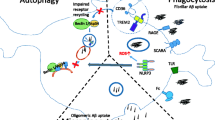
Abstract
Early detection and clinical diagnosis of Alzheimer’s disease (AD) have become an extremely important link in the prevention and treatment of AD. Because of the occult onset, the diagnosis and treatment of AD based on clinical symptoms are increasingly challenged by current severe situations. Therefore, molecular diagnosis models based on early AD pathological markers have received more attention. Among the possible pathological mechanisms, microglia which are necessary for normal brain function are highly expected and have been continuously studied in various models. Several AD biomarkers already exist, but currently there is a paucity of specific and sensitive microglia biomarkers which can accurately measure preclinical AD. Bringing microglia biomarkers into the molecular diagnostic system which is based on fluid and neuroimaging will play an important role in future scientific research and clinical practice. Furthermore, developing novel, more specific, and sensitive microglia biomarkers will make it possible to pharmaceutically target chemical pathways that preserve beneficial microglial functions in response to AD pathology. This review discusses microglia biomarkers in the context of AD.


Similar content being viewed by others
Data Availability
Not applicable
Abbreviations
- Aβ:
-
amyloid β
- ABCA1:
-
ATP-binding cassette transporter A1
- AD:
-
Alzheimer’s disease
- ADAM:
-
a disintegrin and metalloproteinase
- APOE:
-
plaque-associated apolipoprotein E
- ASC:
-
apoptosis-associated speck-like protein containing a caspase recruitment domain
- AT(N):
-
Aβ deposition, pathologic tau, and neurodegeneration
- BBB:
-
blood-brain barrier
- BPND :
-
binding potential
- [11C]-5:
-
ethyl 6-(3-(3-((5-chlorothiophen-2-yl)sulfonyl)[11C]ureido)-azetidin-1-yl)-5-cyano-2-methylnicotinate
- CB1R:
-
cannabinoid receptor 1
- CB2R:
-
cannabinoid receptor 2
- CE:
-
cholesteryl ester
- CNS:
-
central nervous system
- CSF:
-
cerebrospinal fluid
- CSF1:
-
macrophage colony-stimulating factor 1
- CSF1R:
-
macrophage colony-stimulating factor 1 receptor
- CTSB:
-
cathepsin B
- CX3CL1:
-
CX3 chemokine ligand 1
- DAM:
-
disease-associated microglia
- DCI:
-
diffusion compartment imaging
- DTI:
-
diffusion tensor imaging
- DWI:
-
diffusion-weighted imaging
- ELISA:
-
enzyme linked immunosorbent assay
- EOAD:
-
early-onset AD
- FA:
-
fractional anisotropy
- GPNMB:
-
Glycoprotein NMB
- IDE:
-
insulysin
- IL:
-
interleukin
- LOAD:
-
late-onset AD
- MaR1:
-
Maresin1
- MCI:
-
mild cognitive impairment
- MCP1:
-
monocyte chemotactic protein 1
- MRI:
-
magnetic resonance imaging
- MMSE:
-
Mini-Mental State Examination
- NF-kB:
-
nuclear factor-kB
- NFT:
-
neurofibrillary tangles
- NIA-AA:
-
National Institute on Aging and Alzheimer’s Association
- NLRP3:
-
NLR family, pyrin domain containing 3
- P2RY12:
-
recombinant purinergic receptor P2Y
- PET:
-
positron emission tomography
- PGRN:
-
progranulin
- PLCG2:
-
phospholipase-C-γ2
- PPARγ:
-
microglial peroxisome proliferator-activated receptor γ
- PROS1:
-
vitamin k-dependent protein S
- p-tau:
-
phosphorylated tau
- rs-fMRI:
-
resting-state functional magnetic resonance imaging
- SCD:
-
subjective cognitive decline
- sTREM2:
-
soluble TREM2
- SUVR:
-
standard uptake value ratio
- TET2:
-
ten-eleven translocation 2
- TNF-α:
-
Tumor necrosis factor-α
- TREM2:
-
triggering receptor expressed on myeloid cells 2
- TSPO:
-
Translocator protein
- t-tau:
-
total-tau.
References
2020 Alzheimer's disease facts and figures (2020). Alzheimers Dement. https://doi.org/10.1002/alz.12068
DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer's disease. Mol Neurodegener 14(1):32. https://doi.org/10.1186/s13024-019-0333-5
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ et al (2011) The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7(3):263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W et al (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement 14(4):535–562. https://doi.org/10.1016/j.jalz.2018.02.018
Miron VE, Priller J (2020) Investigating Microglia in Health and Disease: Challenges and Opportunities. Trends Immunol 41(9):785–793. https://doi.org/10.1016/j.it.2020.07.002
Bellenguez C, Grenier-Boley B, Lambert JC (2020) Genetics of Alzheimer's disease: where we are, and where we are going. Curr Opin Neurobiol 61:40–48. https://doi.org/10.1016/j.conb.2019.11.024
Johnson ECB, Dammer EB, Duong DM, Ping L, Zhou M, Yin L, Higginbotham LA, Guajardo A et al (2020) Large-scale proteomic analysis of Alzheimer's disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat Med 26(5):769–780. https://doi.org/10.1038/s41591-020-0815-6
Hansen DV, Hanson JE, Sheng M (2018) Microglia in Alzheimer's disease. J Cell Biol 217(2):459–472. https://doi.org/10.1083/jcb.201709069
Hemonnot AL, Hua J, Ulmann L, Hirbec H (2019) Microglia in Alzheimer Disease: Well-Known Targets and New Opportunities. Front Aging Neurosci 11:233. https://doi.org/10.3389/fnagi.2019.00233
Hickman S, Izzy S, Sen P, Morsett L, El Khoury J (2018) Microglia in neurodegeneration. Nat Neurosci 21(10):1359–1369. https://doi.org/10.1038/s41593-018-0242-x
Zhong L, Wang Z, Wang D, Wang Z, Martens YA, Wu L, Xu Y, Wang K et al (2018) Amyloid-beta modulates microglial responses by binding to the triggering receptor expressed on myeloid cells 2 (TREM2). Mol Neurodegener 13(1):15. https://doi.org/10.1186/s13024-018-0247-7
Zhang J, Chen B, Lu J, Wu Y, Wang S, Yao Z, Zhu L, Qiao Y et al (2019) Brains of rhesus monkeys display Aβ deposits and glial pathology while lacking Aβ dimers and other Alzheimer's pathologies. Aging Cell 18(4):e12978. https://doi.org/10.1111/acel.12978
Mecca C, Giambanco I, Donato R, Arcuri C (2018) Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes. Int J Mol Sci 19(1). https://doi.org/10.3390/ijms19010318
Heckmann BL, Teubner BJW, Tummers B, Boada-Romero E, Harris L, Yang M, Guy CS, Zakharenko SS et al (2019) LC3-Associated Endocytosis Facilitates β-Amyloid Clearance and Mitigates Neurodegeneration in Murine Alzheimer's Disease. Cell 178(3):536–551.e514. https://doi.org/10.1016/j.cell.2019.05.056
Lee CYD, Daggett A, Gu X, Jiang LL, Langfelder P, Li X, Wang N, Zhao Y et al (2018) Elevated TREM2 Gene Dosage Reprograms Microglia Responsivity and Ameliorates Pathological Phenotypes in Alzheimer's Disease Models. Neuron 97(5):1032–1048.e1035. https://doi.org/10.1016/j.neuron.2018.02.002
Zhao Y, Wu X, Li X, Jiang LL, Gui X, Liu Y, Sun Y, Zhu B et al (2018) TREM2 Is a Receptor for β-Amyloid that Mediates Microglial Function. Neuron 97(5):1023–1031.e1027. https://doi.org/10.1016/j.neuron.2018.01.031
Claes C, Van Den Daele J, Boon R, Schouteden S, Colombo A, Monasor LS, Fiers M, Ordovás L et al (2019) Human stem cell-derived monocytes and microglia-like cells reveal impaired amyloid plaque clearance upon heterozygous or homozygous loss of TREM2. Alzheimers Dement 15(3):453–464. https://doi.org/10.1016/j.jalz.2018.09.006
Parhizkar S, Arzberger T, Brendel M, Kleinberger G, Deussing M, Focke C, Nuscher B, Xiong M et al (2019) Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat Neurosci 22(2):191–204. https://doi.org/10.1038/s41593-018-0296-9
Kleineidam L, Chouraki V, Próchnicki T, van der Lee SJ, Madrid-Márquez L, Wagner-Thelen H, Karaca I, Weinhold L et al (2020) PLCG2 protective variant p.P522R modulates tau pathology and disease progression in patients with mild cognitive impairment. Acta Neuropathol 139(6):1025–1044. https://doi.org/10.1007/s00401-020-02138-6
Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O'Loughlin E et al (2017) The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 47(3):566–581.e569. https://doi.org/10.1016/j.immuni.2017.08.008
Carrillo-Jimenez A, Deniz Ö, Niklison-Chirou MV, Ruiz R, Bezerra-Salomão K, Stratoulias V, Amouroux R, Yip PK et al (2019) TET2 Regulates the Neuroinflammatory Response in Microglia. Cell Rep 29(3):697–713.e698. https://doi.org/10.1016/j.celrep.2019.09.013
Hsieh YC, Guo C, Yalamanchili HK, Abreha M, Al-Ouran R, Li Y, Dammer EB, Lah JJ et al (2019) Tau-Mediated Disruption of the Spliceosome Triggers Cryptic RNA Splicing and Neurodegeneration in Alzheimer's Disease. Cell Rep 29(2):301–316.e310. https://doi.org/10.1016/j.celrep.2019.08.104
Eftekharzadeh B, Daigle JG, Kapinos LE, Coyne A, Schiantarelli J, Carlomagno Y, Cook C, Miller SJ et al (2018) Tau Protein Disrupts Nucleocytoplasmic Transport in Alzheimer's Disease. Neuron 99(5):925–940.e927. https://doi.org/10.1016/j.neuron.2018.07.039
Dujardin S, Commins C, Lathuiliere A, Beerepoot P, Fernandes AR, Kamath TV, De Los Santos MB, Klickstein N et al (2020) Tau molecular diversity contributes to clinical heterogeneity in Alzheimer's disease. Nat Med 26(8):1256–1263. https://doi.org/10.1038/s41591-020-0938-9
Wang H, Li Y, Ryder JW, Hole JT, Ebert PJ, Airey DC, Qian HR, Logsdon B et al (2018) Genome-wide RNAseq study of the molecular mechanisms underlying microglia activation in response to pathological tau perturbation in the rTg4510 tau transgenic animal model. Mol Neurodegener 13(1):65. https://doi.org/10.1186/s13024-018-0296-y
Leyns CEG, Gratuze M, Narasimhan S, Jain N, Koscal LJ, Jiang H, Manis M, Colonna M et al (2019) TREM2 function impedes tau seeding in neuritic plaques. Nat Neurosci 22(8):1217–1222. https://doi.org/10.1038/s41593-019-0433-0
Yuan P, Condello C, Keene CD, Wang Y, Bird TD, Paul SM, Luo W, Colonna M et al (2016) TREM2 Haplodeficiency in Mice and Humans Impairs the Microglia Barrier Function Leading to Decreased Amyloid Compaction and Severe Axonal Dystrophy. Neuron 90(4):724–739. https://doi.org/10.1016/j.neuron.2016.05.003
Sayed FA, Telpoukhovskaia M, Kodama L, Li Y, Zhou Y, Le D, Hauduc A, Ludwig C et al (2018) Differential effects of partial and complete loss of TREM2 on microglial injury response and tauopathy. Proc Natl Acad Sci U S A 115(40):10172–10177. https://doi.org/10.1073/pnas.1811411115
Leyns CEG, Ulrich JD, Finn MB, Stewart FR, Koscal LJ, Remolina Serrano J, Robinson GO, Anderson E et al (2017) TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc Natl Acad Sci U S A 114(43):11524–11529. https://doi.org/10.1073/pnas.1710311114
Gratuze M, Leyns CE, Sauerbeck AD, St-Pierre MK, Xiong M, Kim N, Serrano JR, Tremblay M et al (2020) Impact of TREM2R47H variant on tau pathology-induced gliosis and neurodegeneration. J Clin Invest 130(9):4954–4968. https://doi.org/10.1172/jci138179
Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A, Schwartz S, Albasset S et al (2019) NLRP3 inflammasome activation drives tau pathology. Nature 575(7784):669–673. https://doi.org/10.1038/s41586-019-1769-z
Marim FM, Franco MMC, Gomes MTR, Miraglia MC, Giambartolomei GH, Oliveira SC (2017) The role of NLRP3 and AIM2 in inflammasome activation during Brucella abortus infection. Semin Immunopathol 39(2):215–223. https://doi.org/10.1007/s00281-016-0581-1
Stancu IC, Cremers N, Vanrusselt H, Couturier J, Vanoosthuyse A, Kessels S, Lodder C, Brône B et al (2019) Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol 137(4):599–617. https://doi.org/10.1007/s00401-018-01957-y
Shi Y, Manis M, Long J, Wang K, Sullivan PM, Remolina Serrano J, Hoyle R, Holtzman DM (2019) Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J Exp Med 216(11):2546–2561. https://doi.org/10.1084/jem.20190980
Dejanovic B, Huntley MA, De Mazière A, Meilandt WJ, Wu T, Srinivasan K, Jiang Z, Gandham V et al (2018) Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron 100(6):1322–1336.e1327. https://doi.org/10.1016/j.neuron.2018.10.014
El Gaamouch F, Audrain M, Lin WJ, Beckmann N, Jiang C, Hariharan S, Heeger PS, Schadt EE et al (2020) VGF-derived peptide TLQP-21 modulates microglial function through C3aR1 signaling pathways and reduces neuropathology in 5xFAD mice. Mol Neurodegener 15(1):4. https://doi.org/10.1186/s13024-020-0357-x
Carvalho K, Faivre E, Pietrowski MJ, Marques X, Gomez-Murcia V, Deleau A, Huin V, Hansen JN et al (2019) Exacerbation of C1q dysregulation, synaptic loss and memory deficits in tau pathology linked to neuronal adenosine A2A receptor. Brain 142(11):3636–3654. https://doi.org/10.1093/brain/awz288
Houtman J, Freitag K, Gimber N, Schmoranzer J, Heppner FL, Jendrach M (2019) Beclin1-driven autophagy modulates the inflammatory response of microglia via NLRP3. Embo J 38(4). https://doi.org/10.15252/embj.201899430
Friker LL, Scheiblich H, Hochheiser IV, Brinkschulte R, Riedel D, Latz E, Geyer M, Heneka MT (2020) β-Amyloid Clustering around ASC Fibrils Boosts Its Toxicity in Microglia. Cell Rep 30(11):3743–3754.e3746. https://doi.org/10.1016/j.celrep.2020.02.025
Zhang M, Qian C, Zheng ZG, Qian F, Wang Y, Thu PM, Zhang X, Zhou Y et al (2018) Jujuboside A promotes Aβ clearance and ameliorates cognitive deficiency in Alzheimer's disease through activating Axl/HSP90/PPARγ pathway. Theranostics 8(15):4262–4278. https://doi.org/10.7150/thno.26164
Nugent AA, Lin K, van Lengerich B, Lianoglou S, Przybyla L, Davis SS, Llapashtica C, Wang J et al (2020) TREM2 Regulates Microglial Cholesterol Metabolism upon Chronic Phagocytic Challenge. Neuron 105(5):837–854.e839. https://doi.org/10.1016/j.neuron.2019.12.007
Niu Z, Shi Q, Zhang W, Shu Y, Yang N, Chen B, Wang Q, Zhao X et al (2017) Caspase-1 cleaves PPARγ for potentiating the pro-tumor action of TAMs. Nat Commun 8(1):766. https://doi.org/10.1038/s41467-017-00523-6
He F, Doucet JA, Stephens JM (2008) Caspase-mediated degradation of PPARgamma proteins in adipocytes. Obesity (Silver Spring) 16(8):1735–1741. https://doi.org/10.1038/oby.2008.269
Molinuevo JL, Ayton S, Batrla R, Bednar MM, Bittner T, Cummings J, Fagan AM, Hampel H et al (2018) Current state of Alzheimer's fluid biomarkers. Acta Neuropathol 136(6):821–853. https://doi.org/10.1007/s00401-018-1932-x
Blennow K, Dubois B, Fagan AM, Lewczuk P, de Leon MJ, Hampel H (2015) Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer's disease. Alzheimers Dement 11(1):58–69. https://doi.org/10.1016/j.jalz.2014.02.004
Bekris LM, Khrestian M, Dyne E, Shao Y, Pillai JA, Rao SM, Bemiller SM, Lamb B et al (2018) Soluble TREM2 and biomarkers of central and peripheral inflammation in neurodegenerative disease. J Neuroimmunol 319:19–27. https://doi.org/10.1016/j.jneuroim.2018.03.003
Suárez-Calvet M, Morenas-Rodríguez E, Kleinberger G, Schlepckow K, Araque Caballero M, Franzmeier N, Capell A, Fellerer K et al (2019) Early increase of CSF sTREM2 in Alzheimer's disease is associated with tau related-neurodegeneration but not with amyloid-β pathology. Mol Neurodegener 14(1):1. https://doi.org/10.1186/s13024-018-0301-5
Heslegrave A, Heywood W, Paterson R, Magdalinou N, Svensson J, Johansson P, Öhrfelt A, Blennow K et al (2016) Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer's disease. Mol Neurodegener 11:3. https://doi.org/10.1186/s13024-016-0071-x
Rauchmann BS, Schneider-Axmann T, Alexopoulos P, Perneczky R (2019) CSF soluble TREM2 as a measure of immune response along the Alzheimer's disease continuum. Neurobiol Aging 74:182–190. https://doi.org/10.1016/j.neurobiolaging.2018.10.022
Ewers M, Franzmeier N, Suárez-Calvet M, Morenas-Rodriguez E, Caballero MAA, Kleinberger G, Piccio L, Cruchaga C et al (2019) Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer's disease. Sci Transl Med 11(507). https://doi.org/10.1126/scitranslmed.aav6221
Nordengen K, Kirsebom BE, Henjum K, Selnes P, Gísladóttir B, Wettergreen M, Torsetnes SB, Grøntvedt GR et al (2019) Glial activation and inflammation along the Alzheimer's disease continuum. J Neuroinflammation 16(1):46. https://doi.org/10.1186/s12974-019-1399-2
Henjum K, Quist-Paulsen E, Zetterberg H, Blennow K, Nilsson LNG, Watne LO (2018) CSF sTREM2 in delirium-relation to Alzheimer's disease CSF biomarkers Aβ42, t-tau and p-tau. J Neuroinflammation 15(1):304. https://doi.org/10.1186/s12974-018-1331-1
Woollacott IOC, Nicholas JM, Heslegrave A, Heller C, Foiani MS, Dick KM, Russell LL, Paterson RW et al (2018) Cerebrospinal fluid soluble TREM2 levels in frontotemporal dementia differ by genetic and pathological subgroup. Alzheimers Res Ther 10(1):79. https://doi.org/10.1186/s13195-018-0405-8
Hou XH, Bi YL, Tan MS, Xu W, Li JQ, Shen XN, Dou KX, Tan CC et al (2019) Genome-wide association study identifies Alzheimer's risk variant in MS4A6A influencing cerebrospinal fluid sTREM2 levels. Neurobiol Aging 84:241.e213–241.e220. https://doi.org/10.1016/j.neurobiolaging.2019.05.008
Halaas NB, Henjum K, Blennow K, Dakhil S, Idland AV, Nilsson LN, Sederevicius D, Vidal-Piñeiro D et al (2020) CSF sTREM2 and Tau Work Together in Predicting Increased Temporal Lobe Atrophy in Older Adults. Cereb Cortex 30(4):2295–2306. https://doi.org/10.1093/cercor/bhz240
Perea JR, Lleó A, Alcolea D, Fortea J, Ávila J, Bolós M (2018) Decreased CX3CL1 Levels in the Cerebrospinal Fluid of Patients With Alzheimer's Disease. Front Neurosci 12:609. https://doi.org/10.3389/fnins.2018.00609
Li A, Zhao J, Fan C, Zhu L, Huang C, Li Q, Gan D, Wen C et al (2020) Delivery of exogenous proteins by mesenchymal stem cells attenuates early memory deficits in a murine model of Alzheimer's disease. Neurobiol Aging 86:81–91. https://doi.org/10.1016/j.neurobiolaging.2019.10.012
Fan Q, He W, Gayen M, Benoit MR, Luo X, Hu X, Yan R (2020) Activated CX3CL1/Smad2 Signals Prevent Neuronal Loss and Alzheimer's Tau Pathology-Mediated Cognitive Dysfunction. J Neurosci 40(5):1133–1144. https://doi.org/10.1523/jneurosci.1333-19.2019
Kulczyńska-Przybik A, Słowik A, Mroczko P, Borawski B, Groblewska M, Borawska R, Mroczko B (2020) Cerebrospinal Fluid and Blood CX3CL1 as a Potential Biomarker in Early Diagnosis and Prognosis of Dementia. Curr Alzheimer Res 17(8):709–721. https://doi.org/10.2174/1567205017666201109095657
Lauridsen C, Sando SB, Møller I, Berge G, Pomary PK, Grøntvedt GR, Salvesen Ø, Bråthen G et al (2017) Cerebrospinal Fluid Aβ43 Is Reduced in Early-Onset Compared to Late-Onset Alzheimer's Disease, But Has Similar Diagnostic Accuracy to Aβ42. Front Aging Neurosci 9:210. https://doi.org/10.3389/fnagi.2017.00210
Körtvélyessy P, Gukasjan A, Sweeney-Reed CM, Heinze HJ, Thurner L, Bittner DM (2015) Progranulin and Amyloid-β Levels: Relationship to Neuropsychology in Frontotemporal and Alzheimer's Disease. J Alzheimers Dis 46(2):375–380. https://doi.org/10.3233/jad-150069
Mendsaikhan A, Tooyama I, Bellier JP, Serrano GE, Sue LI, Lue LF, Beach TG, Walker DG (2019) Characterization of lysosomal proteins Progranulin and Prosaposin and their interactions in Alzheimer's disease and aged brains: increased levels correlate with neuropathology. Acta Neuropathol Commun 7(1):215. https://doi.org/10.1186/s40478-019-0862-8
Suárez-Calvet M, Capell A, Araque Caballero M, Morenas-Rodríguez E, Fellerer K, Franzmeier N, Kleinberger G, Eren E, Deming Y, Piccio L, Karch CM, Cruchaga C, Paumier K, Bateman RJ, Fagan AM, Morris JC, Levin J, Danek A, Jucker M, Masters CL, Rossor MN, Ringman JM, Shaw LM, Trojanowski JQ, Weiner M, Ewers M, Haass C (2018) CSF progranulin increases in the course of Alzheimer's disease and is associated with sTREM2, neurodegeneration and cognitive decline. EMBO Mol Med 10(12). https://doi.org/10.15252/emmm.201809712
Morenas-Rodríguez E, Alcolea D, Suárez-Calvet M, Muñoz-Llahuna L, Vilaplana E, Sala I, Subirana A, Querol-Vilaseca M et al (2019) Different pattern of CSF glial markers between dementia with Lewy bodies and Alzheimer's disease. Sci Rep 9(1):7803. https://doi.org/10.1038/s41598-019-44173-8
Batzu L, Westman E, Pereira JB (2020) Cerebrospinal fluid progranulin is associated with increased cortical thickness in early stages of Alzheimer's disease. Neurobiol Aging 88:61–70. https://doi.org/10.1016/j.neurobiolaging.2019.12.012
Hüttenrauch M, Ogorek I, Klafki H, Otto M, Stadelmann C, Weggen S, Wiltfang J, Wirths O (2018) Glycoprotein NMB: a novel Alzheimer's disease associated marker expressed in a subset of activated microglia. Acta Neuropathol Commun 6(1):108. https://doi.org/10.1186/s40478-018-0612-3
Kamphuis W, Kooijman L, Schetters S, Orre M, Hol EM (2016) Transcriptional profiling of CD11c-positive microglia accumulating around amyloid plaques in a mouse model for Alzheimer's disease. Biochim Biophys Acta 1862(10):1847–1860. https://doi.org/10.1016/j.bbadis.2016.07.007
Ashton NJ, Suárez-Calvet M, Heslegrave A, Hye A, Razquin C, Pastor P, Sanchez-Valle R, Molinuevo JL et al (2019) Plasma levels of soluble TREM2 and neurofilament light chain in TREM2 rare variant carriers. Alzheimers Res Ther 11(1):94. https://doi.org/10.1186/s13195-019-0545-5
Tanaka M, Yamakage H, Masuda S, Inoue T, Ohue-Kitano R, Araki R, Matoba Y, Saito M et al (2019) Serum soluble TREM2 is a potential novel biomarker of cognitive impairment in Japanese non-obese patients with diabetes. Diabetes Metab 45(1):86–89. https://doi.org/10.1016/j.diabet.2017.06.006
Ohara T, Hata J, Tanaka M, Honda T, Yamakage H, Yoshida D, Inoue T, Hirakawa Y et al (2019) Serum Soluble Triggering Receptor Expressed on Myeloid Cells 2 as a Biomarker for Incident Dementia: The Hisayama Study. Ann Neurol 85(1):47–58. https://doi.org/10.1002/ana.25385
Wang Q, Xu Y, Qi C, Liu A, Zhao Y (2020) Association study of serum soluble TREM2 with vascular dementia in Chinese Han population. Int J Neurosci 130(7):708–712. https://doi.org/10.1080/00207454.2019.1702548
Kim TS, Lim HK, Lee JY, Kim DJ, Park S, Lee C, Lee CU (2008) Changes in the levels of plasma soluble fractalkine in patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett 436(2):196–200. https://doi.org/10.1016/j.neulet.2008.03.019
Kim DK, Han D, Park J, Choi H, Park JC, Cha MY, Woo J, Byun MS et al (2019) Deep proteome profiling of the hippocampus in the 5XFAD mouse model reveals biological process alterations and a novel biomarker of Alzheimer's disease. Exp Mol Med 51(11):1–17. https://doi.org/10.1038/s12276-019-0326-z
Tondo G, Iaccarino L, Caminiti SP, Presotto L, Santangelo R, Iannaccone S, Magnani G, Perani D (2020) The combined effects of microglia activation and brain glucose hypometabolism in early-onset Alzheimer's disease. Alzheimers Res Ther 12(1):50. https://doi.org/10.1186/s13195-020-00619-0
Fan Z, Brooks DJ, Okello A, Edison P (2017) An early and late peak in microglial activation in Alzheimer's disease trajectory. Brain 140(3):792–803. https://doi.org/10.1093/brain/aww349
Ismail R, Parbo P, Madsen LS, Hansen AK, Hansen KV, Schaldemose JL, Kjeldsen PL, Stokholm MG et al (2020) The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer's disease: a longitudinal PET study. J Neuroinflammation 17(1):151. https://doi.org/10.1186/s12974-020-01820-6
Parbo P, Madsen LS, Ismail R, Zetterberg H, Blennow K, Eskildsen SF, Vorup-Jensen T, Brooks DJ (2020) Low plasma neurofilament light levels associated with raised cortical microglial activation suggest inflammation acts to protect prodromal Alzheimer's disease. Alzheimers Res Ther 12(1):3. https://doi.org/10.1186/s13195-019-0574-0
Dani M, Wood M, Mizoguchi R, Fan Z, Walker Z, Morgan R, Hinz R, Biju M et al (2018) Microglial activation correlates in vivo with both tau and amyloid in Alzheimer's disease. Brain 141(9):2740–2754. https://doi.org/10.1093/brain/awy188
Cisbani G, Koppel A, Knezevic D, Suridjan I, Mizrahi R, Bazinet RP (2020) Peripheral cytokine and fatty acid associations with neuroinflammation in AD and aMCI patients: An exploratory study. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2020.02.014
Sacher C, Blume T, Beyer L, Biechele G, Sauerbeck J, Eckenweber F, Deussing M, Focke C et al (2020) Asymmetry of fibrillar plaque burden in amyloid mouse models. J Nucl Med. https://doi.org/10.2967/jnumed.120.242750
Blume T, Focke C, Peters F, Deussing M, Albert NL, Lindner S, Gildehaus FJ, von Ungern-Sternberg B et al (2018) Microglial response to increasing amyloid load saturates with aging: a longitudinal dual tracer in vivo μPET-study. J Neuroinflammation 15(1):307. https://doi.org/10.1186/s12974-018-1347-6
Terada T, Yokokura M, Obi T, Bunai T, Yoshikawa E, Ando I, Shimada H, Suhara T et al (2019) In vivo direct relation of tau pathology with neuroinflammation in early Alzheimer's disease. J Neurol 266(9):2186–2196. https://doi.org/10.1007/s00415-019-09400-2
Yamagishi S, Iga Y, Nakamura M, Takizawa C, Fukumoto D, Kakiuchi T, Nishiyama S, Ohba H et al (2019) Upregulation of cannabinoid receptor type 2, but not TSPO, in senescence-accelerated neuroinflammation in mice: a positron emission tomography study. J Neuroinflammation 16(1):208. https://doi.org/10.1186/s12974-019-1604-3
Ahmad R, Postnov A, Bormans G, Versijpt J, Vandenbulcke M, Van Laere K (2016) Decreased in vivo availability of the cannabinoid type 2 receptor in Alzheimer's disease. Eur J Nucl Med Mol Imaging 43(12):2219–2227. https://doi.org/10.1007/s00259-016-3457-7
Wang L, Liu BJ, Cao Y, Xu WQ, Sun DS, Li MZ, Shi FX, Li M et al (2018) Deletion of Type-2 Cannabinoid Receptor Induces Alzheimer's Disease-Like Tau Pathology and Memory Impairment Through AMPK/GSK3β Pathway. Mol Neurobiol 55(6):4731–4744. https://doi.org/10.1007/s12035-017-0676-2
Wang L, Shi FX, Xu WQ, Cao Y, Li N, Li M, Wang Q, Wang JZ et al (2018) The Down-Expression of ACE and IDE Exacerbates Exogenous Amyloid-β Neurotoxicity in CB2R-/- Mice. J Alzheimers Dis 64(3):957–971. https://doi.org/10.3233/jad-180142
Schmöle AC, Lundt R, Toporowski G, Hansen JN, Beins E, Halle A, Zimmer A (2018) Cannabinoid Receptor 2-Deficiency Ameliorates Disease Symptoms in a Mouse Model with Alzheimer's Disease-Like Pathology. J Alzheimers Dis 64(2):379–392. https://doi.org/10.3233/jad-180230
Walker DG, Tang TM, Lue LF (2017) Studies on Colony Stimulating Factor Receptor-1 and Ligands Colony Stimulating Factor-1 and Interleukin-34 in Alzheimer's Disease Brains and Human Microglia. Front Aging Neurosci 9:244. https://doi.org/10.3389/fnagi.2017.00244
Horti AG, Naik R, Foss CA, Minn I, Misheneva V, Du Y, Wang Y, Mathews WB et al (2019) PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R). Proc Natl Acad Sci U S A 116(5):1686–1691. https://doi.org/10.1073/pnas.1812155116
Sosna J, Philipp S, Albay R 3rd, Reyes-Ruiz JM, Baglietto-Vargas D, LaFerla FM, Glabe CG (2018) Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer's disease. Mol Neurodegener 13(1):11. https://doi.org/10.1186/s13024-018-0244-x
Spangenberg E, Severson PL, Hohsfield LA, Crapser J, Zhang J, Burton EA, Zhang Y, Spevak W et al (2019) Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer's disease model. Nat Commun 10(1):3758. https://doi.org/10.1038/s41467-019-11674-z
Mancuso R, Fryatt G, Cleal M, Obst J, Pipi E, Monzón-Sandoval J, Ribe E, Winchester L et al (2019) CSF1R inhibitor JNJ-40346527 attenuates microglial proliferation and neurodegeneration in P301S mice. Brain 142(10):3243–3264. https://doi.org/10.1093/brain/awz241
Walker DG, Tang TM, Mendsaikhan A, Tooyama I, Serrano GE, Sue LI, Beach TG, Lue LF (2020) Patterns of Expression of Purinergic Receptor P2RY12, a Putative Marker for Non-Activated Microglia, in Aged and Alzheimer's Disease Brains. Int J Mol Sci 21(2). https://doi.org/10.3390/ijms21020678
Franco-Bocanegra DK, George B, Lau LC, Holmes C, Nicoll JAR, Boche D (2019) Microglial motility in Alzheimer's disease and after Aβ42 immunotherapy: a human post-mortem study. Acta Neuropathol Commun 7(1):174. https://doi.org/10.1186/s40478-019-0828-x
Mildner A, Huang H, Radke J, Stenzel W, Priller J (2017) P2Y(12) receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. Glia 65(2):375–387. https://doi.org/10.1002/glia.23097
Peters DG, Pollack AN, Cheng KC, Sun D, Saido T, Haaf MP, Yang QX, Connor JR et al (2018) Dietary lipophilic iron alters amyloidogenesis and microglial morphology in Alzheimer's disease knock-in APP mice. Metallomics 10(3):426–443. https://doi.org/10.1039/c8mt00004b
Zeineh MM, Chen Y, Kitzler HH, Hammond R, Vogel H, Rutt BK (2015) Activated iron-containing microglia in the human hippocampus identified by magnetic resonance imaging in Alzheimer disease. Neurobiol Aging 36(9):2483–2500. https://doi.org/10.1016/j.neurobiolaging.2015.05.022
Bulk M, Abdelmoula WM, Nabuurs RJA, van der Graaf LM, Mulders CWH, Mulder AA, Jost CR, Koster AJ et al (2018) Postmortem MRI and histology demonstrate differential iron accumulation and cortical myelin organization in early- and late-onset Alzheimer's disease. Neurobiol Aging 62:231–242. https://doi.org/10.1016/j.neurobiolaging.2017.10.017
van Duijn S, Bulk M, van Duinen SG, Nabuurs RJA, van Buchem MA, van der Weerd L, Natté R (2017) Cortical Iron Reflects Severity of Alzheimer's Disease. J Alzheimers Dis 60(4):1533–1545. https://doi.org/10.3233/jad-161143
Femminella GD, Dani M, Wood M, Fan Z, Calsolaro V, Atkinson R, Edginton T, Hinz R et al (2019) Microglial activation in early Alzheimer trajectory is associated with higher gray matter volume. Neurology 92(12):e1331–e1343. https://doi.org/10.1212/wnl.0000000000007133
Femminella GD, Ninan S, Atkinson R, Fan Z, Brooks DJ, Edison P (2016) Does Microglial Activation Influence Hippocampal Volume and Neuronal Function in Alzheimer's Disease and Parkinson's Disease Dementia? J Alzheimers Dis 51(4):1275–1289. https://doi.org/10.3233/jad-150827
Schmitz TW, Soreq H, Poirier J, Spreng RN (2020) Longitudinal Basal Forebrain Degeneration Interacts with TREM2/C3 Biomarkers of Inflammation in Presymptomatic Alzheimer's Disease. J Neurosci 40(9):1931–1942. https://doi.org/10.1523/jneurosci.1184-19.2019
Snow WM, Dale R, O'Brien-Moran Z, Buist R, Peirson D, Martin M, Albensi BC (2017) In Vivo Detection of Gray Matter Neuropathology in the 3xTg Mouse Model of Alzheimer's Disease with Diffusion Tensor Imaging. J Alzheimers Dis 58(3):841–853. https://doi.org/10.3233/JAD-170136
Liu L, Liu Y, Li N, Huang R, Zheng X, Huang L, Hou S, Yuan Q (2020) Multiple inflammatory profiles of microglia and altered neuroimages in APP/PS1 transgenic AD mice. Brain Res Bull 156:86–104. https://doi.org/10.1016/j.brainresbull.2020.01.003
Taquet M, Jankovski A, Rensonnet G, Jacobs D, des Rieux A, Macq B, Warfield SK, Scherrer B (2019) Extra-axonal restricted diffusion as an in-vivo marker of reactive microglia. Sci Rep 9 (1):13874. https://doi.org/10.1038/s41598-019-50432-5
Yang J, Fu Z, Zhang X, Xiong M, Meng L, Zhang Z (2020) TREM2 ectodomain and its soluble form in Alzheimer's disease. J Neuroinflammation 17(1):204. https://doi.org/10.1186/s12974-020-01878-2
Ma LZ, Tan L, Bi YL, Shen XN, Xu W, Ma YH, Li HQ, Dong Q et al (2020) Dynamic changes of CSF sTREM2 in preclinical Alzheimer's disease: the CABLE study. Mol Neurodegener 15(1):25. https://doi.org/10.1186/s13024-020-00374-8
Suárez-Calvet M, Araque Caballero M, Kleinberger G, Bateman RJ, Fagan AM, Morris JC, Levin J, Danek A et al (2016) Early changes in CSF sTREM2 in dominantly inherited Alzheimer's disease occur after amyloid deposition and neuronal injury. Sci Transl Med 8(369):369ra178. https://doi.org/10.1126/scitranslmed.aag1767
Zhong L, Chen XF, Wang T, Wang Z, Liao C, Wang Z, Huang R, Wang D et al (2017) Soluble TREM2 induces inflammatory responses and enhances microglial survival. J Exp Med 214(3):597–607. https://doi.org/10.1084/jem.20160844
Bemiller SM, Maphis NM, Formica SV, Wilson GN, Miller CM, Xu G, Kokiko-Cochran ON, Kim KW et al (2018) Genetically enhancing the expression of chemokine domain of CX(3)CL1 fails to prevent tau pathology in mouse models of tauopathy. J Neuroinflammation 15(1):278. https://doi.org/10.1186/s12974-018-1310-6
Hanzel CE, Pichet-Binette A, Pimentel LS, Iulita MF, Allard S, Ducatenzeiler A, Do Carmo S, Cuello AC (2014) Neuronal driven pre-plaque inflammation in a transgenic rat model of Alzheimer's disease. Neurobiol Aging 35(10):2249–2262. https://doi.org/10.1016/j.neurobiolaging.2014.03.026
Finneran DJ, Morgan D, Gordon MN, Nash KR (2019) CNS-Wide over Expression of Fractalkine Improves Cognitive Functioning in a Tauopathy Model. J Neuroimmune Pharmacol 14(2):312–325. https://doi.org/10.1007/s11481-018-9822-5
Strobel S, Grünblatt E, Riederer P, Heinsen H, Arzberger T, Al-Sarraj S, Troakes C, Ferrer I et al (2015) Changes in the expression of genes related to neuroinflammation over the course of sporadic Alzheimer's disease progression: CX3CL1, TREM2, and PPARγ. J Neural Transm (Vienna) 122(7):1069–1076. https://doi.org/10.1007/s00702-015-1369-5
Paushter DH, Du H, Feng T, Hu F (2018) The lysosomal function of progranulin, a guardian against neurodegeneration. Acta Neuropathol 136(1):1–17. https://doi.org/10.1007/s00401-018-1861-8
Evers BM, Rodriguez-Navas C, Tesla RJ, Prange-Kiel J, Wasser CR, Yoo KS, McDonald J, Cenik B et al (2017) Lipidomic and Transcriptomic Basis of Lysosomal Dysfunction in Progranulin Deficiency. Cell Rep 20(11):2565–2574. https://doi.org/10.1016/j.celrep.2017.08.056
Xu HM, Tan L, Wan Y, Tan MS, Zhang W, Zheng ZJ, Kong LL, Wang ZX et al (2017) PGRN Is Associated with Late-Onset Alzheimer's Disease: a Case-Control Replication Study and Meta-analysis. Mol Neurobiol 54(2):1187–1195. https://doi.org/10.1007/s12035-016-9698-4
Nicholson AM, Finch NA, Thomas CS, Wojtas A, Rutherford NJ, Mielke MM, Roberts RO, Boeve BF et al (2014) Progranulin protein levels are differently regulated in plasma and CSF. Neurology 82(21):1871–1878. https://doi.org/10.1212/wnl.0000000000000445
Guan Z, Chen Z, Fu S, Dai L, Shen Y (2020) Progranulin Administration Attenuates β-Amyloid Deposition in the Hippocampus of 5xFAD Mice Through Modulating BACE1 Expression and Microglial Phagocytosis. Front Cell Neurosci 14:260. https://doi.org/10.3389/fncel.2020.00260
Takahashi H, Klein ZA, Bhagat SM, Kaufman AC, Kostylev MA, Ikezu T, Strittmatter SM (2017) Opposing effects of progranulin deficiency on amyloid and tau pathologies via microglial TYROBP network. Acta Neuropathol 133(5):785–807. https://doi.org/10.1007/s00401-017-1668-z
Morenas-Rodríguez E, Cervera-Carles L, Vilaplana E, Alcolea D, Carmona-Iragui M, Dols-Icardo O, Ribosa-Nogué R, Muñoz-Llahuna L et al (2016) Progranulin Protein Levels in Cerebrospinal Fluid in Primary Neurodegenerative Dementias. J Alzheimers Dis 50(2):539–546. https://doi.org/10.3233/jad-150746
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T et al (2015) Neuroinflammation in Alzheimer's disease. Lancet Neurol 14(4):388–405. https://doi.org/10.1016/s1474-4422(15)70016-5
Bettcher BM, Johnson SC, Fitch R, Casaletto KB, Heffernan KS, Asthana S, Zetterberg H, Blennow K et al (2018) Cerebrospinal Fluid and Plasma Levels of Inflammation Differentially Relate to CNS Markers of Alzheimer's Disease Pathology and Neuronal Damage. J Alzheimers Dis 62(1):385–397. https://doi.org/10.3233/jad-170602
Budge KM, Neal ML, Richardson JR, Safadi FF (2018) Glycoprotein NMB: an Emerging Role in Neurodegenerative Disease. Mol Neurobiol 55(6):5167–5176. https://doi.org/10.1007/s12035-017-0707-z
Huang JJ, Ma WJ, Yokoyama S (2012) Expression and immunolocalization of Gpnmb, a glioma-associated glycoprotein, in normal and inflamed central nervous systems of adult rats. Brain Behav 2(2):85–96. https://doi.org/10.1002/brb3.39
Kawahara K, Hirata H, Ohbuchi K, Nishi K, Maeda A, Kuniyasu A, Yamada D, Maeda T et al (2016) The novel monoclonal antibody 9F5 reveals expression of a fragment of GPNMB/osteoactivin processed by furin-like protease(s) in a subpopulation of microglia in neonatal rat brain. Glia 64(11):1938–1961. https://doi.org/10.1002/glia.23034
Sala Frigerio C, Wolfs L, Fattorelli N, Thrupp N, Voytyuk I, Schmidt I, Mancuso R, Chen WT et al (2019) The Major Risk Factors for Alzheimer's Disease: Age, Sex, and Genes Modulate the Microglia Response to Aβ Plaques. Cell Rep 27(4):1293–1306.e1296. https://doi.org/10.1016/j.celrep.2019.03.099
Satoh JI, Kino Y, Yanaizu M, Ishida T, Saito Y (2019) Microglia express GPNMB in the brains of Alzheimer's disease and Nasu-Hakola disease. Intractable Rare Dis Res 8(2):120–128. https://doi.org/10.5582/irdr.2019.01049
O'Bryant SE, Mielke MM, Rissman RA, Lista S, Vanderstichele H, Zetterberg H, Lewczuk P, Posner H et al (2017) Blood-based biomarkers in Alzheimer disease: Current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement 13(1):45–58. https://doi.org/10.1016/j.jalz.2016.09.014
Park SA, Han SM, Kim CE (2020) New fluid biomarkers tracking non-amyloid-β and non-tau pathology in Alzheimer's disease. Exp Mol Med 52(4):556–568. https://doi.org/10.1038/s12276-020-0418-9
Hsiung GY, Fok A, Feldman HH, Rademakers R, Mackenzie IR (2011) rs5848 polymorphism and serum progranulin level. J Neurol Sci 300(1-2):28–32. https://doi.org/10.1016/j.jns.2010.10.009
Carecchio M, Fenoglio C, De Riz M, Guidi I, Comi C, Cortini F, Venturelli E, Restelli I et al (2009) Progranulin plasma levels as potential biomarker for the identification of GRN deletion carriers. A case with atypical onset as clinical amnestic Mild Cognitive Impairment converted to Alzheimer's disease. J Neurol Sci 287(1-2):291–293. https://doi.org/10.1016/j.jns.2009.07.011
Piscopo P, Rivabene R, Galimberti D, Crestini A, Talarico G, Vanacore N, Scarpini E, Bruno G et al (2013) Gender effects on plasma PGRN levels in patients with Alzheimer's disease: a preliminary study. J Alzheimers Dis 35(2):313–318. https://doi.org/10.3233/jad-121606
Antonell A, Gil S, Sánchez-Valle R, Balasa M, Bosch B, Prat MC, Chiollaz AC, Fernández M et al (2012) Serum progranulin levels in patients with frontotemporal lobar degeneration and Alzheimer's disease: detection of GRN mutations in a Spanish cohort. J Alzheimers Dis 31(3):581–591. https://doi.org/10.3233/jad-2012-112120
Cooper YA, Nachun D, Dokuru D, Yang Z, Karydas AM, Serrero G, Yue B, Boxer AL et al (2018) Progranulin levels in blood in Alzheimer's disease and mild cognitive impairment. Ann Clin Transl Neurol 5(5):616–629. https://doi.org/10.1002/acn3.560
Shafit-Zagardo B, Gruber RC, DuBois JC (2018) The role of TAM family receptors and ligands in the nervous system: From development to pathobiology. Pharmacol Ther 188:97–117. https://doi.org/10.1016/j.pharmthera.2018.03.002
Nakanishi H (2020) Microglial cathepsin B as a key driver of inflammatory brain diseases and brain aging. Neural Regen Res 15(1):25–29. https://doi.org/10.4103/1673-5374.264444
Sjödin S, Brinkmalm G, Öhrfelt A, Parnetti L, Paciotti S, Hansson O, Hardy J, Blennow K et al (2019) Endo-lysosomal proteins and ubiquitin CSF concentrations in Alzheimer's and Parkinson's disease. Alzheimers Res Ther 11(1):82. https://doi.org/10.1186/s13195-019-0533-9
Bai H, Yang B, Yu W, Xiao Y, Yu D, Zhang Q (2018) Cathepsin B links oxidative stress to the activation of NLRP3 inflammasome. Exp Cell Res 362(1):180–187. https://doi.org/10.1016/j.yexcr.2017.11.015
Yin P, Wang S, Wei Y, Wang X, Zhang J, Yin X, Feng J, Zhu M (2020) Maresin1 Decreased Microglial Chemotaxis and Ameliorated Inflammation Induced by Amyloid-β42 in Neuron-Microglia Co-Culture Models. J Alzheimers Dis 73(2):503–515. https://doi.org/10.3233/jad-190682
Zhu M, Wang X, Hjorth E, Colas RA, Schroeder L, Granholm AC, Serhan CN, Schultzberg M (2016) Pro-Resolving Lipid Mediators Improve Neuronal Survival and Increase Aβ42 Phagocytosis. Mol Neurobiol 53(4):2733–2749. https://doi.org/10.1007/s12035-015-9544-0
Gui Y, Marks JD, Das S, Hyman BT, Serrano-Pozo A (2020) Characterization of the 18 kDa translocator protein (TSPO) expression in post-mortem normal and Alzheimer's disease brains. Brain Pathol 30(1):151–164. https://doi.org/10.1111/bpa.12763
Chiquita S, Ribeiro M, Castelhano J, Oliveira F, Sereno J, Batista M, Abrunhosa A, Rodrigues-Neves AC et al (2019) A longitudinal multimodal in vivo molecular imaging study of the 3xTg-AD mouse model shows progressive early hippocampal and taurine loss. Hum Mol Genet 28(13):2174–2188. https://doi.org/10.1093/hmg/ddz045
Felsky D, Roostaei T, Nho K, Risacher SL, Bradshaw EM, Petyuk V, Schneider JA, Saykin A et al (2019) Neuropathological correlates and genetic architecture of microglial activation in elderly human brain. Nat Commun 10(1):409. https://doi.org/10.1038/s41467-018-08279-3
Yokokura M, Terada T, Bunai T, Nakaizumi K, Takebayashi K, Iwata Y, Yoshikawa E, Futatsubashi M et al (2017) Depiction of microglial activation in aging and dementia: Positron emission tomography with [(11)C]DPA713 versus [(11)C]( R)PK11195. J Cereb Blood Flow Metab 37(3):877–889. https://doi.org/10.1177/0271678x16646788
Keller T, López-Picón FR, Krzyczmonik A, Forsback S, Kirjavainen AK, Takkinen JS, Alzghool O, Rajander J et al (2018) [(18)F]F-DPA for the detection of activated microglia in a mouse model of Alzheimer's disease. Nucl Med Biol 67:1–9. https://doi.org/10.1016/j.nucmedbio.2018.09.001
Deleye S, Waldron AM, Verhaeghe J, Bottelbergs A, Wyffels L, Van Broeck B, Langlois X, Schmidt M et al (2017) Evaluation of Small-Animal PET Outcome Measures to Detect Disease Modification Induced by BACE Inhibition in a Transgenic Mouse Model of Alzheimer Disease. J Nucl Med 58(12):1977–1983. https://doi.org/10.2967/jnumed.116.187625
López-Picón FR, Snellman A, Eskola O, Helin S, Solin O, Haaparanta-Solin M, Rinne JO (2018) Neuroinflammation Appears Early on PET Imaging and Then Plateaus in a Mouse Model of Alzheimer Disease. J Nucl Med 59(3):509–515. https://doi.org/10.2967/jnumed.117.197608
Focke C, Blume T, Zott B, Shi Y, Deussing M, Peters F, Schmidt C, Kleinberger G et al (2019) Early and Longitudinal Microglial Activation but Not Amyloid Accumulation Predicts Cognitive Outcome in PS2APP Mice. J Nucl Med 60(4):548–554. https://doi.org/10.2967/jnumed.118.217703
López A, Aparicio N, Pazos MR, Grande MT, Barreda-Manso MA, Benito-Cuesta I, Vázquez C, Amores M et al (2018) Cannabinoid CB(2) receptors in the mouse brain: relevance for Alzheimer's disease. J Neuroinflammation 15(1):158. https://doi.org/10.1186/s12974-018-1174-9
Navarro G, Borroto-Escuela D, Angelats E, Etayo Í, Reyes-Resina I, Pulido-Salgado M, Rodríguez-Pérez AI, Canela EI et al (2018) Receptor-heteromer mediated regulation of endocannabinoid signaling in activated microglia. Role of CB(1) and CB(2) receptors and relevance for Alzheimer's disease and levodopa-induced dyskinesia. Brain Behav Immun 67:139–151. https://doi.org/10.1016/j.bbi.2017.08.015
Wu J, Hocevar M, Foss JF, Bie B, Naguib M (2017) Activation of CB(2) receptor system restores cognitive capacity and hippocampal Sox2 expression in a transgenic mouse model of Alzheimer's disease. Eur J Pharmacol 811:12–20. https://doi.org/10.1016/j.ejphar.2017.05.044
Li C, Shi J, Wang B, Li J, Jia H (2019) CB2 cannabinoid receptor agonist ameliorates novel object recognition but not spatial memory in transgenic APP/PS1 mice. Neurosci Lett 707:134286. https://doi.org/10.1016/j.neulet.2019.134286
Askari VR, Shafiee-Nick R (2019) The protective effects of β-caryophyllene on LPS-induced primary microglia M(1)/M(2) imbalance: A mechanistic evaluation. Life Sci 219:40–73. https://doi.org/10.1016/j.lfs.2018.12.059
Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P et al (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34(36):11929–11947. https://doi.org/10.1523/JNEUROSCI.1860-14.2014
Casali BT, MacPherson KP, Reed-Geaghan EG, Landreth GE (2020) Microglia depletion rapidly and reversibly alters amyloid pathology by modification of plaque compaction and morphologies. Neurobiol Dis 142:104956. https://doi.org/10.1016/j.nbd.2020.104956
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K et al (2017) A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease. Cell 169(7):1276–1290 e1217. https://doi.org/10.1016/j.cell.2017.05.018
Villa A, Klein B, Janssen B, Pedragosa J, Pepe G, Zinnhardt B, Vugts DJ, Gelosa P et al (2018) Identification of new molecular targets for PET imaging of the microglial anti-inflammatory activation state. Theranostics 8(19):5400–5418. https://doi.org/10.7150/thno.25572
Angelova DM, Brown DR (2018) Altered Processing of β-Amyloid in SH-SY5Y Cells Induced by Model Senescent Microglia. ACS Chem Neurosci 9(12):3137–3152. https://doi.org/10.1021/acschemneuro.8b00334
McIntosh A, Mela V, Harty C, Minogue AM, Costello DA, Kerskens C, Lynch MA (2019) Iron accumulation in microglia triggers a cascade of events that leads to altered metabolism and compromised function in APP/PS1 mice. Brain Pathol 29(5):606–621. https://doi.org/10.1111/bpa.12704
Lancaster TM, Hill MJ, Sims R, Williams J (2019) Microglia - mediated immunity partly contributes to the genetic association between Alzheimer's disease and hippocampal volume. Brain Behav Immun 79:267–273. https://doi.org/10.1016/j.bbi.2019.02.011
Bouchon A, Hernández-Munain C, Cella M, Colonna M (2001) A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med 194(8):1111–1122. https://doi.org/10.1084/jem.194.8.1111
Hopperton KE, Mohammad D, Trépanier MO, Giuliano V, Bazinet RP (2018) Markers of microglia in post-mortem brain samples from patients with Alzheimer's disease: a systematic review. Mol Psychiatry 23(2):177–198. https://doi.org/10.1038/mp.2017.246
Code Availability
Not applicable
Funding
This study was supported by grants from the Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01), ZJLab, Shanghai Center for Brain Science and Brain-Inspired Technology, Tianqiao and Chrissy Chen Institute, and the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Fudan University.
Author information
Authors and Affiliations
Contributions
PFZ, HH, LT, and JTY did the manuscript preparation and drafting. LT and JTY are responsible for the study conception and design. PFZ and HH wrote the first draft of the manuscript. All authors have contributed to the manuscript revising and editing critically for important intellectual content and given final approval of the version and agreed to be accountable for all aspects of the work presented here.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
All authors have given final approval of the version and agreed with the publication of this study here.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, PF., Hu, H., Tan, L. et al. Microglia Biomarkers in Alzheimer’s Disease. Mol Neurobiol 58, 3388–3404 (2021). https://doi.org/10.1007/s12035-021-02348-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02348-3