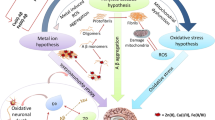
Abstract
Autotaxin (ATX) is a soluble extracellular enzyme that is abundant in mammalian plasma and cerebrospinal fluid (CSF). It has two known enzymatic activities, acting as both a phosphodiesterase and a phospholipase. The majority of its biological effects have been associated with its ability to liberate lysophosphatidic acid (LPA) from its substrate, lysophosphatidylcholine (LPC). LPA has diverse pleiotropic effects in the central nervous system (CNS) and other tissues via the activation of a family of six cognate G protein-coupled receptors. These LPA receptors (LPARs) are expressed in some combination in all known cell types in the CNS where they mediate such fundamental cellular processes as proliferation, differentiation, migration, chronic inflammation, and cytoskeletal organization. As a result, dysregulation of LPA content may contribute to many CNS and PNS disorders such as chronic inflammatory or neuropathic pain, glioblastoma multiforme (GBM), hemorrhagic hydrocephalus, schizophrenia, multiple sclerosis, Alzheimer’s disease, metabolic syndrome-induced brain damage, traumatic brain injury, hepatic encephalopathy-induced cerebral edema, macular edema, major depressive disorder, stress-induced psychiatric disorder, alcohol-induced brain damage, HIV-induced brain injury, pruritus, and peripheral nerve injury. ATX activity is now known to be the primary biological source of this bioactive signaling lipid, and as such, represents a potentially high-value drug target. There is currently one ATX inhibitor entering phase III clinical trials, with several additional preclinical compounds under investigation. This review discusses the physiological and pathological significance of the ATX-LPA-LPA receptor signaling axis and summarizes the evidence for targeting this pathway for the treatment of CNS diseases.


Similar content being viewed by others
References
Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, Liotta LA (1992) Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem 267(4):2524–2529
Clair T, Lee HY, Liotta LA, Stracke ML (1997) Autotaxin is an exoenzyme possessing 5′-nucleotide phosphodiesterase/ATP pyrophosphatase and ATPase activities. J Biol Chem 272(2):996–1001. https://doi.org/10.1074/jbc.272.2.996
Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K (2002) Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem 277(42):39436–39442. https://doi.org/10.1074/jbc.M205623200
Houben AJ, Moolenaar WH (2011) Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev 30(3–4):557–565. https://doi.org/10.1007/s10555-011-9319-7
Herr DR, Ong JH, Ong WY (2018) Potential therapeutic applications for inhibitors of autotaxin, a bioactive lipid-producing lysophospholipase D, in disorders affecting the nervous system. ACS Chem Neurosci 9(3):398–400. https://doi.org/10.1021/acschemneuro.8b00057
Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST et al (2010) LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 50:157–186. https://doi.org/10.1146/annurev.pharmtox.010909.105753
Lin ME, Herr DR, Chun J (2010) Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat 91(3–4):130–138. https://doi.org/10.1016/j.prostaglandins.2009.02.002
van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ et al (2006) Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol 26(13):5015–5022. https://doi.org/10.1128/MCB.02419-05
Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y et al (2006) Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem 281(35):25822–25830. https://doi.org/10.1074/jbc.M605142200
Yung YC, Stoddard NC, Chun J (2014) LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res 55(7):1192–1214. https://doi.org/10.1194/jlr.R046458
Thalman C, Horta G, Qiao L, Endle H, Tegeder I, Cheng H, Laube G, Sigrudsson T et al (2018) Synaptic phospholipids as a new target for cortical hyperexcitability and E/I balance in psychiatric disorders. Mol Psychiatry. 2018 Aug;23(8):1699-1710. https://doi.org/10.1038/s41380-018-0053-1.
Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, Kingsbury MA, Zhang G et al (2001) Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J Biol Chem 276(36):33697–33704. https://doi.org/10.1074/jbc.M104441200
Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J (2000) Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci U S A 97(24):13384–13389. https://doi.org/10.1073/pnas.97.24.13384
Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J (2002) Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol Cell Biol 22(19):6921–6929. https://doi.org/10.1128/mcb.22.19.6921-6929.2002
Lee Z, Cheng CT, Zhang H, Subler MA, Wu J, Mukherjee A, Windle JJ, Chen CK et al (2008) Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol Biol Cell 19(12):5435–5445. https://doi.org/10.1091/mbc.E08-03-0316
Lin ME, Rivera RR, Chun J (2012) Targeted deletion of LPA5 identifies novel roles for lysophosphatidic acid signaling in development of neuropathic pain. J Biol Chem 287(21):17608–17617. https://doi.org/10.1074/jbc.M111.330183
Hata E, Sasaki N, Takeda A, Tohya K, Umemoto E, Akahoshi N, Ishii S, Bando K et al (2016) Lysophosphatidic acid receptors LPA4 and LPA6 differentially promote lymphocyte transmigration across high endothelial venules in lymph nodes. Int Immunol 28(6):283–292. https://doi.org/10.1093/intimm/dxv072
Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T et al (2005) LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 435(7038):104–108. https://doi.org/10.1038/nature03505
Ye X, Herr DR, Diao H, Rivera R, Chun J (2011) Unique uterine localization and regulation may differentiate LPA3 from other lysophospholipid receptors for its role in embryo implantation. Fertil Steril 95(6):2107–2113, 2113 e2101-2104. https://doi.org/10.1016/j.fertnstert.2011.02.024
Ye X, Diao H, Chun J (2012) 11-deoxy prostaglandin F(2alpha), a thromboxane A2 receptor agonist, partially alleviates embryo crowding in Lpar3((−/−)) females. Fertil Steril 97(3):757–763. https://doi.org/10.1016/j.fertnstert.2011.12.004
Pasternack SM, von Kugelgen I, Al Aboud K, Lee YA, Ruschendorf F, Voss K, Hillmer AM, Molderings GJ et al (2008) G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet 40(3):329–334. https://doi.org/10.1038/ng.84
Shimomura Y, Garzon MC, Kristal L, Shapiro L, Christiano AM (2009) Autosomal recessive woolly hair with hypotrichosis caused by a novel homozygous mutation in the P2RY5 gene. Exp Dermatol 18(3):218–221. https://doi.org/10.1111/j.1600-0625.2008.00788.x
Shinkuma S, Akiyama M, Inoue A, Aoki J, Natsuga K, Nomura T, Arita K, Abe R et al (2010) Prevalent LIPH founder mutations lead to loss of P2Y5 activation ability of PA-PLA1alpha in autosomal recessive hypotrichosis. Hum Mutat 31(5):602–610. https://doi.org/10.1002/humu.21235
Nahum S, Morice-Picard F, Taieb A, Sprecher E (2011) A novel mutation in LPAR6 causes autosomal recessive hypotrichosis of the scalp. Clin Exp Dermatol 36(2):188–194. https://doi.org/10.1111/j.1365-2230.2010.03944.x
van Meeteren LA, Moolenaar WH (2007) Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res 46(2):145–160. https://doi.org/10.1016/j.plipres.2007.02.001
Moolenaar WH, Houben AJ, Lee SJ, van Meeteren LA (2013) Autotaxin in embryonic development. Biochim Biophys Acta 1831(1):13–19. https://doi.org/10.1016/j.bbalip.2012.09.013
Bachner D, Ahrens M, Betat N, Schroder D, Gross G (1999) Developmental expression analysis of murine autotaxin (ATX). Mech Dev 84(1–2):121–125
Ohuchi H, Hayashibara Y, Matsuda H, Onoi M, Mitsumori M, Tanaka M, Aoki J, Arai H et al (2007) Diversified expression patterns of autotaxin, a gene for phospholipid-generating enzyme during mouse and chicken development. Dev Dyn 236(4):1134–1143. https://doi.org/10.1002/dvdy.21119
Fotopoulou S, Oikonomou N, Grigorieva E, Nikitopoulou I, Paparountas T, Thanassopoulou A, Zhao Z, Xu Y et al (2010) ATX expression and LPA signalling are vital for the development of the nervous system. Dev Biol 339(2):451–464. https://doi.org/10.1016/j.ydbio.2010.01.007
Koike S, Yutoh Y, Keino-Masu K, Noji S, Masu M, Ohuchi H (2011) Autotaxin is required for the cranial neural tube closure and establishment of the midbrain–hindbrain boundary during mouse development. Dev Dyn 240(2):413–421
Greenman R, Gorelik A, Sapir T, Baumgart J, Zamor V, Segal-Salto M, Levin-Zaidman S, Aidinis V et al (2015) Non-cell autonomous and non-catalytic activities of ATX in the developing brain. Front Neurosci 9:53. https://doi.org/10.3389/fnins.2015.00053
Sato K, Malchinkhuu E, Muraki T, Ishikawa K, Hayashi K, Tosaka M, Mochiduki A, Inoue K et al (2005) Identification of autotaxin as a neurite retraction-inducing factor of PC12 cells in cerebrospinal fluid and its possible sources. J Neurochem 92(4):904–914
Fukushima N, Kimura Y, Chun J (1998) A single receptor encoded by vzg-1/lpA1/edg-2 couples to G proteins and mediates multiple cellular responses to lysophosphatidic acid. Proc Natl Acad Sci U S A 95(11):6151–6156. https://doi.org/10.1073/pnas.95.11.6151
Contos JJ, Chun J (2001) The mouse lp(A3)/Edg7 lysophosphatidic acid receptor gene: genomic structure, chromosomal localization, and expression pattern. Gene 267(2):243–253. https://doi.org/10.1016/s0378-1119(01)00410-3
Lee CW, Rivera R, Dubin AE, Chun J (2007) LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J Biol Chem 282(7):4310–4317. https://doi.org/10.1074/jbc.M610826200
Spohr TC, Choi JW, Gardell SE, Herr DR, Rehen SK, Gomes FC, Chun J (2008) Lysophosphatidic acid receptor-dependent secondary effects via astrocytes promote neuronal differentiation. J Biol Chem 283(12):7470–7479. https://doi.org/10.1074/jbc.M707758200
Yuelling LW, Waggener CT, Afshari FS, Lister JA, Fuss B (2012) Autotaxin/ENPP2 regulates oligodendrocyte differentiation in vivo in the developing zebrafish hindbrain. Glia 60(10):1605–1618. https://doi.org/10.1002/glia.22381
Wheeler NA, Lister JA, Fuss B (2015) The autotaxin–lysophosphatidic acid axis modulates histone acetylation and gene expression during oligodendrocyte differentiation. J Neurosci 35(32):11399–11414
Choi JW, Chun J (2013) Lysophospholipids and their receptors in the central nervous system. Biochim Biophys Acta 1831(1):20–32. https://doi.org/10.1016/j.bbalip.2012.07.015
Weiner JA, Hecht JH, Chun J (1998) Lysophosphatidic acid receptor gene vzg-1/lpA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J Comp Neurol 398(4):587–598
Garcia-Diaz B, Riquelme R, Varela-Nieto I, Jimenez AJ, de Diego I, Gomez-Conde AI, Matas-Rico E, Aguirre JA et al (2015) Loss of lysophosphatidic acid receptor LPA1 alters oligodendrocyte differentiation and myelination in the mouse cerebral cortex. Brain Struct Funct 220(6):3701–3720. https://doi.org/10.1007/s00429-014-0885-7
Nogaroli L, Yuelling LM, Dennis J, Gorse K, Payne SG, Fuss B (2009) Lysophosphatidic acid can support the formation of membranous structures and an increase in MBP mRNA levels in differentiating oligodendrocytes. Neurochem Res 34(1):182–193. https://doi.org/10.1007/s11064-008-9772-z
Fox MA, Colello RJ, Macklin WB, Fuss B (2003) Phosphodiesterase-Ialpha/autotaxin: a counteradhesive protein expressed by oligodendrocytes during onset of myelination. Mol Cell Neurosci 23(3):507–519
Fox MA, Alexander JK, Afshari FS, Colello RJ, Fuss B (2004) Phosphodiesterase-I alpha/autotaxin controls cytoskeletal organization and FAK phosphorylation during myelination. Mol Cell Neurosci 27(2):140–150. https://doi.org/10.1016/j.mcn.2004.06.002
Dennis J, White MA, Forrest AD, Yuelling LM, Nogaroli L, Afshari FS, Fox MA, Fuss B (2008) Phosphodiesterase-Ialpha/autotaxin’s MORFO domain regulates oligodendroglial process network formation and focal adhesion organization. Mol Cell Neurosci 37(2):412–424. https://doi.org/10.1016/j.mcn.2007.10.018
Yuelling LM, Fuss B (2008) Autotaxin (ATX): a multi-functional and multi-modular protein possessing enzymatic lysoPLD activity and matricellular properties. Biochim Biophys Acta 1781(9):525–530. https://doi.org/10.1016/j.bbalip.2008.04.009
Clair T, Aoki J, Koh E, Bandle RW, Nam SW, Ptaszynska MM, Mills GB, Schiffmann E et al (2003) Autotaxin hydrolyzes sphingosylphosphorylcholine to produce the regulator of migration, sphingosine-1-phosphate. Cancer Res 63(17):5446–5453
Novgorodov AS, El-Alwani M, Bielawski J, Obeid LM, Gudz TI (2007) Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J 21(7):1503–1514. https://doi.org/10.1096/fj.06-7420com
Cui QL, Fang J, Kennedy TE, Almazan G, Antel JP (2014) Role of p38MAPK in S1P receptor-mediated differentiation of human oligodendrocyte progenitors. Glia 62(8):1361–1375. https://doi.org/10.1002/glia.22688
Jung CG, Kim HJ, Miron VE, Cook S, Kennedy TE, Foster CA, Antel JP, Soliven B (2007) Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. Glia 55(16):1656–1667. https://doi.org/10.1002/glia.20576
Awada R, Saulnier-Blache JS, Gres S, Bourdon E, Rondeau P, Parimisetty A, Orihuela R, Harry GJ et al (2014) Autotaxin downregulates LPS-induced microglia activation and pro-inflammatory cytokines production. J Cell Biochem 115(12):2123–2132. https://doi.org/10.1002/jcb.24889
Awada R, Rondeau P, Gres S, Saulnier-Blache JS, Lefebvre d'Hellencourt C, Bourdon E (2012) Autotaxin protects microglial cells against oxidative stress. Free Radic Biol Med 52(2):516–526. https://doi.org/10.1016/j.freeradbiomed.2011.11.014
Mouratis MA, Magkrioti C, Oikonomou N, Katsifa A, Prestwich GD, Kaffe E, Aidinis V (2015) Autotaxin and endotoxin-induced acute lung injury. PLoS One 10(7):e0133619. https://doi.org/10.1371/journal.pone.0133619
Mirzoyan K, Denis C, Casemayou A, Gilet M, Marsal D, Goudouneche D, Faguer S, Bascands JL et al (2017) Lysophosphatidic acid protects against endotoxin-induced acute kidney injury. Inflammation 40(5):1707–1716. https://doi.org/10.1007/s10753-017-0612-7
Swaney JS, Chapman C, Correa LD, Stebbins KJ, Bundey RA, Prodanovich PC, Fagan P, Baccei CS et al (2010) A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol 160(7):1699–1713. https://doi.org/10.1111/j.1476-5381.2010.00828.x
Robering JW, Gebhardt L, Wolf K, Kuhn H, Kremer AE, Fischer MJM (2019) Lysophosphatidic acid activates satellite glia cells and Schwann cells. Glia 67(5):999–1012. https://doi.org/10.1002/glia.23585
Fujita R, Kiguchi N, Ueda H (2007) LPA-mediated demyelination in ex vivo culture of dorsal root. Neurochem Int 50(2):351–355. https://doi.org/10.1016/j.neuint.2006.09.003
Tsukahara R, Ueda H (2016) Myelin-related gene silencing mediated by LPA1 - Rho/ROCK signaling is correlated to acetylation of NFkappaB in S16 Schwann cells. J Pharmacol Sci 132(2):162–165. https://doi.org/10.1016/j.jphs.2016.07.010
Inoue M, Xie W, Matsushita Y, Chun J, Aoki J, Ueda H (2008) Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience 152(2):296–298. https://doi.org/10.1016/j.neuroscience.2007.12.041
Ueda H, Matsunaga H, Olaposi OI, Nagai J (2013) Lysophosphatidic acid: chemical signature of neuropathic pain. Biochim Biophys Acta 1831(1):61–73. https://doi.org/10.1016/j.bbalip.2012.08.014
McArthur S, Gobbetti T, Kusters DH, Reutelingsperger CP, Flower RJ, Perretti M (2015) Definition of a novel pathway centered on lysophosphatidic acid to recruit monocytes during the resolution phase of tissue inflammation. J Immunol 195(3):1139–1151. https://doi.org/10.4049/jimmunol.1500733
Ng W, Pébay A, Drummond K, Burgess A, Kaye AH, Morokoff A (2014) Complexities of lysophospholipid signalling in glioblastoma. 21. https://doi.org/10.1016/j.jocn.2014.02.013
Ray R, Rai V (2017) Lysophosphatidic acid converts monocytes into macrophages in both mice and humans. Blood. 2017 Mar 2;129(9):1177-1183.https://doi.org/10.1182/blood-2016-10-743757.
Tabuchi S, Kume K, Aihara M, Shimizu T (2000) Expression of lysophosphatidic acid receptor in rat astrocytes: mitogenic effect and expression of neurotrophic genes. Neurochem Res 25(5):573–582. https://doi.org/10.1023/A:1007542532395
Bouchareb R, Mahmut A, Nsaibia MJ, Boulanger MC, Dahou A, Lepine JL, Laflamme MH, Hadji F et al (2015) Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation 132(8):677–690. https://doi.org/10.1161/CIRCULATIONAHA.115.016757
Park GY, Lee YG, Berdyshev E, Nyenhuis S, Du J, Fu P, Gorshkova IA, Li Y et al (2013) Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am J Respir Crit Care Med 188(8):928–940. https://doi.org/10.1164/rccm.201306-1014OC
Zheng H, Hogberg J, Stenius U (2017) ATM-activated autotaxin (ATX) propagates inflammation and DNA damage in lung epithelial cells: a new mode of action for silica-induced DNA damage? Carcinogenesis 38(12):1196–1206. https://doi.org/10.1093/carcin/bgx100
Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Aoki J, Hama K, Okudaira S et al (2007) Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J Clin Gastroenterol 41(6):616–623. https://doi.org/10.1097/01.mcg.0000225642.90898.0e
Li S, Xiong C, Zhang J (2012) ATX and LPA receptor 3 are coordinately up-regulated in lipopolysaccharide-stimulated THP-1 cells through PKR and SPK1-mediated pathways. FEBS Lett 586(6):792–797. https://doi.org/10.1016/j.febslet.2012.01.044
Li S, Zhang J (2009) Lipopolysaccharide induces autotaxin expression in human monocytic THP-1 cells. Biochem Biophys Res Commun 378(2):264–268. https://doi.org/10.1016/j.bbrc.2008.11.047
Nakasaki T, Tanaka T, Okudaira S, Hirosawa M, Umemoto E, Otani K, Jin S, Bai Z et al (2008) Involvement of the lysophosphatidic acid-generating enzyme autotaxin in lymphocyte-endothelial cell interactions. Am J Pathol 173(5):1566–1576. https://doi.org/10.2353/ajpath.2008.071153
Benesch MG, Ko YM, Tang X, Dewald J, Lopez-Campistrous A, Zhao YY, Lai R, Curtis JM et al (2015) Autotaxin is an inflammatory mediator and therapeutic target in thyroid cancer. Endocr Relat Cancer 22(4):593–607. https://doi.org/10.1530/ERC-15-0045
Wu JM, Xu Y, Skill NJ, Sheng H, Zhao Z, Yu M, Saxena R, Maluccio MA (2010) Autotaxin expression and its connection with the TNF-alpha-NF-kappaB axis in human hepatocellular carcinoma. Mol Cancer 9:71. https://doi.org/10.1186/1476-4598-9-71
Benesch MG, Tang X, Dewald J, Dong WF, Mackey JR, Hemmings DG, McMullen TP, Brindley DN (2015) Tumor-induced inflammation in mammary adipose tissue stimulates a vicious cycle of autotaxin expression and breast cancer progression. FASEB J 29(9):3990–4000. https://doi.org/10.1096/fj.15-274480
Brindley DN, Lin FT, Tigyi GJ (2013) Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim Biophys Acta 1831(1):74–85. https://doi.org/10.1016/j.bbalip.2012.08.015
Leblanc R, Peyruchaud O (2015) New insights into the autotaxin/LPA axis in cancer development and metastasis. Exp Cell Res 333(2):183–189. https://doi.org/10.1016/j.yexcr.2014.11.010
Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB et al (2002) Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol 158(2):227–233. https://doi.org/10.1083/jcb.200204026
Benesch MGK, Ko YM, McMullen TPW, Brindley DN (2014) Autotaxin in the crosshairs: Taking aim at cancer and other inflammatory conditions. 588. https://doi.org/10.1016/j.febslet.2014.02.009
Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, Yu S, Stephens LC et al (2009) Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell 15(6):539–550. https://doi.org/10.1016/j.ccr.2009.03.027
St-Coeur PD, Ferguson D, Morin P Jr, Touaibia M (2013) PF-8380 and closely related analogs: synthesis and structure-activity relationship towards autotaxin inhibition and glioma cell viability. Arch Pharm (Weinheim) 346(2):91–97. https://doi.org/10.1002/ardp.201200395
Hoelzinger DB, Nakada M, Demuth T, Rosensteel T, Reavie LB, Berens ME (2008) Autotaxin: a secreted autocrine/paracrine factor that promotes glioma invasion. J Neuro-Oncol 86(3):297–309. https://doi.org/10.1007/s11060-007-9480-6
Kishi Y, Okudaira S, Tanaka M, Hama K, Shida D, Kitayama J, Yamori T, Aoki J et al (2006) Autotaxin is overexpressed in glioblastoma multiforme and contributes to cell motility of glioblastoma by converting lysophosphatidylcholine to lysophosphatidic acid. J Biol Chem 281(25):17492–17500
Reeves VL, Trybula JS, Wills RC, Goodpaster BH, Dubé JJ, Kienesberger PC, Kershaw EE (2015) Serum autotaxin/ENPP 2 correlates with insulin resistance in older humans with obesity. Obesity 23(12):2371–2376
Parimisetty A, Dorsemans AC, Awada R, Ravanan P, Diotel N, Lefebvre d’Hellencourt C (2016) Secret talk between adipose tissue and central nervous system via secreted factors—an emerging frontier in the neurodegenerative research. J Neuroinflammation 13(1):67. https://doi.org/10.1186/s12974-016-0530-x
Tokumura A, Kanaya Y, Kitahara M, Miyake M, Yoshioka Y, Fukuzawa K (2002) Increased formation of lysophosphatidic acids by lysophospholipase D in serum of hypercholesterolemic rabbits. J Lipid Res 43(2):307–315
Federico L, Ren H, Mueller PA, Wu T, Liu S, Popovic J, Blalock EM, Sunkara M et al (2012) Autotaxin and its product lysophosphatidic acid suppress brown adipose differentiation and promote diet-induced obesity in mice. Mol Endocrinol 26(5):786–797. https://doi.org/10.1210/me.2011-1229
Nishimura S, Nagasaki M, Okudaira S, Aoki J, Ohmori T, Ohkawa R, Nakamura K, Igarashi K et al (2014) ENPP2 contributes to adipose tissue expansion and insulin resistance in diet-induced obesity. Diabetes 63(12):4154–4164. https://doi.org/10.2337/db13-1694
Kim SE, Choo J, Yoon J, Chu JR, Bae YJ, Lee S, Park T, Sung MK (2017) Genome-wide analysis identifies colonic genes differentially associated with serum leptin and insulin concentrations in C57BL/6J mice fed a high-fat diet. PLoS One 12(2):e0171664. https://doi.org/10.1371/journal.pone.0171664
Nam SW, Clair T, Kim Y-S, McMarlin A, Schiffmann E, Liotta LA, Stracke ML (2001) Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res 61(18):6938–6944
Ptaszynska MM, Pendrak ML, Stracke ML, Roberts DD (2010) Autotaxin signaling via lysophosphatidic acid receptors contributes to vascular endothelial growth factor–induced endothelial cell migration. Mol Cancer Res. 2010 Mar;8(3):309-21. https://doi.org/10.1158/1541-7786.MCR-09-0288.
Yukiura H, Kano K, Kise R, Inoue A, Aoki J (2015) Autotaxin overexpression causes embryonic lethality and vascular defects. PLoS One 10(5):e0126734. https://doi.org/10.1371/journal.pone.0126734
Im E, Motiejunaite R, Aranda J, Park EY, Federico L, Kim TI, Clair T, Stracke ML et al (2010) Phospholipase Cgamma activation drives increased production of autotaxin in endothelial cells and lysophosphatidic acid-dependent regression. Mol Cell Biol 30(10):2401–2410. https://doi.org/10.1128/MCB.01275-09
Motiejūnaitė R, Aranda J, Kazlauskas A (2014) Pericytes prevent regression of endothelial cell tubes by accelerating metabolism of lysophosphatidic acid. Microvasc Res 93:62–71
Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN et al (2010) Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res 51(11):3299–3305. https://doi.org/10.1194/jlr.M009449
Illingworth DR, Glover J (1971) The composition of lipids in cerebrospinal fluid of children and adults. J Neurochem 18(5):769–776. https://doi.org/10.1111/j.1471-4159.1971.tb12006.x
Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H (2004) Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med 10(7):712–718. https://doi.org/10.1038/nm1060
Ueda H (2006) Molecular mechanisms of neuropathic pain-phenotypic switch and initiation mechanisms. Pharmacol Ther 109(1–2):57–77. https://doi.org/10.1016/j.pharmthera.2005.06.003
Kuwajima K, Sumitani M, Kurano M, Kano K, Nishikawa M, Uranbileg B, Tsuchida R, Ogata T et al (2018) Lysophosphatidic acid is associated with neuropathic pain intensity in humans: an exploratory study. PLoS One 13(11):e0207310. https://doi.org/10.1371/journal.pone.0207310
Nagai J, Uchida H, Matsushita Y, Yano R, Ueda M, Niwa M, Aoki J, Chun J et al (2010) Autotaxin and lysophosphatidic acid1 receptor-mediated demyelination of dorsal root fibers by sciatic nerve injury and intrathecal lysophosphatidylcholine. Mol Pain 6:78. https://doi.org/10.1186/1744-8069-6-78
Inoue M, Ma L, Aoki J, Chun J, Ueda H (2008) Autotaxin, a synthetic enzyme of lysophosphatidic acid (LPA), mediates the induction of nerve-injured neuropathic pain. Mol Pain 4(1):6. https://doi.org/10.1186/1744-8069-4-6
Nagai J, Ueda H (2011) Pre-emptive morphine treatment abolishes nerve injury-induced lysophospholipid synthesis in mass spectrometrical analysis. J Neurochem 118(2):256–265. https://doi.org/10.1111/j.1471-4159.2011.07297.x
Ma L, Uchida H, Nagai J, Inoue M, Chun J, Aoki J, Ueda H (2009) Lysophosphatidic acid-3 receptor-mediated feed-forward production of lysophosphatidic acid: an initiator of nerve injury-induced neuropathic pain. Mol Pain 5:64. https://doi.org/10.1186/1744-8069-5-64
Velasco M, O'Sullivan C, Sheridan GK (2017) Lysophosphatidic acid receptors (LPARs): Potential targets for the treatment of neuropathic pain. Neuropharmacology 113(Pt B):608–617. https://doi.org/10.1016/j.neuropharm.2016.04.002
Su J, Delaney A, Matteo R, Bartlett B, Kultima K, Hökfelt T, Svensson C (2015) Blockage of lysophosphatidic acid reverses arthritis-induced hypersensitivity and Cavα2δ1 and P2X3 expression in dorsal root ganglia. Scand J Pain 8(1):53–54
Srikanth M, Chew WS, Hind T, Lim SM, Hay NWJ, Lee JHM, Rivera R, Chun J et al (2018) Lysophosphatidic acid and its receptor LPA1 mediate carrageenan induced inflammatory pain in mice. Eur J Pharmacol 841:49–56. https://doi.org/10.1016/j.ejphar.2018.10.005
Inoue M, Ma L, Aoki J, Ueda H (2008) Simultaneous stimulation of spinal NK1 and NMDA receptors produces LPC which undergoes ATX-mediated conversion to LPA, an initiator of neuropathic pain. J Neurochem 107(6):1556–1565. https://doi.org/10.1111/j.1471-4159.2008.05725.x
Ma L, Uchida H, Nagai J, Inoue M, Aoki J, Ueda H (2010) Evidence for de novo synthesis of lysophosphatidic acid in the spinal cord through phospholipase A2 and autotaxin in nerve injury-induced neuropathic pain. J Pharmacol Exp Ther 333(2):540–546. https://doi.org/10.1124/jpet.109.164830
Yeo JF, Ong WY, Ling SF, Farooqui AA (2004) Intracerebroventricular injection of phospholipases A2 inhibitors modulates allodynia after facial carrageenan injection in mice. Pain 112(1–2):148–155. https://doi.org/10.1016/j.pain.2004.08.009
Kakiuchi Y, Nagai J, Gotoh M, Hotta H, Murofushi H, Ogawa T, Ueda H, Murakami-Murofushi K (2011) Antinociceptive effect of cyclic phosphatidic acid and its derivative on animal models of acute and chronic pain. Mol Pain 7:33. https://doi.org/10.1186/1744-8069-7-33
Jones SB, Pfeifer LA, Bleisch TJ, Beauchamp TJ, Durbin JD, Klimkowski VJ, Hughes NE, Rito CJ et al (2016) Novel autotaxin inhibitors for the treatment of osteoarthritis pain: lead optimization via structure-based drug design. ACS Med Chem Lett 7(9):857–861. https://doi.org/10.1021/acsmedchemlett.6b00207
Thirunavukkarasu K, Swearingen CA, Oskins JL, Lin C, Bui HH, Jones SB, Pfeifer LA, Norman BH et al (2017) Identification and pharmacological characterization of a novel inhibitor of autotaxin in rodent models of joint pain. Osteoarthr Cartil 25(6):935–942. https://doi.org/10.1016/j.joca.2016.09.006
Gierse J, Thorarensen A, Beltey K, Bradshaw-Pierce E, Cortes-Burgos L, Hall T, Johnston A, Murphy M et al (2010) A novel autotaxin inhibitor reduces lysophosphatidic acid levels in plasma and the site of inflammation. J Pharmacol Exp Ther 334(1):310–317. https://doi.org/10.1124/jpet.110.165845
Kawagoe H, Stracke ML, Nakamura H, Sano K (1997) Expression and transcriptional regulation of the PD-Ialpha/autotaxin gene in neuroblastoma. Cancer Res 57(12):2516–2521
Yao M, Li S, Wu X, Diao S, Zhang G, He H, Bian L, Lu Y (2018) Cellular origin of glioblastoma and its implication in precision therapy. Cell Mol Immunol 15(8):737–739. https://doi.org/10.1038/cmi.2017.159
Tabuchi S (2015) The autotaxin-lysophosphatidic acid-lysophosphatidic acid receptor cascade: proposal of a novel potential therapeutic target for treating glioblastoma multiforme. Lipids Health Dis 14:56. https://doi.org/10.1186/s12944-015-0059-5
Hoelzinger DB, Mariani L, Weis J, Woyke T, Berens TJ, McDonough WS, Sloan A, Coons SW et al (2005) Gene expression profile of glioblastoma multiforme invasive phenotype points to new therapeutic targets. Neoplasia 7(1):7–16. https://doi.org/10.1593/neo.04535
Annabi B, Lachambre MP, Plouffe K, Sartelet H, Beliveau R (2009) Modulation of invasive properties of CD133+ glioblastoma stem cells: a role for MT1-MMP in bioactive lysophospholipid signaling. Mol Carcinog 48(10):910–919. https://doi.org/10.1002/mc.20541
Shimura T, Kurano M, Morita Y, Yoshikawa N, Nishikawa M, Igarashi K, Shimamoto S, Aoki J et al (2019) Autotaxin and soluble IL-2 receptor concentrations in cerebrospinal fluids are useful for the diagnosis of central nervous system invasion caused by haematological malignancies. Ann Clin Biochem 56(2):240–246. https://doi.org/10.1177/0004563218818197
Bhave SR, Dadey DY, Karvas RM, Ferraro DJ, Kotipatruni RP, Jaboin JJ, Hallahan AN, Dewees TA et al (2013) Autotaxin inhibition with PF-8380 enhances the radiosensitivity of human and murine glioblastoma cell lines. Front Oncol 3:236. https://doi.org/10.3389/fonc.2013.00236
Schleicher SM, Thotala DK, Linkous AG, Hu R, Leahy KM, Yazlovitskaya EM, Hallahan DE (2011) Autotaxin and LPA receptors represent potential molecular targets for the radiosensitization of murine glioma through effects on tumor vasculature. PLoS One 6(7):e22182. https://doi.org/10.1371/journal.pone.0022182
Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K, New South W, Australian Capital Territory Neonatal Intensive Care Units’ Data C (2014) Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 133(1):55–62. https://doi.org/10.1542/peds.2013-0372
Mirendil H, Thomas EA, De Loera C, Okada K, Inomata Y, Chun J (2015) LPA signaling initiates schizophrenia-like brain and behavioral changes in a mouse model of prenatal brain hemorrhage. Transl Psychiatry 5:e541. https://doi.org/10.1038/tp.2015.33
Yung YC, Mutoh T, Lin ME, Noguchi K, Rivera RR, Choi JW, Kingsbury MA, Chun J (2011) Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci Transl Med 3(99):99ra87. https://doi.org/10.1126/scitranslmed.3002095
Park R, Moon UY, Park JY, Hughes LJ, Johnson RL, Cho SH, Kim S (2016) Yap is required for ependymal integrity and is suppressed in LPA-induced hydrocephalus. Nat Commun 7:10329. https://doi.org/10.1038/ncomms10329
Herr KJ, Herr DR, Lee CW, Noguchi K, Chun J (2011) Stereotyped fetal brain disorganization is induced by hypoxia and requires lysophosphatidic acid receptor 1 (LPA1) signaling. Proc Natl Acad Sci U S A 108(37):15444–15449. https://doi.org/10.1073/pnas.1106129108
Wang HY, Liu CB, Wu HW, Kuo JS (2010) Direct profiling of phospholipids and lysophospholipids in rat brain sections after ischemic stroke. Rapid Commun Mass Spectrom 24(14):2057–2064. https://doi.org/10.1002/rcm.4620
Vogt J, Yang JW, Mobascher A, Cheng J, Li Y, Liu X, Baumgart J, Thalman C et al (2015) Molecular cause and functional impact of altered synaptic lipid signaling due to a prg-1 gene SNP. EMBO Mol Med. 2016 Jan 1;8(1):25-38. https://doi.org/10.15252/emmm.201505677.
Hammack BN, Fung KY, Hunsucker SW, Duncan MW, Burgoon MP, Owens GP, Gilden DH (2004) Proteomic analysis of multiple sclerosis cerebrospinal fluid. Mult Scler 10(3):245–260. https://doi.org/10.1191/1352458504ms1023oa
Zahednasab H, Balood M, Harirchian MH, Mesbah-Namin SA, Rahimian N, Siroos B (2014) Increased autotaxin activity in multiple sclerosis. J Neuroimmunol 273(1–2):120–123. https://doi.org/10.1016/j.jneuroim.2014.06.006
Schmitz K, Brunkhorst R, de Bruin N, Mayer CA, Haussler A, Ferreiros N, Schiffmann S, Parnham MJ et al (2017) Dysregulation of lysophosphatidic acids in multiple sclerosis and autoimmune encephalomyelitis. Acta Neuropathol Commun 5(1):42. https://doi.org/10.1186/s40478-017-0446-4
Thirunavukkarasu K, Tan B, Swearingen CA, Rocha G, Bui HH, McCann DJ, Jones SB, Norman BH et al (2016) Pharmacological characterization of a potent inhibitor of autotaxin in animal models of inflammatory bowel disease and multiple sclerosis. J Pharmacol Exp Ther 359(1):207–214. https://doi.org/10.1124/jpet.116.234013
Ramesh S, Govindarajulu M, Suppiramaniam V, Moore T, Dhanasekaran M (2018) Autotaxin–lysophosphatidic acid signaling in Alzheimer’s disease. 19. https://doi.org/10.3390/ijms19071827
Umemura K, Yamashita N, Yu X, Arima K, Asada T, Makifuchi T, Murayama S, Saito Y et al (2006) Autotaxin expression is enhanced in frontal cortex of Alzheimer-type dementia patients. Neurosci Lett 400(1–2):97–100. https://doi.org/10.1016/j.neulet.2006.02.008
McLimans KE, Willette AA, Alzheimer’s disease neuroimaging I (2017) Autotaxin is related to metabolic dysfunction and predicts Alzheimer’s disease outcomes. J Alzheimers Dis 56(1):403–413. https://doi.org/10.3233/JAD-160891
Mulder C, Wahlund LO, Teerlink T, Blomberg M, Veerhuis R, van Kamp GJ, Scheltens P, Scheffer PG (2003) Decreased lysophosphatidylcholine/phosphatidylcholine ratio in cerebrospinal fluid in Alzheimer’s disease. J Neural Transm (Vienna) 110(8):949–955. https://doi.org/10.1007/s00702-003-0007-9
Sun YX, Minthon L, Wallmark A, Warkentin S, Blennow K, Janciauskiene S (2003) Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 16(3):136–144. https://doi.org/10.1159/000071001
Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G et al (1999) Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A 96(12):6931–6936. https://doi.org/10.1073/pnas.96.12.6931
Farooqui AA, Horrocks LA (2007) Glycerophospholipids in the brain. https://doi.org/10.1007/978-0-387-49931-4
Shi J, Dong Y, Cui MZ, Xu X (2013) Lysophosphatidic acid induces increased BACE1 expression and abeta formation. Biochim Biophys Acta 1832(1):29–38. https://doi.org/10.1016/j.bbadis.2012.09.010
Sayas CL, Moreno-Flores MT, Avila J, Wandosell F (1999) The neurite retraction induced by lysophosphatidic acid increases Alzheimer’s disease-like Tau phosphorylation. J Biol Chem 274(52):37046–37052. https://doi.org/10.1074/jbc.274.52.37046
de la Torre JC (2004) Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol 3(3):184–190
Li HY, Oh YS, Choi JW, Jung JY, Jun HS (2017) Blocking lysophosphatidic acid receptor 1 signaling inhibits diabetic nephropathy in db/db mice. Kidney Int 91(6):1362–1373. https://doi.org/10.1016/j.kint.2016.11.010
Savaskan NE, Rocha L, Kotter MR, Baer A, Lubec G, van Meeteren LA, Kishi Y, Aoki J et al (2007) Autotaxin (NPP-2) in the brain: cell type-specific expression and regulation during development and after neurotrauma. Cell Mol Life Sci 64(2):230–243. https://doi.org/10.1007/s00018-006-6412-0
Crack PJ, Zhang M, Morganti-Kossmann MC, Morris AJ, Wojciak JM, Fleming JK, Karve I, Wright D et al (2014) Anti-lysophosphatidic acid antibodies improve traumatic brain injury outcomes. J Neuroinflammation 11:37. https://doi.org/10.1186/1742-2094-11-37
Goldshmit Y, Matteo R, Sztal T, Ellett F, Frisca F, Moreno K, Crombie D, Lieschke GJ et al (2012) Blockage of lysophosphatidic acid signaling improves spinal cord injury outcomes. Am J Pathol 181(3):978–992. https://doi.org/10.1016/j.ajpath.2012.06.007
Frugier T, Crombie D, Conquest A, Tjhong F, Taylor C, Kulkarni T, McLean C, Pebay A (2011) Modulation of LPA receptor expression in the human brain following neurotrauma. Cell Mol Neurobiol 31(4):569–577. https://doi.org/10.1007/s10571-011-9650-0
Farooqui AA, Horrocks LA (2006) Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist 12(3):245–260. https://doi.org/10.1177/1073858405285923
Masago K, Kihara Y, Yanagida K, Hamano F, Nakagawa S, Niwa M, Shimizu T (2018) Lysophosphatidic acid receptor, LPA6, regulates endothelial blood-brain barrier function: Implication for hepatic encephalopathy. Biochem Biophys Res Commun 501(4):1048–1054. https://doi.org/10.1016/j.bbrc.2018.05.106
Dacheva I, Ullmer C, Ceglowska K, Nogoceke E, Hartmann G, Muller S, Rejdak R, Nowomiejska K et al (2016) Lysophosphatidic acids and autotaxin in retinal vein occlusion. Retina 36(12):2311–2318. https://doi.org/10.1097/IAE.0000000000001112
Aston C, Jiang L, Sokolov BP (2005) Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry 10(3):309–322. https://doi.org/10.1038/sj.mp.4001565
Itagaki K, Takebayashi M, Abe H, Shibasaki C, Kajitani N, Okada-Tsuchioka M, Hattori K, Yoshida S et al (2019) Reduced serum and cerebrospinal fluid levels of autotaxin in major depressive disorder. Int J Neuropsychopharmacol 22(4):261–269. https://doi.org/10.1093/ijnp/pyz005
Manzardo A, Gunewardena S, Butler M (2013) Over-expression of the miRNA cluster at chromosome 14q32 in the alcoholic brain correlates with suppression of predicted target mRNA required for oligodendrocyte proliferation. Gene 526(2):356–363
Manzardo AM, Gunewardena S, Wang K, Butler MG (2014) Exon microarray analysis of human dorsolateral prefrontal cortex in alcoholism. Alcohol Clin Exp Res 38(6):1594–1601. https://doi.org/10.1111/acer.12429
Wheeler NA, Fuss B, Knapp PE, Zou S (2016) HIV-1 tat inhibits autotaxin lysophospholipase D activity and modulates oligodendrocyte differentiation. ASN Neuro 8(5):1759091416669618. https://doi.org/10.1177/1759091416669618
Oude Elferink RP, Bolier R, Beuers UH (2015) Lysophosphatidic acid and signaling in sensory neurons. Biochim Biophys Acta 1851(1):61–65. https://doi.org/10.1016/j.bbalip.2014.09.004
Kremer AE, Gonzales E, Schaap FG, Oude Elferink RP, Jacquemin E, Beuers U (2016) Serum autotaxin activity correlates with pruritus in pediatric cholestatic disorders. J Pediatr Gastroenterol Nutr 62(4):530–535. https://doi.org/10.1097/MPG.0000000000001044
Kremer AE, Martens JJ, Kulik W, Rueff F, Kuiper EM, van Buuren HR, van Erpecum KJ, Kondrackiene J et al (2010) Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology 139(3):1008–1018, 1018 e1001. https://doi.org/10.1053/j.gastro.2010.05.009
Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EM, Mettang T, Reiners KS, Raap U et al (2012) Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology 56(4):1391–1400. https://doi.org/10.1002/hep.25748
Kremer AE, Bolier R, Dixon PH, Geenes V, Chambers J, Tolenaars D, Ris-Stalpers C, Kaess BM et al (2015) Autotaxin activity has a high accuracy to diagnose intrahepatic cholestasis of pregnancy. J Hepatol 62(4):897–904. https://doi.org/10.1016/j.jhep.2014.10.041
Nakao M, Sugaya M, Suga H, Kawaguchi M, Morimura S, Kai H, Ohmatsu H, Fujita H et al (2014) Serum autotaxin levels correlate with pruritus in patients with atopic dermatitis. 134. https://doi.org/10.1038/jid.2014.24
Beuers U, Gerken G, Pusl T (2006) Biliary drainage transiently relieves intractable pruritus in primary biliary cirrhosis [3]. 44. https://doi.org/10.1002/hep.21271
Shimizu Y, Morikawa Y, Okudaira S, Kimoto S, Tanaka T, Aoki J, Tokumura A (2014) Potentials of the circulating pruritogenic mediator lysophosphatidic acid in development of allergic skin inflammation in mice: role of blood cell-associated lysophospholipase D activity of autotaxin. Am J Pathol 184(5):1593–1603. https://doi.org/10.1016/j.ajpath.2014.01.029
Renback K, Inoue M, Yoshida A, Nyberg F, Ueda H (2000) Vzg-1/lysophosphatidic acid-receptor involved in peripheral pain transmission. Brain Res Mol Brain Res 75(2):350–354
Oude Elferink RP, Kremer AE, Beuers U (2011) Mediators of pruritus during cholestasis. Curr Opin Gastroenterol 27(3):289–293. https://doi.org/10.1097/MOG.0b013e32834575e8
Szepanowski F, Derksen A, Steiner I, Meyer Zu Horste G, Daldrup T, Hartung HP, Kieseier BC (2016) Fingolimod promotes peripheral nerve regeneration via modulation of lysophospholipid signaling. J Neuroinflammation 13(1):143. https://doi.org/10.1186/s12974-016-0612-9
van Meeteren LA, Brinkmann V, Saulnier-Blache JS, Lynch KR, Moolenaar WH (2008) Anticancer activity of FTY720: phosphorylated FTY720 inhibits autotaxin, a metastasis-enhancing and angiogenic lysophospholipase D. Cancer Lett 266(2):203–208. https://doi.org/10.1016/j.canlet.2008.02.052
Castagna D, Budd DC, Macdonald SJ, Jamieson C, Watson AJ (2016) Development of autotaxin inhibitors: an overview of the patent and primary literature. J Med Chem 59(12):5604–5621. https://doi.org/10.1021/acs.jmedchem.5b01599
Desroy N, Housseman C, Bock X, Joncour A, Bienvenu N, Cherel L, Labeguere V, Rondet E et al (2017) Discovery of 2-[[2-ethyl-6-[4-[2-(3-hydroxyazetidin-1-yl)-2-oxoethyl]piperazin-1-yl]-8-methyli midazo[1,2-a]pyridin-3-yl]methylamino]-4-(4-fluorophenyl)thiazole-5-carbonitrile (GLPG1690), a first-in-class autotaxin inhibitor undergoing clinical evaluation for the treatment of idiopathic pulmonary fibrosis. J Med Chem 60(9):3580–3590. https://doi.org/10.1021/acs.jmedchem.7b00032
Fagard L, Desrivot J, Dupont S, Heckmann B, Blanque R, Gheyle L, Ralic J, Vanhoutte F Favorable human safety, pharmacokinetics and pharmacodynamics of the autotaxin inhibitor GLPG1690, a potential new treatment in idiopathic pulmonary fibrosis. In: A103. IPF: MORE ABOUT THERAPY AND OUTCOMES. pp A2701-A2701. https://doi.org/10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A2701
Maher TM, van der Aar EM, Van de Steen O, Allamassey L, Desrivot J, Dupont S, Fagard L, Ford P et al (2018) Safety, tolerability, pharmacokinetics, and pharmacodynamics of GLPG1690, a novel autotaxin inhibitor, to treat idiopathic pulmonary fibrosis (FLORA): a phase 2a randomised placebo-controlled trial. Lancet Respir Med 6:627–635
Salous AK, Panchatcharam M, Sunkara M, Mueller P, Dong A, Wang Y, Graf GA, Smyth SS et al (2013) Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice. J Lipid Res 54(10):2775–2784. https://doi.org/10.1194/jlr.M039685
Navab M, Chattopadhyay A, Hough G, Meriwether D, Fogelman SI, Wagner AC, Grijalva V, Su F et al (2015) Source and role of intestinally derived lysophosphatidic acid in dyslipidemia and atherosclerosis. J Lipid Res 56(4):871–887. https://doi.org/10.1194/jlr.M056614
Katsifa A, Kaffe E, Nikolaidou-Katsaridou N, Economides AN, Newbigging S, McKerlie C, Aidinis V (2015) The bulk of autotaxin activity is dispensable for adult mouse life. PLoS One 10(11):e0143083. https://doi.org/10.1371/journal.pone.0143083
Saga H, Ohhata A, Hayashi A, Katoh M, Maeda T, Mizuno H, Takada Y, Komichi Y et al (2014) A novel highly potent autotaxin/ENPP2 inhibitor produces prolonged decreases in plasma lysophosphatidic acid formation in vivo and regulates urethral tension. PLoS One 9(4):e93230. https://doi.org/10.1371/journal.pone.0093230
Benesch MG, Tang X, Maeda T, Ohhata A, Zhao YY, Kok BP, Dewald J, Hitt M et al (2014) Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice. FASEB J 28(6):2655–2666. https://doi.org/10.1096/fj.13-248641
Aikawa S, Kano K, Inoue A, Aoki J (2017) Proliferation of mouse endometrial stromal cells in culture is highly sensitive to lysophosphatidic acid signaling. Biochem Biophys Res Commun 484(1):202–208. https://doi.org/10.1016/j.bbrc.2016.12.154
Venkatraman G, Benesch MG, Tang X, Dewald J, McMullen TP, Brindley DN (2015) Lysophosphatidate signaling stabilizes Nrf2 and increases the expression of genes involved in drug resistance and oxidative stress responses: implications for cancer treatment. FASEB J 29(3):772–785. https://doi.org/10.1096/fj.14-262659
Ferry G, Moulharat N, Pradere JP, Desos P, Try A, Genton A, Giganti A, Beucher-Gaudin M et al (2008) S32826, a nanomolar inhibitor of autotaxin: discovery, synthesis and applications as a pharmacological tool. J Pharmacol Exp Ther 327(3):809–819. https://doi.org/10.1124/jpet.108.141911
Gupte R, Patil R, Liu J, Wang Y, Lee SC, Fujiwara Y, Fells J, Bolen AL et al (2011) Benzyl and naphthalene methylphosphonic acid inhibitors of autotaxin with anti-invasive and anti-metastatic activity. ChemMedChem 6(5):922–935. https://doi.org/10.1002/cmdc.201000425
Katakai T, Kondo N, Ueda Y, Kinashi T (2014) Autotaxin produced by stromal cells promotes LFA-1-independent and Rho-dependent interstitial T cell motility in the lymph node paracortex. J Immunol 193(2):617–626. https://doi.org/10.4049/jimmunol.1400565
Li S, Wang B, Xu Y, Zhang J (2011) Autotaxin is induced by TSA through HDAC3 and HDAC7 inhibition and antagonizes the TSA-induced cell apoptosis. Mol Cancer 10:18. https://doi.org/10.1186/1476-4598-10-18
Iyer P, Lalane R 3rd, Morris C, Challa P, Vann R, Rao PV (2012) Autotaxin-lysophosphatidic acid axis is a novel molecular target for lowering intraocular pressure. PLoS One 7(8):e42627. https://doi.org/10.1371/journal.pone.0042627
Kato K, Ikeda H, Miyakawa S, Futakawa S, Nonaka Y, Fujiwara M, Okudaira S, Kano K et al (2016) Structural basis for specific inhibition of autotaxin by a DNA aptamer. Nat Struct Mol Biol 23(5):395–401. https://doi.org/10.1038/nsmb.3200
Woodcock JM, Coolen C, Goodwin KL, Baek DJ, Bittman R, Samuel MS, Pitson SM, Lopez AF (2015) Destabilisation of dimeric 14-3-3 proteins as a novel approach to anti-cancer therapeutics. Oncotarget 6(16):14522–14536. https://doi.org/10.18632/oncotarget.3995
Hausmann J, Kamtekar S, Christodoulou E, Day JE, Wu T, Fulkerson Z, Albers HM, van Meeteren LA et al (2011) Structural basis of substrate discrimination and integrin binding by autotaxin. Nat Struct Mol Biol 18(2):198–204. https://doi.org/10.1038/nsmb.1980
Albers HM, Hendrickx LJ, van Tol RJ, Hausmann J, Perrakis A, Ovaa H (2011) Structure-based design of novel boronic acid-based inhibitors of autotaxin. J Med Chem 54(13):4619–4626. https://doi.org/10.1021/jm200310q
Fulkerson Z, Wu T, Sunkara M, Kooi CV, Morris AJ, Smyth SS (2011) Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J Biol Chem 286(40):34654–34663. https://doi.org/10.1074/jbc.M111.276725
Chew WS, Wang W, Herr DR (2016) To fingolimod and beyond: the rich pipeline of drug candidates that target S1P signaling. Pharmacol Res 113(Pt A):521–532. https://doi.org/10.1016/j.phrs.2016.09.025
van Meeteren LA, Ruurs P, Christodoulou E, Goding JW, Takakusa H, Kikuchi K, Perrakis A, Nagano T et al (2005) Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J Biol Chem 280(22):21155–21161. https://doi.org/10.1074/jbc.M413183200
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Herr, D.R., Chew, W.S., Satish, R.L. et al. Pleotropic Roles of Autotaxin in the Nervous System Present Opportunities for the Development of Novel Therapeutics for Neurological Diseases. Mol Neurobiol 57, 372–392 (2020). https://doi.org/10.1007/s12035-019-01719-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01719-1
Keywords
- Autotaxin
- Lysophospholipase D
- Inflammatory neuropathic pain
- Glioblastoma multiforme
- Hemorrhagic hydrocephalus
- Schizophrenia
- Multiple sclerosis
- Alzheimer’s disease
- Metabolic syndrome-induced brain damage
- Traumatic brain injury
- Hepatic encephalopathy induced cerebral edema
- Macular edema
- Major depressive disorder
- Stress-induced psychiatric disorder
- Alcohol-induced brain damage
- HIV-induced brain injury
- Pruritus
- Peripheral nerve injury