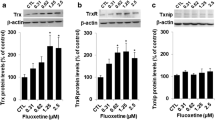
Abstract
Omega-3 polyunsaturated fatty acids (n-3 or omega-3 PUFAs) and melatonin receptor agonist ramelteon (RMT) both display antidepressant effects, while their cellular effects on anti-oxidative and neuroprotective mechanisms might be different. In this study, we aimed to decipher the individual and synergistic actions of n-3 PUFAs and RMT, as compared with the conventional antidepressant fluoxetine (FLX), in a cellular model of oxidative stress, which might play an important role in the pathophysiology of depression and associated disorders. We investigated the rescue and prevention effects of FLX, RMT, and n-3 PUFAs, e.g., eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), by using cell viability in SH-SY5Y cells under oxidative stress along with measurements of key cellular markers of oxidative stress, inflammatory, and neuroprotection. The results revealed that the RMT and EPA combination significantly increased the cell viability in a dose-dependent manner. RMT showed preventive effects, FLX and DHA possessed rescue effects, while EPA showed both rescue and preventive effects. We observed the dose-dependent activation and translocation of nuclear factor-κB to the nucleus augmented by the expressions of peroxisome proliferator activator receptor-gamma, tyrosine hydroxylase, c-Fos expression, and reactive oxygen species, implying that RMT and EPA combination reversed oxidative and neuroinflammatory pathophysiology and protected the neuronal cells from further damage. The results demonstrated that RMT and EPA synergistically provide effective neuroprotective, anti-oxidative/inflammatory effect against oxidative stress. Our study provides pre-clinical evidence to conduct future clinical trials of using n-3 PUFAs/RMT combination in depressive disorders.






Similar content being viewed by others
References
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE et al (2013) Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet (London, England) 382(9904):1575–1586. https://doi.org/10.1016/s0140-6736(13)61611-6
Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE et al (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289(23):3095–3105. https://doi.org/10.1001/jama.289.23.3095
Chung CP, Schmidt D, Stein CM, Morrow JD, Salomon RM (2013) Increased oxidative stress in patients with depression and its relationship to treatment. Psychiatry Res 206(0):213–216. https://doi.org/10.1016/j.psychres.2012.10.018
Caballero-Martinez F, Leon-Vazquez F, Paya-Pardo A, Diaz-Holgado A (2014) Use of health care resources and loss of productivity in patients with depressive disorders seen in Primary Care: INTERDEP Study. Actas espanolas de psiquiatria 42(6):281–291
Puri BK, Counsell SJ, Richardson AJ, Horrobin DF (2002) Eicosapentaenoic acid in treatment-resistant depression. Arch Gen Psychiatry 59(1):91–92
Fava M (2003) Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 53(8):649–659. https://doi.org/10.1016/S0006-3223(03)00231-2
Jones SG, Benca RM (2015) Circadian disruption in psychiatric disorders. Sleep medicine clinics 10(4):481–493. https://doi.org/10.1016/j.jsmc.2015.07.004
Monteleone P, Martiadis V, Maj M (2011) Circadian rhythms and treatment implications in depression. Prog Neuro-Psychopharmacol Biol Psychiatry 35(7):1569–1574. https://doi.org/10.1016/j.pnpbp.2010.07.028
Martynhak BJ, Pereira M, de Souza CP, Andreatini R (2015) Stretch, shrink, and shatter the rhythms: the intrinsic circadian period in mania and depression. CNS & Neurological Disorders Drug Targets 14(8):963–969. https://doi.org/10.2174/1871527314666150909115203
Fava M (2004) Daytime sleepiness and insomnia as correlates of depression. J Clin Psychiatry 65(Suppl 16):27–32
Brown GM, McIntyre RS, Rosenblat J, Hardeland R Depressive disorders: processes leading to neurogeneration and potential novel treatments. Prog Neuro-Psychopharmacol Biol Psychiatry. https://doi.org/10.1016/j.pnpbp.2017.04.023
Cardinali DP, Srinivasan V, Brzezinski A, Brown GM (2012) Melatonin and its analogs in insomnia and depression. J Pineal Res 52(4):365–375. https://doi.org/10.1111/j.1600-079X.2011.00962.x
Goldsmith DR, Rapaport MH, Miller BJ (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21(12):1696–1709. https://doi.org/10.1038/mp.2016.3
Maes M, Fisar Z, Medina M, Scapagnini G, Nowak G, Berk M (2012) New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates—Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology 20(3):127–150. https://doi.org/10.1007/s10787-011-0111-7
Vellante F, Cornelio M, Acciavatti T, Cinosi E, Marini S, Dezi S, De Risio L, Di Iorio G et al (2013) Treatment of resistant insomnia and major depression. La Clinica terapeutica 164(5):429–435. https://doi.org/10.7417/ct.2013.1609
Joshi N, Biswas J, Nath C, Singh S (2015) Promising role of melatonin as Neuroprotectant in neurodegenerative pathology. Mol Neurobiol 52(1):330–340. https://doi.org/10.1007/s12035-014-8865-8
Spadoni G, Bedini A, Lucarini S, Mor M, Rivara S (2015) Pharmacokinetic and pharmacodynamic evaluation of ramelteon: an insomnia therapy. Expert Opin Drug Metab Toxicol 11(7):1145–1156. https://doi.org/10.1517/17425255.2015.1045487
Srinivasan V, Brzezinski A, Pandi-Perumal SR, Spence DW, Cardinali DP, Brown GM (2011) Melatonin agonists in primary insomnia and depression-associated insomnia: are they superior to sedative-hypnotics? Prog Neuro-Psychopharmacol Biol Psychiatry 35(4):913–923. https://doi.org/10.1016/j.pnpbp.2011.03.013
McElroySL, WinstanleyEL, MartensB, PatelNC, MoriN, MoellerD, McCoyJ, KeckPE, Jr. (2011) A randomized, placebo-controlled study of adjunctive ramelteon in ambulatory bipolar I disorder with manic symptoms and sleep disturbance. Int Clin Psychopharmacol26 (1):48–53. doi:https://doi.org/10.1097/YIC.0b013e3283400d35
Parada E, Buendia I, Leon R, Negredo P, Romero A, Cuadrado A, Lopez MG, Egea J (2014) Neuroprotective effect of melatonin against ischemia is partially mediated by alpha-7 nicotinic receptor modulation and HO-1 overexpression. J Pineal Res 56(2):204–212. https://doi.org/10.1111/jpi.12113
Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML (2016) MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu Rev Pharmacol Toxicol 56(1):361–383. https://doi.org/10.1146/annurev-pharmtox-010814-124742
Chen S, Shi L, Liang F, Xu L, Desislava D, Wu Q, Zhang J (2016) Exogenous melatonin for delirium prevention: a meta-analysis of randomized controlled trials. Mol Neurobiol 53(6):4046–4053. https://doi.org/10.1007/s12035-015-9350-8
Spadoni G, Bedini A, Rivara S, Mor M (2011) Melatonin receptor agonists: new options for insomnia and depression treatment. CNS Neurosci Therapeutics 17(6):733–741. https://doi.org/10.1111/j.1755-5949.2010.00197.x
Norris ER, Karen B, Correll JR, Zemanek KJ, Lerman J, Primelo RA, Kaufmann MW (2013) A double-blind, randomized, placebo-controlled trial of adjunctive ramelteon for the treatment of insomnia and mood stability in patients with euthymic bipolar disorder. J Affect Disord 144(1–2):141–147. https://doi.org/10.1016/j.jad.2012.06.023
Su K-P, Matsuoka Y, Pae C-U (2015) Omega-3 polyunsaturated fatty acids in prevention of mood and anxiety disorders. Clin Psychopharmacol Neurosci 13(2):129–137. https://doi.org/10.9758/cpn.2015.13.2.129
AppletonKM, SallisHM, PerryR, NessAR, ChurchillR (2015) Omega-3 fatty acids for depression in adults The Cochrane database of systematic reviews (11):Cd004692. doi:https://doi.org/10.1002/14651858.CD004692.pub4
Su KP, Shen WW, Huang SY (2000) Are omega3 fatty acids beneficial in depression but not mania? Arch Gen Psychiatry 57(7):716–717. https://doi.org/10.1001/archpsyc.57.7.716
Su K-P (2012) Inflammation in psychopathology of depression: clinical, biological, and therapeutic implications. Biomedicine 2(2):68–74. https://doi.org/10.1016/j.biomed.2012.03.002
Su KP, Wang SM, Pae CU (2013) Omega-3 polyunsaturated fatty acids for major depressive disorder. Expert Opin Investig Drugs 22(12):1519–1534. https://doi.org/10.1517/13543784.2013.836487
Song C, Shieh CH, Wu YS, Kalueff A, Gaikwad S, Su KP (2016) The role of omega-3 polyunsaturated fatty acids eicosapentaenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer’s disease: Acting separately or synergistically? Prog Lipid Res 62:41–54. https://doi.org/10.1016/j.plipres.2015.12.003
Huang SY, Yang HT, Chiu CC, Pariante CM, Su KP (2008) Omega-3 fatty acids on the forced-swimming test. J PsychiatrRes 42(1):58–63
Song C, Zhang XY, Manku M (2009) Increased phospholipase A2 activity and inflammatory response but decreased nerve growth factor expression in the olfactory bulbectomized rat model of depression: effects of chronic ethyl-eicosapentaenoate treatment. J Neurosci 29(1):14–22. https://doi.org/10.1523/JNEUROSCI.3569-08.2009
Corsi L, Dongmo BM, Avallone R (2015) Supplementation of omega 3 fatty acids improves oxidative stress in activated BV2 microglial cell line. Int J Food Sci Nutr 66(3):293–299. https://doi.org/10.3109/09637486.2014.986073
Graciano MF, Leonelli M, Curi R, RC A (2016) Omega-3 fatty acids control productions of superoxide and nitrogen oxide and insulin content in INS-1E cells. J Physiol Biochem 72(4):699–710. https://doi.org/10.1007/s13105-016-0509-1
Lu DY, Tsao YY, Leung YM, Su KP (2010) Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology 35(11):2238–2248. https://doi.org/10.1038/npp.2010.98
Carabelli B, Delattre AM, Pudell C, Mori MA, Suchecki D, Machado RB, Venancio DP, Piazzetta SR et al (2015) The antidepressant-like effect of fish oil: Possible role of ventral hippocampal 5-HT1A post-synaptic receptor. Mol Neurobiol 52(1):206–215. https://doi.org/10.1007/s12035-014-8849-8
Su KP (2009) Biological mechanism of antidepressant effect of omega-3 fatty acids: how does fish oil act as a ‘Mind-Body Interface’? Neurosignals 17(2):144–152. https://doi.org/10.1159/000198167
Su KP (2012) Inflammation in psychopathology of depression: clinical, biological, and therapeutic implications. Biomedicine 2(2):68–74. https://doi.org/10.1016/j.biomed.2012.03.002
Lu DY, Leung YM, Su KP (2013) Interferon-alpha induces nitric oxide synthase expression and haem oxygenase-1 down-regulation in microglia: implications of cellular mechanism of IFN-alpha-induced depression. Int J Neuropsychopharmacol 16(2):433–444. https://doi.org/10.1017/S1461145712000338
Pandya CD, Howell KR, Pillai A (2013) Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog Neuro-Psychopharmacol Biol Psychiatry 46:214–223. https://doi.org/10.1016/j.pnpbp.2012.10.017
Su KP, Matsuoka Y, Pae CU (2015) Omega-3 polyunsaturated fatty acids in prevention of mood and anxiety disorders. Clin Psychopharmacol Neurosci : Off Sci Jo Korean Coll Neuropsychopharmacol 13(2):129–137. https://doi.org/10.9758/cpn.2015.13.2.129
Calder PC (2006) n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. The Am J Clin Nutr 83(6 Suppl):1505s–1519s
Scorza FA, Cavalheiro EA, Scorza CA, Galduróz JCF, Tufik S, Andersen ML (2013) Sleep apnea and inflammation—getting a good night’s sleep with omega-3 supplementation. Front Neurol 4:193. https://doi.org/10.3389/fneur.2013.00193
Yehuda S, Rabinovitz-Shenkar S, Carasso RL (2011) Effects of essential fatty acids in iron deficient and sleep-disturbed attention deficit hyperactivity disorder (ADHD) children. Eur J Clin Nutr 65(10):1167–1169. https://doi.org/10.1038/ejcn.2011.80
Huss M, Volp A, Stauss-Grabo M (2010) Supplementation of polyunsaturated fatty acids, magnesium and zinc in children seeking medical advice for attention-deficit/hyperactivity problems—an observational cohort study. Lipids Health Dis 9(1):105. https://doi.org/10.1186/1476-511x-9-105
Lu D-Y, Tsao Y-Y, Leung Y-M, Su K-P (2010) Docosahexaenoic acid suppresses Neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology 35(11):2238–2248. https://doi.org/10.1038/npp.2010.98
Shi Z, Ren H, Huang Z, Peng Y, He B, Yao X, Yuan TF, Su H (2016) Fish oil prevents lipopolysaccharide-induced depressive-like behavior by inhibiting neuroinflammation. Mol Neurobiol 54(9):7327–7334. https://doi.org/10.1007/s12035-016-0212-9
Liu Z, Gan L, Chen Y, Luo D, Zhang Z, Cao W, Zhou Z, Lin X et al (2016) Mark4 promotes oxidative stress and inflammation via binding to PPARγ and activating NF-κB pathway in mice adipocytes. Sci Rep 6(1):21382. https://doi.org/10.1038/srep21382
Wang X, Wang Y, Hu JP, Yu S, Li BK, Cui Y, Ren L, Zhang LD (2017) Astragaloside IV, a natural PPARgamma agonist, reduces Abeta production in Alzheimer’s disease through inhibition of BACE1. Mol Neurobiol 54(4):2939–2949. https://doi.org/10.1007/s12035-016-9874-6
Wu JS, Tsai HD, Cheung WM, Hsu CY, Lin TN (2016) PPAR-gamma ameliorates neuronal apoptosis and ischemic brain injury via suppressing NF-kappaB-driven p22phox transcription. Mol Neurobiol 53(6):3626–3645. https://doi.org/10.1007/s12035-015-9294-z
Ye J, Han Y, Chen X, Xie J, Liu X, Qiao S, Wang C (2014) l-carnitine attenuates H2O2-induced neuron apoptosis via inhibition of endoplasmic reticulum stress. Neurochem Int 78:86–95. https://doi.org/10.1016/j.neuint.2014.08.009
Sun X, Min D, Wang Y, Hao L (2015) Potassium aspartate inhibits SH-SY5Y cell damage and apoptosis induced by ouabain and H2O2. Mol Med Rep 12(2):2842–2848. https://doi.org/10.3892/mmr.2015.3741
Luchtman DW, Meng Q, Wang X, Shao D, Song C (2013) Omega-3 fatty acid eicosapentaenoic acid attenuates MPP+-induced neurodegeneration in fully differentiated human SH-SY5Y and primary mesencephalic cells. J Neurochem 124(6):855–868. https://doi.org/10.1111/jnc.12068
Bartl J, Walitza S, Grünblatt E (2014) Enhancement of cell viability after treatment with polyunsaturated fatty acids. Neurosci Lett 559:56–60. https://doi.org/10.1016/j.neulet.2013.11.023
Rovito D, Giordano C, Plastina P, Barone I, De Amicis F, Mauro L, Rizza P, Lanzino M et al (2015) Omega-3 DHA- and EPA–dopamine conjugates induce PPARγ-dependent breast cancer cell death through autophagy and apoptosis. Biochim Biophys Acta Gen Subj 1850(11):2185–2195. https://doi.org/10.1016/j.bbagen.2015.08.004
Zhang Y-P, Brown RE, Zhang P-C, Zhao Y-T, Ju X-H, Song C (2017) DHA, EPA and their combination at various ratios differently modulated Aβ25-35-induced neurotoxicity in SH-SY5Y cells. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA). https://doi.org/10.1016/j.plefa.2017.07.003
Rescigno T, Capasso A, Tecce MF (2016) Effect of docosahexaenoic acid on cell cycle pathways in breast cell lines with different transformation degree. J Cell Physiol 231(6):1226–1236. https://doi.org/10.1002/jcp.25217
Hampel U, Krüger M, Kunnen C, Garreis F, Willcox M, Paulsen F (2015) In vitro effects of docosahexaenoic and eicosapentaenoic acid on human meibomian gland epithelial cells. Exp Eye Res 140:139–148. https://doi.org/10.1016/j.exer.2015.08.024
Horrocks LA, Farooqui AA (2004) Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fat Acids 70(4):361–372. https://doi.org/10.1016/j.plefa.2003.12.011
Tan JW, Ho CF, Ng YK, Ong WY (2016) Docosahexaenoic acid and L-Carnitine prevent ATP loss in SH-SY5Y neuroblastoma cells after exposure to silver nanoparticles. Environ Toxicol 31(2):224–232. https://doi.org/10.1002/tox.22037
Delattre AM, Carabelli B, Mori MA, Kempe PG, Rizzo de Souza LE, Zanata SM, Machado RB, Suchecki D et al (2017) Maternal omega-3 supplement improves dopaminergic system in pre- and postnatal inflammation-induced neurotoxicity in Parkinson’s disease model. Mol Neurobiol 54(3):2090–2106. https://doi.org/10.1007/s12035-016-9803-8
Rahmani A, Kheradmand D, Keyhanvar P, Shoae-Hassani A, Darbandi-Azar A (2013) Neurogenesis and increase in differentiated neural cell survival via phosphorylation of Akt1 after fluoxetine treatment of stem cells. Biomed Res Int 2013:582526. https://doi.org/10.1155/2013/582526
Schaz U, Fohr KJ, Liebau S, Fulda S, Koelch M, Fegert JM, Boeckers TM, Ludolph AG (2011) Dose-dependent modulation of apoptotic processes by fluoxetine in maturing neuronal cells: an in vitro study. World J Biol Psychiatry : Off J World Fed Soc Biol Psychiatry 12(2):89–98. https://doi.org/10.3109/15622975.2010.506927
Song JD, Lee SK, Kim KM, Kim JW, Kim JM, Yoo YH, Park YC (2008) Redox factor-1 mediates NF-κB nuclear translocation for LPS-induced iNOS expression in murine macrophage cell line RAW 264.7. Immunology 124(1):58–67. https://doi.org/10.1111/j.1365-2567.2007.02736.x
CharneyDS, ManjiHK (2004) Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Science's STKE : signal transduction knowledge environment 2004 (225):re5. doi:https://doi.org/10.1126/stke.2252004re5
Li B, Zhang S, Zhang H, Nu W, Cai L, Hertz L, Peng L (2008) Fluoxetine-mediated 5-HT2B receptor stimulation in astrocytes causes EGF receptor transactivation and ERK phosphorylation. Psychopharmacology 201(3):443–458. https://doi.org/10.1007/s00213-008-1306-5
Mercier G, Lennon AM, Renouf B, Dessouroux A, Ramauge M, Courtin F, Pierre M (2004) MAP kinase activation by fluoxetine and its relation to gene expression in cultured rat astrocytes. J Mol Neurosci : MN 24(2):207–216. https://doi.org/10.1385/jmn:24:2:207
Draper E, Reynolds CM, Canavan M, Mills KH, Loscher CE, Roche HM (2011) Omega-3 fatty acids attenuate dendritic cell function via NF-κB independent of PPARγ. J Nutr Biochem 22(8):784–790. https://doi.org/10.1016/j.jnutbio.2010.06.009
Hu X-L, Niu Y-X, Zhang Q, Tian X, Gao L-Y, Guo L-P, Meng W-H, Zhao Q-C (2015) Neuroprotective effects of Kukoamine B against hydrogen peroxide-induced apoptosis and potential mechanisms in SH-SY5Y cells. Environ Toxicol Pharmacol 40(1):230–240. https://doi.org/10.1016/j.etap.2015.06.017
Sullivan-Gunn MJ, Lewandowski PA (2013) Elevated hydrogen peroxide and decreased catalase and glutathione peroxidase protection are associated with aging sarcopenia. BMC Geriatr 13(1):104. https://doi.org/10.1186/1471-2318-13-104
Sompol P, Xu Y, Ittarat W, Daosukho C, St Clair D (2006) NF-kappaB-associated MnSOD induction protects against beta-amyloid-induced neuronal apoptosis. J Mol Neurosci : MN 29(3):279–288. https://doi.org/10.1385/JMN:29:3:279
Snow WM, Stoesz BM, Kelly DM, Albensi BC (2014) Roles for NF-kappaB and gene targets of NF-kappaB in synaptic plasticity, memory, and navigation. Mol Neurobiol 49(2):757–770. https://doi.org/10.1007/s12035-013-8555-y
Fenton WS, Hibbeln J, Knable M (2000) Essential fatty acids, lipid membrane abnormalities, and the diagnosis and treatment of schizophrenia. Biol Psychiatry 47(1):8–21
Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP (2003) Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot Essent Fat Acids 69(6):393–399. https://doi.org/10.1016/j.plefa.2003.08.010
Im DS (2012) Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog Lipid Res 51(3):232–237. https://doi.org/10.1016/j.plipres.2012.02.003
Mathes AM, Kubulus D, Waibel L, Weiler J, Heymann P, Wolf B, Rensing H (2008) Selective activation of melatonin receptors with ramelteon improves liver function and hepatic perfusion after hemorrhagic shock in rat. Crit Care Med 36(10):2863–2870. https://doi.org/10.1097/CCM.0b013e318187b863
Reale M, Pesce M, Priyadarshini M, Kamal MA, Patruno A (2012) Mitochondria as an easy target to oxidative stress events in Parkinson’s disease. CNS & Neurol Disord Drug Targets 11(4):430–438. https://doi.org/10.2174/187152712800792875
Kaewsuk S, Sae-ung K, Phansuwan-Pujito P, Govitrapong P (2009) Melatonin attenuates methamphetamine-induced reduction of tyrosine hydroxylase, synaptophysin and growth-associated protein-43 levels in the neonatal rat brain. Neurochem Int 55(6):397–405. https://doi.org/10.1016/j.neuint.2009.04.010
Ghaffari H, Venkataramana M, Jalali Ghassam B, Chandra Nayaka S, Nataraju A, Geetha NP, Prakash HS (2014) Rosmarinic acid mediated neuroprotective effects against H2O2-induced neuronal cell damage in N2A cells. Life Sci 113(1–2):7–13. https://doi.org/10.1016/j.lfs.2014.07.010
Yabuki Y, Takahata I, Matsuo K, Owada Y, Fukunaga K (2017) Ramelteon improves post-traumatic stress disorder-like behaviors exhibited by fatty acid-binding protein 3 null mice. https://doi.org/10.1007/s12035-017-0587-2
Fraga IC, Fregoneze JB, Carvalho FL, Dantas KB, Azevedo CS, Pinho CB, de Castro ESE (2005) Acute fluoxetine administration differentially affects brain C-Fos expression in fasted and refed rats. Neuroscience 134(1):327–334. https://doi.org/10.1016/j.neuroscience.2005.03.010
Torres G, Horowitz JM, Laflamme N, Rivest S (1998) Fluoxetine induces the transcription of genes encoding c-fos, corticotropin-releasing factor and its type 1 receptor in rat brain. Neuroscience 87(2):463–477. https://doi.org/10.1016/S0306-4522(98)00147-X
Shang T, Liu L, Zhou J, Zhang M, Hu Q, Fang M, Wu Y, Yao P et al (2017) Protective effects of various ratios of DHA/EPA supplementation on high-fat diet-induced liver damage in mice. Lipids Health Dis 16(1):65. https://doi.org/10.1186/s12944-017-0461-2
Nakanishi A, Tsukamoto I (2015) n-3 polyunsaturated fatty acids stimulate osteoclastogenesis through PPARgamma-mediated enhancement of c-Fos expression, and suppress osteoclastogenesis through PPARgamma-dependent inhibition of NFkB activation. J Nutr Biochem 26(11):1317–1327. https://doi.org/10.1016/j.jnutbio.2015.06.007
BorsiniA, AlboniS, HorowitzMA, TojoLM, CannazzaG, SuK-P, ParianteCM, ZunszainPA (2017) Rescue of IL-1β-induced reduction of human neurogenesis by omega-3 fatty acids and antidepressants. Brain, Behavior, and Immunity 65 (Supplement C):230-238. doi: https://doi.org/10.1016/j.bbi.2017.05.006
Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ (2003) A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry 160(5):996–998. https://doi.org/10.1176/appi.ajp.160.5.996
Mischoulon D, Best-Popescu C, Laposata M, Merens W, Murakami JL, Wu SL, Papakostas GI, Dording CM et al (2008) A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur Neuropsychopharmacol 18(9):639–645. https://doi.org/10.1016/j.euroneuro.2008.04.011
Mocking RJ, Harmsen I, Assies J, Koeter MW, Ruhe HG, Schene AH (2016) Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl Psychiatry 6(3):e756. https://doi.org/10.1038/tp.2016.29
Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, Drago F, Caraci F (2014) Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One 9(5):e96905. https://doi.org/10.1371/journal.pone.0096905
Funding
The work was supported by the China Medical University under the Aim for Top University Plan of the Ministry of Education, Taiwan, and by the following Grants: MOST 106-2314-B-038-049; MOST 106-2314-B-039-027-MY3; 106-2314-B-038-049; 106-2314-B-039-031; 106-2314-B-039-035; 104-2314-B-039-022-MY2, and 104-2314-B-039-050-MY3 from the Ministry of Science and Technology, Taiwan; NHRI-EX105-10528NI from the National Health Research Institutes, Taiwan; and CMU104-S-1603 and CMU104-S44 from the China Medical University, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Siegfried Kasper received grants/research support, consulting fees, and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, AstraZeneca, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, and Servier. The other authors declare no potential conflict of interest.
Electronic Supplementary Material
Supplementary Fig. 1
Cell viability as detected by the MTT assay in SH-SY5Y neuronal cells with increasing H2O2, ramelteon (RMT), fluoxetine (FLX) and n-3 PUFAs (EPA & DHA) doses for 24h. Data were normalized to control values that were taken as 100% of cell viability. The arrow indicates the optimal concentration chosen for further experiments. Error bars indicate the standard error. (JPEG 113kb)
Supplementary Fig. 2
(a-l): Quantitative analysis of the effects of ramelteon (RMT), fluoxetine (FLX), n-3 PUFAs (EPA & DHA) and their combination under oxidative stress (H2O2) on c-Fos, tyrosine hydroxylase (TH), proliferators-activator receptor-gamma (PPAR-γ), catalase, superoxide dismutase (SOD1 and SOD2) protein markers. Protein band intensities, normalized to GAPDH. Histogram of densitometry data showing the individual and synergistic effects of RMT, FLX, EPA and DHA treatment; rescue effect (a, c, e, g, i, k) and prevention effect (b, d, f, h, j, l). The values presented are the means ± SEM of three independent experiments, *p<0.05 or **p<0.01 or ***p<0.001, versus H2O2 treatment group, ##p<0.01 or ### p<0.001 versus control group. (JPEG 604kb)
ESM 1
(JPEG 677kb)
Rights and permissions
About this article
Cite this article
Satyanarayanan, S.K., Shih, YH., Chien, YC. et al. Anti-Oxidative Effects of Melatonin Receptor Agonist and Omega-3 Polyunsaturated Fatty Acids in Neuronal SH-SY5Y Cells: Deciphering Synergic Effects on Anti-Depressant Mechanisms. Mol Neurobiol 55, 7271–7284 (2018). https://doi.org/10.1007/s12035-018-0899-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0899-x