Abstract
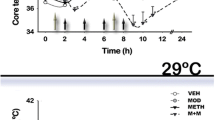
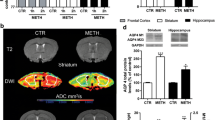
In this study, we examined the influence of cold and hot environments on methamphetamine (METH) neurotoxicity in both drug-naive rats and animals previously exposed to different types of nanoparticles (NPs). Since METH induces oxidative stress, we also examined how a potential chain-breaking antioxidant H-290/51 (Astra-Zeneca, Mölndal, Sweden) affects METH-induced neurotoxicity. Exposure of drug-naive rats to METH (9 mg/kg, s.c.) at 4, 21, or 34 °C for 3 h resulted in breakdown of the blood-brain barrier (BBB), brain edema, and neuronal injuries, which all differed in severity depending upon ambient temperatures. The changes were moderate at 21 °C, 120–180 % larger at 34 °C, and almost absent at 4 °C. In rats chronically treated with NPs (SiO2, Cu, or Ag; 50–60 nm, 50 mg/kg, i.p. for 7 days), METH-induced brain alterations showed a two- to fourfold increase at 21 °C, a four- to sixfold increase at 34 °C, and three- to fourfold increase at 4 °C. SiO2 exposure showed the most pronounced METH-induced brain pathology at all temperatures followed by Ag and Cu NPs. Pretreatment with a potent antioxidant compound H-290/51 (50 mg/kg, p.o., 30 min before METH) significantly reduced brain pathology in naive animals exposed to METH at 21 and 34 °C. In NPs-treated animals, however, attenuation of METH-induced brain pathology occurred only after repeated exposure of H-290/51 (−30 min, 0 min, and +30 min). These observations are the first to show that NPs exacerbate METH-induced brain pathology in both cold and hot environments and demonstrate that timely intervention with antioxidant H-290/51 could have neuroprotective effects.

Similar content being viewed by others
References
Ellinwood EH, Cohen S (1971) Amphetamine abuse. Science 171:420–421
Marquine MJ, Iudicello JE, Morgan EE, Brown GG, Letendre SL, Ellis RJ, Deutsch R, Woods SP et al (2014) “Frontal systems” behaviors in comorbid human immunodeficiency virus infection and methamphetamine dependency. Psychiatry Res 215:208–216
Lecomte T, Mueser KT, MacEwan W, Thornton AE, Buchanan T, Bouchard V, Goldner E, Brink J et al (2013) Predictors of persistent psychotic symptoms in persons with methamphetamine abuse receiving psychiatric treatment. J Nerv Ment Dis 201:1085–1089
Fletcher JB, Reback CJ (2013) Antisocial personality disorder predicts methamphetamine treatment outcomes in homeless, substance-dependent men who have sex with men. J Subst Abus Treat 45:266–272
Embry D, Hankins M, Biglan A, Boles S (2009) Behavioral and social correlates of methamphetamine use in a population-based sample of early and later adolescents. Addict Behav 34:343–351
Iritani BJ, Hallfors DD, Bauer DJ (2007) Crystal methamphetamine use among young adults in the USA. Addiction 102:1102–1113
Jones HE, Myers B, O’Grady KE, Gebhardt S, Theron GB, Wechsberg WM (2014) Initial feasibility and acceptability of a comprehensive intervention for methamphetamine-using pregnant women in South Africa. Psychiatr J 2014:929767. doi:10.1155/2014/929767
Werb D, Kerr T, Buxton J, Shoveller J, Richardson C, Montaner J, Wood E (2013) Crystal methamphetamine and initiation of injection drug use among street-involved youth in a Canadian setting. CMAJ 185:1569–1575
Shariatirad S, Maarefvand M, Ekhtiari H (2013) Emergence of a methamphetamine crisis in Iran. Drug Alcohol Rev 32:223–224
Li L, Assanangkornchai S, Duo L, McNeil E, Li J (2014) Cross-border activities and association with current methamphetamine use among Chinese injection drug users (IDUs) in a China-Myanmar border region. Drug Alcohol Depend 138:48–53
Shukla RK, Crump JL, Chrisco ES (2012) An evolving problem: methamphetamine production and trafficking in the United States. Int J Drug Policy 23:426–435
Verdichevski M, Burns R, Cunningham JK, Tavares J, Callaghan RC (2011) Trends in primary methamphetamine-related admissions to youth residential substance abuse treatment facilities in Canada, 2005–2006 and 2009–2010. Can J Psychiatry 56:696–700
Forcehimes AA, Venner KL, Bogenschutz MP, Foley K, Davis MP, Houck JM, Willie EL, Begaye P (2011) American Indian methamphetamine and other drug use in the southwestern United States. Cult Divers Ethn Minor Psychol 17:366–376
Platteborze PL, Kippenberger DJ, Martin TM (2013) Drug positive rates for the army, army reserve, and army national guard from fiscal year 2001 through 2011. Mil Med 10:1078–1084
Lacy BW, Ditzler TF, Wilson RS, Martin TM, Ochikubo JT, Roussel RR, Pizarro-Matos JM, Vazquez R (2008) Regional methamphetamine use among U.S. Army personnel stationed in the continental United States and Hawaii: a six-year retrospective study (2000–2005). Mil Med 173:353–358
Defalque RJ, Wright AJ (2011) Methamphetamine for Hitler’s Germany: 1937 to 1945. Bull Anesth Hist 29(21-4):32
Patnaik R, Sharma A, Kiyatkin EA, Nozari A, Sharma HS (2013) Nanoparticles exacerbate methamphetamine neurotoxicity in both hot and cold environment. Neuroprotective effects of an antioxidant compound H-290/51. Soc Neurosci Abstr 43:813.27/L18
Sharma HS, Muresanu DF, Patnaik R, Sharma A (2013) Exacerbation of brain pathology after partial restraint in hypertensive rats following SiO2 nanoparticles exposure at high ambient temperature. Mol Neurobiol 48:368–379
Sharma HS, Muresanu DF, Sharma A, Patnaik R, Lafuente JV (2009) Nanoparticles influence pathophysiology of spinal cord injury and repair. Prog Brain Res 180:154–180
Sharma HS, Ali SF, Tian ZR, Hussain SM, Schlager JJ, Sjöquist PO, Sharma A, Muresanu DF (2009) Chronic treatment with nanoparticles exacerbate hyperthermia induced blood-brain barrier breakdown, cognitive dysfunction and brain pathology in the rat. Neuroprotective effects of nanowired antioxidant compound H-290/51. J Nanosci Nanotechnol 9:5073–5090
Kiyatkin EA, Brown PL, Sharma HS (2007) Brain edema and breakdown of the blood-brain barrier during methamphetamine intoxication: critical role of brain hyperthermia. Eur J Neurosci 26:1242–1253
Sharma HS, Kiyatkin EA (2009) Rapid morphological brain abnormalities during acute methamphetamine intoxication in the rat: an experimental study using light and electron microscopy. J Chem Neuroanat 37:18–32
Kiyatkin EA, Sharma HS (2009) Acute methamphetamine intoxication: brain hyperthermia, blood-brain barrier, brain edema, and morphological cell abnormalities. Int Rev Neurobiol 88:65–100
Toborek M, Seelbach MJ, Rashid CS, András IE, Chen L, Park M, Esser KA (2013) Voluntary exercise protects against methamphetamine-induced oxidative stress in brain microvasculature and disruption of the blood-brain barrier. Mol Neurodegener 8:22. doi:10.1186/1750-1326-8-22
Solhi H, Malekirad A, Kazemifar AM, Sharifi F (2014) Oxidative stress and lipid peroxidation in prolonged users of methamphetamine. Drug Metab Lett 7:79–82
Patlolla AK, Hackett D, Tchounwou PB (2014) Silver nanoparticle-induced oxidative stress-dependent toxicity in Sprague-Dawley rats. Mol Cell Biochem 399:257–268
Han JW, Gurunathan S, Jeong JK, Choi YJ, Kwon DN, Park JK, Kim JH (2014) Oxidative stress mediated cytotoxicity of biologically synthesized silver nanoparticles in human lung epithelial adenocarcinoma cell line. Nanoscale Res Lett 9(1):459. doi:10.1186/1556-276X-9-459. eCollection 2014
Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) 8th edn. The National Academic Press, Washington DC. http://www.nap.edu
Sharma HS, Ali SF, Hussain SM, Schlager JJ, Sharma A (2009) Influence of engineered nanoparticles from metals on the blood-brain barrier permeability, cerebral blood flow, brain edema and neurotoxicity. An experimental study in the rat and mice using biochemical and morphological approaches. J Nanosci Nanotechnol 9:5055–5072
Sharma HS, Sharma A (2007) Nanoparticles aggravate heat stress induced cognitive deficits, blood-brain barrier disruption, edema formation and brain pathology. Prog Brain Res 162:245–273
Elliott KA, Jasper HH (1949) Measurement of experimentally induced brain swelling and shrinkage. Am J Physiol 157:122–129
Sharma HS, Sjöquist P-O (2002) A new antioxidant compound H-290/51 modulates glutamate and GABA immunoreactivity in the rat spinal cord following trauma. Amino Acids 23:261–272
Sharma HS, Olsson Y, Cervós-Navarro J (1993) p-Chlorophenylalanine, a serotonin synthesis inhibitor, reduces the response of glial fibrillary acidic protein induced by trauma to the spinal cord. Acta Neuropathol (Berlin) 86:422–427
Sharma HS, Zimmer C, Westman J, Cervós-Navarro J (1992) Acute systemic heat stress increases glial fibrillary acidic protein immunoreactivity in brain. An experimental study in the conscious normotensive young rats. Neuroscience 48:889–901
Zimmer C, Sampaolo S, Sharma HS, Cervós-Navarro J (1991) Glial fibrillary acidic protein immunoreactivity in rat brain following chronic hypoxia. Neuroscience 40:353–361
Mustafa A, Sharma HS, Olsson Y, Gordh T, Thoren P, Sjoquist PO, Roos P, Adem A et al (1995) Vascular permeability to growth hormone in the rat central nervous system after focal spinal cord injury. Influence of a new anti-oxidant H 290/51 and age. Neurosci Res 23:185–194
Sharma HS, Dey PK (1987) Influence of long-term acute heat exposure on regional blood-brain barrier permeability, cerebral blood flow and 5-HT level in conscious normotensive young rats. Brain Res 424:153–162
Sharma HS, Dey PK (1986) Influence of long-term immobilization stress on regional blood-brain barrier permeability, cerebral blood flow and 5-HT level in conscious normotensive young rats. J Neurol Sci 72:61–76
Sharma HS, Dey PK (1986) Probable involvement of 5-hydroxytryptamine in increased permeability of blood-brain barrier under heat stress. Neuropharmacology 25:161–167
Sharma HS, Ali SF (2006) Alterations in blood-brain barrier function by morphine and methamphetamine. Ann N Y Acad Sci 1074:198–224
Sharma HS, Sjöquist PO, Ali SF (2007) Drugs of abuse-induced hyperthermia, blood-brain barrier dysfunction and neurotoxicity: neuroprotective effects of a new antioxidant compound H-290/51. Curr Pharm Des 13:1903–1923
Sharma HS, Menon P, Lafuente JV, Muresanu DF, Tian ZR, Patnaik R, Sharma A (2014) Development of in vivo drug-induced neurotoxicity models. Expert Opin Drug Metab Toxicol 13:1–25
Kiyatkin EA, Sharma HS (2012) Environmental conditions modulate neurotoxic effects of psychomotor stimulant drugs of abuse. Int Rev Neurobiol 102:147–171
Kiyatkin EA, Sharma HS (2009) Permeability of the blood-brain barrier depends on brain temperature. Neuroscience 161:926–939
Sharma HS, Cervós-Navarro J, Dey PK (1991) Increased blood-brain barrier permeability following acute short-term forced-swimming exercise in conscious normotensive young rats. Neurosci Res 10:211–221
Sharma HS, Westman J, Cervós-Navarro J, Dey PK, Nyberg F (1995) Probable involvement of serotonin in the increased permeability of the blood-brain barrier by forced swimming. An experimental study using Evans blue and 131I-sodium tracers in the rat. Behav Brain Res 72:189–196
Sharma A, Muresanu DF, Patnaik R, Sharma HS (2013) Size- and age-dependent neurotoxicity of engineered metal nanoparticles in rats. Mol Neurobiol 48:386–396
Sharma HS, Sharma A (2012) Nanowired drug delivery for neuroprotection in central nervous system injuries: modulation by environmental temperature, intoxication of nanoparticles, and comorbidity factors. Nanomed Nanobiotechnol 4:184–203
Sharma HS, Sharma A (2013) New perspectives of nanoneuroprotection, nanoneuropharmacology and nanoneurotoxicity: modulatory role of amino acid neurotransmitters, stress, trauma, and co-morbidity factors in nanomedicine. Amino Acids 45:1055–1071
Wang XZ, Yang Y, Li R, McGuinnes C, Adamson J, Megson IL, Donaldson K (2014) Principal component and causal analysis of structural and acute in vitro toxicity data for nanoparticles. Nanotoxicology 8:465–476
Sharma HS, Patnaik R, Sharma A, Sjöquist PO, Lafuente JV (2009) Silicon dioxide nanoparticles (SiO2, 40–50 nm) exacerbate pathophysiology of traumatic spinal cord injury and deteriorate functional outcome in the rat. An experimental study using pharmacological and morphological approaches. J Nanosci Nanotechnol 9:4970–4980
Sharma HS, Sharma A (2012) Neurotoxicity of engineered nanoparticles from metals. CNS Neurol Disord Drug Targets 11:65–80
Sharma A, Muresanu DF, Mössler H, Sharma HS (2012) Superior neuroprotective effects of cerebrolysin in nanoparticle-induced exacerbation of hyperthermia-induced brain pathology. CNS Neurol Disord Drug Targets 11:7–25
Sharma HS (2011) Early microvascular reactions and blood-spinal cord barrier disruption are instrumental in pathophysiology of spinal cord injury and repair: novel therapeutic strategies including nanowired drug delivery to enhance neuroprotection. J Neural Transm 118:155–176
Tian ZR, Sharma A, Nozari A, Subramaniam R, Lundstedt T, Sharma HS (2012) Nanowired drug delivery to enhance neuroprotection in spinal cord injury. CNS Neurol Disord Drug Targets 11:86–95
Muresanu DF, Sharma A, Tian ZR, Smith MA, Sharma HS (2012) Nanowired drug delivery of antioxidant compound H-290/51 enhances neuroprotection in hyperthermia-induced neurotoxicity. CNS Neurol Disord Drug Targets 11:50–64
Sharma A, Muresanu DF, Lafuente JV, Menon P, Patnaik R, Tian ZR, Mössler H, Sharma HS (2014) Nanodelivery of cerebrolysin as adjunct therapy with functionalized magnetic iron oxide nanoparticles enhances neuroprotection following whole body hyperthermia. In Proceedings National Innovations Summit & Showcase, vol. 2. National Harbor, Washington DC, June 15-18, 2014, Technical Innovation Nr. 613. Advanced Materials & Applications, NSTI-Nanotech 2014. www.nsti.org. ISBN 978-1-4822-5827-1, pp 363–366
Ruozi B, Belletti D, Forni F, Sharma A, Muresanu D, Mössler H, Vandelli MA, Tosi G et al (2014) Poly (D, L-lactide-co-glycolide) nanoparticles loaded with cerebrolysin display neuroprotective activity in a rat model of concussive head injury. CNS Neurol Disord Drug Targets 13:1475–1482
Acknowledgments
This investigation is supported by grants from the Air Force Office of Scientific Research (EOARD, London, UK) and Air Force Material Command, USAF, under grant number FA8655-05-1-3065; Swedish Medical Research Council (Nr 2710-HSS), Swedish Strategic Research Foundation, Stockholm, Sweden; Göran Gustafsson Foundation, Stockholm, Sweden (HSS), Astra-Zeneca, Mölndal, Sweden (HSS/AS), The University Grants Commission, New Delhi, India (HSS/AS), Ministry of Science and Technology, Gov’t. of India and Gov’t. of Sweden (HSS/AS), Indian Medical Research Council, New Delhi, India (HSS/AS); India-EU Research Co-operation Program (RP/AS/HSS) and IT 794/13 (JVL), Government of Basque Country and UFI 11/32 (JVL); University of Basque Country, Spain. This study also is supported in part by the National Institute on Drug Abuse—Intramural Research Program, NIH (EAK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, H.S., Kiyatkin, E.A., Patnaik, R. et al. Exacerbation of Methamphetamine Neurotoxicity in Cold and Hot Environments: Neuroprotective Effects of an Antioxidant Compound H-290/51. Mol Neurobiol 52, 1023–1033 (2015). https://doi.org/10.1007/s12035-015-9252-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9252-9