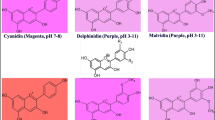
Abstract
The carotenoid pathway in plants has been altered through metabolic engineering to enhance their nutritional value and generate keto-carotenoids, which are widely sought after in the food, feed, and human health industries. In this study, the aim was to produce keto-carotenoids by manipulating the native carotenoid pathway in tobacco plants through chloroplast engineering. Transplastomic tobacco plants were generated that express a synthetic multigene operon composed of three heterologous genes, with Intercistronic Expression Elements (IEEs) for effective mRNA splicing. The metabolic changes observed in the transplastomic plants showed a significant shift towards the xanthophyll cycle, with only a minor production of keto-lutein. The use of a ketolase gene in combination with the lycopene cyclase and hydroxylase genes was a novel approach and demonstrated a successful redirection of the carotenoid pathway towards the xanthophyll cycle and the production of keto-lutein. This study presents a scalable molecular genetic platform for the development of novel keto-carotenoids in tobacco using the Design–Build–Test–Learn (DBTL) approach.
Graphical Abstract
This study corroborates chloroplast metabolic engineering using a synthetic biology approach for producing novel metabolites belonging to carotenoid class in industrially important tobacco plant. The synthetic multigene construct resulted in producing a novel metabolite, keto-lutein with high accumulation of xanthophyll metabolites. This figure was drawn using BioRender (https://www.biorender.com).





Similar content being viewed by others
Data availability
Datasets generated from this study are provided in the main article and as supplemental files.
Abbreviations
- IEE:
-
Intercistronic expression element
- aadA:
-
Spectinomycin
- CrtW/bkt:
-
β-Carotene ketolase
- CrtZ/bhy:
-
β-Carotene hydroxylase
- lcy:
-
β-Lycopene cyclase
- CrtE:
-
GGPP synthase
- CrtB:
-
Phytoene synthase
- CrtI:
-
Phytoene synthase
- CrtY:
-
Lycopene cyclase
- LB:
-
Luria–Bertani media
- SBMSN:
-
Super broth with ammonium and sucrose medium
- 2YT:
-
Yeast extract and tryptone media
- TB:
-
Tryptone broth
- MS:
-
Murashige and Skoog medium
References
Ruiz-Sola, M. Á., & Rodríguez-Concepción, M. (2012). Carotenoid biosynthesis in Arabidopsis: A colorful pathway. Arabidopsis Book, 10, e0158.
Mann, V., Harker, M., Pecker, I., & Hirschberg, J. (2000). Metabolic engineering of astaxanthin production in tobacco flowers. Nature Biotechnology, 18(8), 888–892.
Kim, S. H., Ahn, Y. O., Ahn, M.-J., Lee, H.-S., & Kwak, S.-S. (2012). Down-regulation of β-carotene hydroxylase increases β-carotene and total carotenoids enhancing salt stress tolerance in transgenic cultured cells of sweet potato. Phytochemistry, 74, 69–78.
Swapnil, P., Meena, M., Singh, S. K., Dhuldhaj, U. P., Harish, & Marwal, A. (2021). Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Current Plant Biology, 26, 100203.
Aziz, E., Batool, R., Akhtar, W., Rehman, S., Shahzad, T., Malik, A., Shariati, M. A., Laishevtcev, A., Plygun, S., Heydari, M., Rauf, A., & Ahmed Arif, S. (2020). Xanthophyll: Health benefits and therapeutic insights. Life Sciences, 240, 117104.
Naguib, Y. M. (2000). Antioxidant activities of astaxanthin and related carotenoids. Journal of Agricultural and Food Chemistry, 48(4), 1150–1154.
Misawa, N. (2009). Pathway engineering of plants toward astaxanthin production. Plant Biotechnology, 26(1), 93–99.
Jin, S., & Daniell, H. (2015). The engineered chloroplast genome just got smarter. Trends in Plant Science, 20(10), 622–640.
Llorente, B., Torres-Montilla, S., Morelli, L., Florez-Sarasa, I., Matus, J. T., Ezquerro, M., D’andrea, L., Houhou, F., Majer, E., & Picó, B. (2020). Synthetic conversion of leaf chloroplasts into carotenoid-rich plastids reveals mechanistic basis of natural chromoplast development. Proceedings of the National Academy of Sciences of USA, 117(35), 21796–21803.
Hasunuma, T., Miyazawa, S. I., Yoshimura, S., Shinzaki, Y., Tomizawa, K. I., Shindo, K., Choi, S. K., Misawa, N., & Miyake, C. (2008). Biosynthesis of astaxanthin in tobacco leaves by transplastomic engineering. The Plant Journal, 55(5), 857–868.
Harada, H., Maoka, T., Osawa, A., Hattan, J.-I., Kanamoto, H., Shindo, K., Otomatsu, T., & Misawa, N. (2014). Construction of transplastomic lettuce (Lactuca sativa) dominantly producing astaxanthin fatty acid esters and detailed chemical analysis of generated carotenoids. Transgenic Research, 23(2), 303–315.
Jayaraj, J., Devlin, R., & Punja, Z. (2008). Metabolic engineering of novel ketocarotenoid production in carrot plants. Transgenic Research, 17(4), 489–501.
Lu, Y., Rijzaani, H., Karcher, D., Ruf, S., & Bock, R. (2013). Efficient metabolic pathway engineering in transgenic tobacco and tomato plastids with synthetic multigene operons. Proceedings of the National Academy of Sciences of USA, 110(8), E623–E632.
Zhou, F., Karcher, D., & Bock, R. (2007). Identification of a plastid intercistronic expression element (IEE) facilitating the expression of stable translatable monocistronic mRNAs from operons. The Plant Journal, 52(5), 961–972.
Apel, W., & Bock, R. (2009). Enhancement of carotenoid biosynthesis in transplastomic tomatoes by induced lycopene-to-provitamin A conversion. Plant Physiology, 151(1), 59–66.
Zhou, P., Ye, L., Xie, W., Lv, X., & Yu, H. (2015). Highly efficient biosynthesis of astaxanthin in Saccharomyces cerevisiae by integration and tuning of algal crtZ and bkt. Applied Microbiology and Biotechnology, 99(20), 8419–8428.
Maliga, P., Tungsuchat-Huang, T., & Lutz, K. A. (2021). Transformation of the plastid genome in tobacco: The model system for chloroplast genome engineering. Methods in Molecular Biology, 2317, 135–153.
Ruf, S., Hermann, M., Berger, I. J., Carrer, H., & Bock, R. (2001). Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nature Biotechnology, 19(9), 870.
McBride, K. E., Svab, Z., Schaaf, D. J., Hogan, P. S., Stalker, D. M., & Maliga, P. (1995). Amplification of a chimeric Bacillus gene in chloroplasts leads to an extraordinary level of an insecticidal protein in tobacco. Bio/Technology, 13(4), 362–365.
Daniell, H., Jin, S., Zhu, X. G., Gitzendanner, M. A., Soltis, D. E., & Soltis, P. S. (2021). Green giant—A tiny chloroplast genome with mighty power to produce high-value proteins: History and phylogeny. Plant Biotechnology Journal, 19(3), 430–447.
Ruf, S., & Bock, R. (2014). Plastid transformation in tomato. In Chloroplast biotechnology (pp. 265–276). Springer.
Verma, D., Samson, N. P., Koya, V., & Daniell, H. (2008). A protocol for expression of foreign genes in chloroplasts. Nature Protocols, 3(4), 739–758.
Day, A., & Goldschmidt-Clermont, M. (2011). The chloroplast transformation toolbox: Selectable markers and marker removal. Plant Biotechnology Journal, 9(5), 540–553.
Doyle, J. (1991). DNA protocols for plants. In Molecular techniques in taxonomy (pp. 283–293). Springer.
Lichtenthaler, H. K., & Buschmann, C. (2001). Chlorophylls and carotenoids: Measurement and characterization by UV–Vis spectroscopy. Current Protocols in Food Analytical Chemistry, 1(1), F4.3.1-F4.3.8.
Zielewicz, W., Wróbel, B., & Niedbała, G. (2020). Quantification of chlorophyll and carotene pigments content in Mountain Melick (Melica nutans L.) in relation to edaphic variables. Forests, 11(11), 1197.
Huang, J.-C., Zhong, Y.-J., Liu, J., Sandmann, G., & Chen, F. (2013). Metabolic engineering of tomato for high-yield production of astaxanthin. Metabolic Engineering, 17, 59–67.
Campbell, R., Morris, W. L., Mortimer, C. L., Misawa, N., Ducreux, L. J., Morris, J. A., Hedley, P. E., Fraser, P. D., & Taylor, M. A. (2015). Optimising ketocarotenoid production in potato tubers: Effect of genetic background, transgene combinations and environment. Plant Science, 234, 27–37.
Zhu, C., Naqvi, S., Breitenbach, J., Sandmann, G., Christou, P., & Capell, T. (2008). Combinatorial genetic transformation generates a library of metabolic phenotypes for the carotenoid pathway in maize. Proceedings of the National Academy of Sciences of USA, 105(47), 18232–18237.
Pierce, E. C., LaFayette, P. R., Ortega, M. A., Joyce, B. L., Kopsell, D. A., & Parrott, W. A. (2015). Ketocarotenoid production in soybean seeds through metabolic engineering. PLoS ONE, 10(9), e0138196.
Fujisawa, M., Takita, E., Harada, H., Sakurai, N., Suzuki, H., Ohyama, K., Shibata, D., & Misawa, N. (2009). Pathway engineering of Brassica napus seeds using multiple key enzyme genes involved in ketocarotenoid formation. Journal of Experimental Botany, 60(4), 1319–1332.
León, R., Couso, I., & Fernández, E. (2007). Metabolic engineering of ketocarotenoids biosynthesis in the unicellular microalga Chlamydomonas reinhardtii. Journal of Biotechnology, 130(2), 143–152.
Stålberg, K., Lindgren, O., Ek, B., & Höglund, A. S. (2003). Synthesis of ketocarotenoids in the seed of Arabidopsis thaliana. The Plant Journal, 36(6), 771–779.
Ralley, L., Enfissi, E. M., Misawa, N., Schuch, W., Bramley, P. M., & Fraser, P. D. (2004). Metabolic engineering of ketocarotenoid formation in higher plants. The Plant Journal, 39(4), 477–486.
Zhang, Y., & Fernie, A. R. (2021). Metabolons, enzyme–enzyme assemblies that mediate substrate channeling, and their roles in plant metabolism. Plant Communications, 2(1), 100081.
Sun, T., Rao, S., Zhou, X., & Li, L. (2022). Plant carotenoids: Recent advances and future perspectives. Molecular Horticulture, 2(1), 3.
Grossman, A. R., Harris, E. E., Hauser, C., Lefebvre, P. A., Martinez, D., Rokhsar, D., Shrager, J., Silflow, C. D., Stern, D., & Vallon, O. (2003). Chlamydomonas reinhardtii at the crossroads of genomics. Eukaryotic Cell, 2(6), 1137–1150.
Allen, Q. M., Febres, V. J., Rathinasabapathi, B., & Chaparro, J. X. (2022). Engineering a plant-derived astaxanthin synthetic pathway into Nicotiana benthamiana. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2021.831785
Acknowledgements
The authors are grateful to Professor Dr. Ralph Bock and Dr. Stephanie Ruf from Max Planck Institute for Molecular Plant Physiology, Germany for providing us the chloroplast-specific vector pRB96 and resolving few queries for chloroplast transformation. We thank Professor K. C. Bansal for his guidance for the project and Professor Dr. Damodhar Reddy from ICAR-Central Tobacco Research Institute, India for sending us tobacco seeds for the study. We would also like to extend our gratitude to Dr. P. Maheswara Reddy and his research students for helping us to carry out this work in lab.
Funding
The authors thank Deakin University, Australia for partially funding the research and providing Post-Graduate Research Scholarship DUPRS to NT. We also thank TERI-Nanobiotechnology Centre, India to provide research infrastructure and internal funding support for the study.
Author information
Authors and Affiliations
Contributions
NT conducted experiments, analysed data and drafted the MS. JER and DMC participated in drafting and data analysis and contributed reagents/chemicals. SKL participated in drafting and data analysis, contributed reagents/chemicals and designed experiments. All the authors read and approved the final MS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest or any conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors. Further, the authors have considered Committee on Publication Ethics (COPE) guidelines for preparing this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanwar, N., Rookes, J.E., Cahill, D.M. et al. Carotenoid Pathway Engineering in Tobacco Chloroplast Using a Synthetic Operon. Mol Biotechnol 65, 1923–1934 (2023). https://doi.org/10.1007/s12033-023-00693-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00693-3