Abstract
Novel effective drugs or therapeutic vaccines have been already developed to eradicate viral infections. Some non-viral carriers have been used for effective drug delivery to a target cell or tissue. Among them, cell penetrating peptides (CPPs) attracted a special interest to enhance drug delivery into the cells with low toxicity. They were also applied to transfer peptide/protein-based and nucleic acids-based therapeutic vaccines against viral infections. CPPs-conjugated drugs or vaccines were investigated in several viral infections including poliovirus, Ebola, coronavirus, herpes simplex virus, human immunodeficiency virus, hepatitis B virus, hepatitis C virus, Japanese encephalitis virus, and influenza A virus. Some studies showed that the uptake of CPPs or CPPs-conjugated drugs can be performed through both non-endocytic and endocytic pathways. Despite high potential of CPPs for cargo delivery, there are some serious drawbacks such as non-tissue-specificity, instability, and suboptimal pharmacokinetics features that limit their clinical applications. At present, some solutions are utilized to improve the CPPs properties such as conjugation of CPPs with targeting moieties, the use of fusogenic lipids, generation of the proton sponge effect, etc. Up to now, no CPP or composition containing CPPs has been approved by the Food and Drug Administration (FDA) due to the lack of sufficient in vivo studies on stability, immunological assays, toxicity, and endosomal escape of CPPs. In this review, we briefly describe the properties, uptake mechanisms, advantages and disadvantages, and improvement of intracellular delivery, and bioavailability of cell penetrating peptides. Moreover, we focus on their application as an effective drug carrier to combat viral infections.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell penetrating peptides (CPPs) with 5–30 amino acids can deliver a variety of biomolecules into the cells [1] such as small molecules, small interfering RNA, proteins, peptide-nucleic acid (PNA), imaging agents, and vaccines [2,3,4]. Human immunodeficiency virus transactivating regulatory protein (HIV TAT) was reported as the first CPP with the membrane translocating property [5,6,7]. After that, other CPPs with different properties in charge, polarity, and structure were reported to deliver bioactive molecules as classified in the CPPsite 2.0 database (https://webs.iiitd.edu.in/raghava/cppsite/index.html). CPPs are capable of delivering therapeutic substances (drugs or vaccines) into cellular compartments using the covalent or non-covalent linkage [8,9,10,11].
In recent years, different viral infections with high variability have been represented as major threats to the global health. Some of them led to a significant public health burden and huge economic loss [12,13,14]. Viruses can latch onto host cells as seen in several outbreaks including current coronavirus (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [15]. Antiviral drugs including small molecules that may interfere with viral replication, mRNA-, DNA-, or RNA interference (RNAi)-based vaccines that is said to be the potential vaccines to fight against viruses, and using antibodies that could act as both therapeutic and prophylactic have the capacity to prevent or treat viral infections [15, 16]. The diagnosis of viral infections can be performed by viral culture, antigen detection, serological tests, and viral nucleic acid detection. The molecular technology performed by polymerase chain reaction (PCR) is widely used in the detection and identification of pathogenic viruses [17, 18]. Recently, innovative methods such as biosensing technology (e.g., graphene/zinc oxide nanocomposite-based electrochemical genosensors) enable the researchers to detect nucleic acids in a faster and inexpensive way [19]. In spite of advances in the development of therapeutic strategies, it is recommended to optimize the delivery system rather than investing time and resources into developing new antiviral therapeutic agents. As known, most of the therapeutic targets of antiviral agents (drug or vaccine) are located inside the cells, thus development of a potent and safe intracellular delivery vehicle (such as CPPs) is necessary for increasing their effects in vivo [9]. For example, the incorporation of CPPs in subunit or nucleic acid-based vaccines could improve antigen uptake by antigen-presenting cells (APCs). Furthermore, CPPs could be harnessed to develop new therapeutics and considered as a safe alternative or additive to classical adjuvant formulations. Indeed, they can improve the properties of current antiviral drugs and vaccines [20, 21]. In this review, we briefly describe the properties of CPPs, their mechanisms, and their variety of applications in drug delivery with a focus on the management of viral infections.
Classification of CPPs
Cell penetrating peptides were classified based on their type of origin, chemical charge (cationic, amphipathic, and hydrophobic groups related to their uptake mechanism), physicochemical properties (e.g., charge, hydrophobicity, and distribution of the residues in the peptide sequence), and the extent of modifications and design efforts (Fig. 1). Cationic CPPs are the largest group of cell penetrating peptides containing basic amino acids that are responsible for their cellular uptake and suppression of helical folding formation [e.g., polyarginine, TAT, and DNA- and RNA- and heparan-binding proteins of viruses, protamines, histones, and nuclear localization signal (NLS)] [22,23,24,25]. Some studies showed that oligoarginine peptides (the optimal length: 8–10 residues) penetrate into cells remarkably better than oligolysine peptides with the same length and charge [26, 27]. Increasing the length of oligoarginine peptides decreased their potency of delivery due to the cell toxicity and irreversible interaction with plasma membrane [28]. In addition, more than 40% of CPPs are amphipathic peptides with negative overall charge [29]. These CPPs were divided into primary (mainly chimeric or synthetic peptides derived from natural proteins such as tumor-suppressor p14ARF protein or prion proteins) [30,31,32], and secondary (e.g., MAP or M918) [33] groups based on the peptide sequence, length, and association with lipids. In general, transition to a β-sheet structure led to forming the amphipathic structure and cell penetration [34]. The lowest number of CPPs contains hydrophobic CPPs with only apolar residues derived from signal peptide sequences (e.g., transportan, stapled peptides, prenylated peptides, pepducins, SG3, Pep-7, and fibroblast-growth factor) [35,36,37,38,39,40,41]. On the other hand, CPPs can be classified based on the linkage with therapeutic agent into covalently (as fused to a recombinant protein cargo or conjugated by a linker to the cargo including TAT, penetratin, polyarginine, VP22, Buforin I, transportan, and SynB peptides) [42, 43] or non-covalently (as stable complexes with peptide/protein and oligonucleotide cargos through non-covalent electrostatic and hydrophobic interactions including Pep-1 and MPG peptides)-bonded forms [44, 45]. Table 1 provides a summary of main CPPs and their characteristics. Additionally, the classification of CPPs as protein-derived CPPs (e.g., viral proteins, mammalian DNA/RNA-binding proteins, and transcription factors), chimeric CPPs, and synthetic CPPs is very useful to design more efficient CPPs being able to increase the cellular uptake, and delivery of the related cargos. In recent years, chemical modification of CPPs was used to enhance cargo delivery [46,47,48,49,50,51,52,53,54,55,56].
A schematic diagram illustrating the types of CPPs and their cargos: CPPs can be classified based on chemical charge, linkage with cargo, and also their origin. Different kinds of antiviral cargos including vaccines, small molecules, and peptide/ protein and nucleic acid therapeutics can conjugate with CPPs via covalent or non-covalent binding
Applications of CPPs
The intracellular delivery of therapeutic agents (e.g., nucleic acids or proteins) is difficult due to their large size and hydrophilic nature. Several physical (e.g., electroporation or microinjection), viral (e.g., lentiviral vectors), and non-viral/ chemical (e.g., polymers and liposomes) approaches were applied to enhance their cellular uptake [77]. However, some drawbacks such as low efficiency and immunogenicity, high toxicity, and poor specificity limited their use for delivery of different cargos [78]. In recent years, CPPs have been used to promote the delivery of drugs into cells due to their high efficiency in crossing cell membranes without the aid of any specific receptor, and low toxicity [79]. Moreover, CPPs have been applied in many fields of medicinal applications including imaging and biosensing applications, enzyme replacement therapy, anti-inflammation therapy using antisense peptide nucleic acids (PNAs), cancer therapy, and vaccine development [80]. Among these applications, antiviral drug delivery using CPPs attracted a special interest as follows. In general, current antiviral therapy mainly relies on small molecules to inhibit multiple targets involved in the viruses’ life cycle. The main limitation of currently used antiviral drugs is inefficient delivery into the infected cells in vivo due to their pharmacokinetics and pharmacodynamics properties which limit their applications [81]. Therefore, CPPs were proposed as an effective delivery system for different types of biotherapeutics such as peptides/proteins and nucleic acids in antiviral therapy [82].
Delivery of Therapeutic Small Molecule Drugs
Small molecule drugs inhibit the activity of cellular or viral proteins involved in different stages of the virus life cycles [83]. Although most of small molecule drugs can pass the cell membrane, in some cases, bioavailability of these molecules is limited by their high degree of hydrophilicity which diminishes the ability of crossing the cell membrane [84]. Thus, conjugation of CPPs with these small molecule drugs could enhance their cellular uptake [e.g., delivery of small molecules through the blood–brain barrier (BBB)], and improve their pharmacokinetics and pharmacodynamics profile (e.g., the increased solubility and bioavailability of small molecule drugs in body fluids) [85]. For instance, porphyrin antiviral drug conjugated with CPPs can cross the BBB, and inhibit brain-resident HIV virus causing HIV-associated neurocognitive disorders (HAND) in vitro [86].
Delivery of Peptide/Protein-Based Therapeutics
A large number of peptide/protein-based therapeutics (e.g., enzymes and antibodies) were clinically used to treat various viral diseases. However, some physiochemical properties of peptides/proteins such as size and hydrophilicity could limit their intracellular accumulation. Thus, CPPs were extensively utilized as shuttles for intracellular delivery of a variety of peptides/proteins Incorporation of CPPs (e.g., penetratin, SynB, and TAT) in cytotoxic T lymphocytes (CTL)-inducing therapeutic vaccines is one of their interesting applications for delivery of peptide/protein-based therapeutics [87,88,89]. Moreover, several antiviral peptides (i.e., natural proteins-derived peptides or synthetic peptides) have emerged as ideal therapeutic agents against viral infections in clinical trials. Many of these peptides could exert their antiviral activity by interfering with various steps of virus life cycles. Enfuvirtide is the only approved peptide drug for HIV-1 treatment. Conjugation of antiviral peptide drugs with CPPs was highly suggested to overcome their poor cell permeability [90]. Table 2 shows successful delivery of CPP-conjugated peptides/proteins against related viral infections.
On the other hand, remarkable target specificity and low immunogenicity of antibodies make them as a promising therapeutic agent. More than 80 therapeutic antibodies were approved by the Food and Drug Administration (FDA) and hundreds more are in various phases of clinical trials. Thus, therapeutic antibodies have become one of the predominant and fastest-growing classes of new drugs developed in recent years [91]. However, large molecules including antibodies are notoriously hard to be delivered. TAT cell penetrating peptide showed the significant translocation potency for delivery of antibodies into the cells (e.g., delivery of monoclonal antibodies (mAb) for radioimmunotherapy and radioimmunodetection). Moreover, CPPs were used as shuttles for delivery of single-chain variable fragments (ScFv) which are the engineered proteins generated by fusion of the variable heavy (VH) and light (VL) domains of an antibody. The delivered antibody could maintain its functional conformation to interact with the target in the cell [92]. Some studies have demonstrated effective internalization, and significant antiviral activity of antibody fragments fused to CPPs. For instance, one study showed that mAb targeting HIV capsid protein p24, fused to ĸFGF-MTS CPP (KAAVALLPAVLLALLP) efficiently internalized into the cells, and inhibited the HIV-1 replication in cell culture [93]. Another study indicated that the TAT-fused antibody targeting intracellular HBV X protein (HBx) effectively internalized into the cells, and reduced intracellular HBx in vitro and in vivo [94]. Table 2 represents successful delivery of antibodies by CPPs against related viral infections.
Delivery of Nucleic Acids and Oligonucleotides in Gene Therapy
Gene therapy is efficient delivery of genetic material into the cell, tissue or whole organ without causing pathogenic effects [95]. However, poor permeability of the plasma membrane of eukaryotic cells to DNA led to low concentration of DNA and other oligonucleotides at their targets. To overcome this issue, polylysine and polyarginine peptide carriers with the membrane-destabilizing properties could bind to DNA through electrostatic interaction (i.e., non-covalently bonded form), and facilitate gene transfer into cells [96]. For example, cationic polymers such as polyethylenimine (25 kDa PEI) were utilized for gene delivery due to the formation of nanometer-sized particles with the negatively charged plasmid DNA. The linkage of PEI to TAT CPP through a hetero-bifunctional polyethylenglycol (PEG) spacer (i.e., the TAT-PEG-PEI conjugate) could significantly increase the efficiency of gene delivery in lung, and reduce in vivo toxicity [97].
On the other hand, the therapeutic potential of small interfering RNA (siRNA) and microRNA (miRNA) against various types of viruses (e.g., HIV, HCV, HBV, influenza, Ebola, HSV, and poliovirus) was reported through sequence-specific suppression of gene expression (e.g., transcription or translation) [98]. Cell penetrating peptides could easily be conjugated covalently and non-covalently with siRNAs for their effective delivery into the cells. However, non-covalent strategies were more effective than covalent strategies for siRNA delivery. For example, MPG peptide could significantly improve the efficiency of siRNA delivery and its safety in the target cells [99, 100]. Additionally, MPG-based particles enter the cell independently of the endosomal pathway, and can efficiently deliver siRNAs in a fully biological active form into a variety of cell lines and in vivo [101].
Due to overcoming some problems of siRNAs and miRNAs (e.g., poor pharmacokinetics and sensitivity to enzymatic degradation), a series of antisense oligonucleotides (AOs) with improved properties (i.e., high stability, high affinity, and low toxicity) has been developed for antisense therapy. Peptide nucleic acids (PNAs) and phosphorodiamidate morpholino oligomers (PMO) are such AOs widely used for therapeutic applications. For instance, the CPP-PMO conjugates (PPMOs) were shown to reduce viral replication, and increase the survival rate of infected mice. These in vitro and in vivo therapeutic experiments were performed against various types of viruses (e.g., poliovirus, Ebola, SARS, HSV, HIV, HBV, HCV, measles, Japanese encephalitis virus, and influenza A virus) [102,103,104,105,106,107,108,109,110]. Some studies showed that the efficacy of PNA and PMO conjugated to CPPs was only significant in the presence of endosomolytic agents (e.g., chloroquine and calcium ions) [111, 112]. However, most of the endosomolytic agents are too toxic for in vivo applications limiting the use of CPP-PNA or CPP-PMO [113, 114]. Table 2 includes successful delivery of oligonucleotide-based antiviral therapeutics.
CPPs with Antiviral and Antimicrobial Properties
Antimicrobial peptides (AMPs) are promising antimicrobial agents that influence cellular membrane of microorganisms. Some of CPPs have antibacterial properties. Antibacterial CPPs can penetrate to cytoplasm, bind to nucleic acids, inhibit protein synthesis, and finally cause bacterial cell death [115]. For example, PEP27-2 (MWKWFHNVLSWGWLLADKRPARDYNRK-NH2) is a potent antimicrobial CPP which reduced skin abscess formation during Staphylococcus aureus infection in mouse when used as combined with antibiotics. Indeed, PEP27-2 inhibited cellular processes by interrupting DNA metabolism in bacterial cells [116]. Moreover, some of CPPs possess antiviral properties. For instance, interaction of TAT peptide with CXCR4 co-receptor inhibited the replication efficiency of the virus strains such as HIV-1 and HSV, and showed the antiviral activity [117]. The advantage of these CPPs with antiviral activity is their synergistic antiviral effects, and their role as a delivery vehicle for other antiviral agents. Indeed, the net positive charge of these peptides could interact with the negatively charged components of viruses, and prevent viral infection at the attachment or entry step [106]. For example, TAT-I24 (GRKKRRQRRRPPQCLAFYACFC) demonstrated inhibitory activity against DNA viruses. This peptide could suppress the early step of viral replication cycle at the level of viral entry, and gene expression. The mechanism of action is the direct binding of the peptide to the viral envelope and/or affecting the membrane structures [118]. Another study showed that the anti-HIV-1 virucidal activity of Transportan-PNA conjugate was efficient to block HIV-1 infection or inactivate virus in the plasma before attachment and entry the cells [119].
Mechanism of CPP Internalization
Although the exact mechanism of CPP internalization with or without cargo has not been completely revealed, their uptake pathways have been classified as non-endocytic (i.e., the energy-independent direct delivery of cargo to cytoplasm) and endocytic (i.e., the energy-dependent delivery of cargo to lysosomes) pathways (Fig. 2). Most CPPs utilize two or more uptake mechanisms depending on the type of CPP, cell line, cargo, concentration, temperature and time of incubation. For example, TAT peptide was internalized through three endocytic pathways such as macropinocytosis, clathrin- and caveolae/lipid raft-mediated endocytosis based on cargo type. TAT peptide conjugated with protein was internalized through lipid raft-medicated endocytosis, while TAT peptide embedded with fluorophore used clathrin-dependent endocytosis mechanism [141].
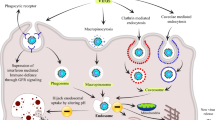
Schematic illustration of uptake mechanisms of CPPs, and some major examples: The CPP uptake mechanism can be directed by non-endocytic (direct penetration) and endocytic pathways. As the cell membrane is impermeable to hydrophilic substances, the delivery into cells can be facilitated by linking the cargos to CPPs. Most CPPs utilize two or more uptake mechanisms depending on the type of CPP, cell line, cargo, concentration, temperature, and time of incubation
The non-endocytic pathway or direct translocation includes the interaction of positively charged CPPs with negatively charged components of phospholipid bilayer [142]. Direct translocation was divided into inverted micelle formation (e.g., penetratin) [48, 143], pore formation mechanisms through barrel-stave or toroidal models (e.g., Pep-1) [144, 145], and the carpet model (e.g., TAT, and dermaseptin as an antimicrobial and amphipathic peptide) [146]. In contrast, endocytic pathway was classified into four dominant pathways including macropinocytosis (a lipid raft-dependent and receptor-independent endocytic pathway; e.g., CPPs-attached growth factors) [147], clathrin-mediated (a receptor-dependent and dynamin-required process; e.g., arginine-rich CPPs) [148] or caveolae-mediated endocytosis (a lipid raft endocytosis associated with caveolin and cavin-1 interaction; e.g., proline-rich CPPs, transportan and transportan-10) [149, 150], and phagocytosis (e.g., the CPP cargo tagged by opsonins such as IgG and complement components enabling CPPs to be recognized by immune cells such as macrophages and dendritic cells) [151].
CPPs with endocytic uptake pathways should escape from endosomes. For example, positively charged CPPs (e.g., TAT) could interact with negatively charged phospholipids in the endosomal membrane leading to a pore/leakage, and finally the release of CPPs [152]. Moreover, the formation of ionic pairs between CPPs and negatively charged membrane lipids occurred in oligo-arginine peptides. Three strategies are available for improving the endosomal release of CPPs including: a) the use of fusogenic lipids (e.g., dioleoylphosphatidyl-ethanolamine (DOPE)) [153], conjugation of the carrier with viral fusion sequence (e.g., HA2 peptide as a pH-sensitive fusogenic peptide derived from influenza virus) [154], and increasing the osmotic pressure within endosomes through the proton sponge effect (e.g., histidine residues) [153].
Advances in Enhancing the CPP Potency
CPPs showed high potential for delivery of a wide range of molecules, but they have some drawbacks including non-specific internalization, fast elimination from the body, and intracellular/vesicular entrapment. However, our knowledge regarding the mechanism and structure–activity relationship of internalization is growing. The most common feature among CPPs is the presence of positive charges such as arginine and lysine. In addition, hydrophobicity plays a major role in the translocation process [56]. The studies showed that the replacement of arginine residues of polyarginine peptides (e.g., TAT, penetratin, transportan, MPG, Pep-1, and pVEC) with lysine residues showed a weaker affinity to the cell surface, and remarkably reduced intracellular translocation. Thus, the presence of arginine in the peptide chain is a desirable modification [25, 155,156,157].
On the other hand, a specific class of CPPs was based on polyproline secondary structure (e.g., SAP and PR39 as an AMP). Indeed, proline residue has a secondary amine chain that does not participate in intra- or inter-molecular hydrogen bonding. Thus, the internalization of polyproline peptides is due to the amphipathicity of the secondary structure rather than of the primary sequence [73, 149]. Additionally, Tryptophan is a very important amino acid in CPP structure being able to transport cargo across cell membranes (e.g., CADY and Pep-1). In fact, the number of tryptophan residues, their position in the helix, and the size of the hydrophobic face formed by them are important for the cell internalization [158, 159]. For example, increasing the number of tryptophan up to three amino acids could enhance cellular uptake. Above this number, the intracellular translocation activity was decreased due to low solubility of the peptide [160]. Tryptophan substitution can also enhance the antimicrobial activity of CPPs. For example, PEP27-2, an analog of PEP27 with Trp substitution, showed stronger antimicrobial activity against a variety of bacteria [116]. Furthermore, polyhistidine in CPP structure (e.g., LAH4) facilitated direct membrane translocation of peptides into cells. Indeed, substitution of amino acids with histidine residues could provide endosomal disruption by the proton sponge effect in the acidic endosomal compartments provoking the endosomal escape [161]. For example, protonated histidines enhanced the delivery of nucleic acids to their targets through osmotic swelling with lysis of endosomes, unpacking of the carrier complex, and the release of nucleic acids [162, 163]. Other properties of histidine include its ability to stabilize nanoparticles through hydrogen bonding and aromatic interactions [162].
Generally, different modifications on CPPs could increase their internalization and/or change the mechanism of penetration such as amino acid substitute [157, 160, 161], and functional group modification [2, 164]. Amino acid substitution of CPPs is one of the ways of achieving variability of the physicochemical parameters (e.g., hydrophobicity or cationic nature) especially changes in dissolution properties [157]. Modification of functional groups (e.g., the alteration of CPPs, α-helicity through hydrocarbon stapling) is another approach for increasing the CPP efficiency [2]. For example, modification of MAP CPP with citraconic anhydride blocked forming acid-labile amide linkage to mask the cationic charge, decreased the non-specific binding and uptake, and thus significantly improved the targeted drug delivery [164].
Also, there is a need for improvement in rational design of CPPs to tackle the possibility of in vivo toxicity. Synthetic tools have paved the way to explore new approaches to improve the cell penetration of CPPs and CPP-therapeutic conjugates. For example, cyclization and stapling increases the metabolic stability and internalization efficiency due to increased structural and conformational rigidity. Thus, multivalency of covalent dimers (primary structure), stabilized helices (secondary and tertiary structure) and even quaternary structure can help to improve the internalization [165]. However, the lack of methodologies for systemic rational design and optimization of new CPPs is one of the key impediments. Fortunately, molecular dynamics (MD) simulation (a simulation-guided rational design approach) is now able to predict structure-based rational fine-tuning of functional properties. Although the next challenge of designing suitable CPPs is to find proper engineering techniques to control the morphology to improve selectivity and specificity [165]. Thus, chemistry and rational design could contribute to the CPP field.
Current Status and Challenges of CPPs in Viral Infections
CPPs have been used to increase drug delivery efficiency [166]. However, it is required to determine the pharmacokinetic properties of CPPs through assessing toxicity, tissue distribution, cell selectivity, solubility and stability, immunogenicity and endosomal degradation [167]. On the other hand, the mechanism of CPP internalization is non-specific binding to bilayer phospholipids on the cell membrane, which severely limits the clinical application of CPPs. There are several solutions for enhancing the CPP’s specificity, and decreasing possible adverse effects of therapeutics such as: a) the design of cell and tissue-specific CPPs. For example, two tumor targeting peptides including RGD (Arg-Gly-Asp) and NGR (Asn-Gly-Arg) can be used for improving specificity in virus-related tumors [168], b) conjugating/ coupling of the CPP with various cell-specific targeting ligands (e.g., folic acid, specific antibodies, and transferrin) through covalent and non-covalent bonds [169,170,171,172], and c) masking the cell-penetrating effect with a stimulus-sensitive cleavable linker (e.g., pH-sensitive, enzyme-sensitive, temperature-sensitive, and magnetism-sensitive or light sensitive cleavable linkers). These linkers can be cleaved, and the CPP restores its normal activity. However, the activation process is generally irreversible and often occurs at off-target sites instead of on-target sites [172].
To date, no CPP-conjugated drugs have been approved by FDA; although several clinical trials on cancer have been evaluating them, one of the issues is the lack of sufficient in vivo studies on stability [149]. The rapid blood clearance of therapeutic agents may be a drawback as the enzymic degradation is happening before reaching the therapeutic site. Moreover, the assessment of immunological and pharmacokinetic studies of CPPs need validation in animal models [21]. The polypeptide CPPs may increase the risk of undesired immune response [173]. Also, the off-target absorption of the therapeutics by any normal tissues and cells can cause cellular toxicity. Thus, the exact dosing of each CPP should be measured in animal models before officially getting applied to the patients [174]. On the other hand, the endosomal degradation is another drawback and CPPs should be designed to have effective endosomal escape to speed up the release of the carriers [175]. Molecular imaging of intracellular and intranuclear targets can be helpful for understanding the CPP internalization mechanism and intracellular trafficking [176]. To our best of knowledge, there is no ongoing clinical trial to investigate the delivery system of CPPs in viral infections.
Conclusion and Future Prospects
CPPs have the potential to transport numerous types of therapeutic agents into a variety of cells. Several biophysical factors including charge, amphipathicity, shape, complexity, and compactness of the structures play an important role in entry of CPP/cargo into the cells. Extensive in vitro and in vivo studies have shown the successful delivery of nucleic acid- and peptide/protein-based drugs and vaccines. To date, no CPP or composition containing CPPs has been approved by the FDA due to the lack of sufficient in vivo studies on stability, immunological assays, toxicity, and endosomal escape of CPPs; but several ongoing clinical trials in different phases are evaluating them (mainly for drug delivery in tumor cells). Despite the many advantages, some serious drawbacks such as non-target tissue-specificity, instability and suboptimal pharmacokinetics features limit their clinical applications. However, three distinct delivery strategies are proposed to enhance the CPP’s specificity including designing cell/ tissue-specific CPPs, conjugation of CPPs with targeting moieties, and modulation of CPP uptake by a stimulus-sensitive signal. Also, the endosomal entrapment issue of CPPs can be solved by the use of fusogenic lipids via destabilizing the endosome membrane, and the use of histidine by creating the proton sponge effect and generating lysosome osmotic swelling. In general, the success of the CPP-based strategy for clinical use depends on their efficiency, safety, and also ultimate cost. Large-scale applications and new methodologies are being implemented to increase the yield and reduce cost. With increasing our knowledge of various aspects of CPPs, along with the development of new efficient CPPs overcoming some limitations, CPPs are expected to become an important part of pharmaceutical agents, especially in antiviral therapies.
Data availability
All data are available in the manuscript.
References
Kurrikoff, K., Gestin, M., & Langel, Ü. (2016). Recent in vivo advances in cell-penetrating peptide-assisted drug delivery. Expert Opinion on Drug Delivery, 13, 373–387.
Kersemans, V., & Cornelissen, B. (2010). Targeting the tumour: Cell penetrating peptides for molecular imaging and radiotherapy. Pharmaceuticals, 3, 600–620.
Guo, Z., Peng, H., Kang, J., & Sun, D. (2016). Cell-penetrating peptides: Possible transduction mechanisms and therapeutic applications. Biomedical Reports, 4, 528–534.
Wang, F., Wang, Y., Zhang, X., Zhang, W., Guo, S., & Jin, F. (2014). Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. Journal of Controlled Release, 174, 126–136.
Schmidt, N., Mishra, A., Lai, G. H., & Wong, G. C. (2010). Arginine-rich cell-penetrating peptides. FEBS Letters, 584, 1806–1813.
Green, M., & Loewenstein, P. M. (1988). Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell, 55, 1179–1188.
Frankel, A. D., & Pabo, C. O. (1988). Cellular uptake of the tat protein from human immunodeficiency virus. Cell, 55, 1189–1193.
Kauffman, W. B., Fuselier, T., He, J., & Wimley, W. C. (2015). Mechanism matters: A taxonomy of cell penetrating peptides. Trends in Biochemical Sciences., 40, 749–764.
Guidotti, G., Brambilla, L., & Rossi, D. (2017). Cell-penetrating peptides: From basic research to clinics. Trends in Pharmacological Sciences., 38, 406–424.
Agrawal, P., Bhalla, S., Usmani, S. S., Singh, S., Chaudhary, K., Raghava, G. P., & Gautam, A. (2016). CPPsite 2.0: A repository of experimentally validated cell-penetrating peptides. Nucleic Acids Research, 44, D1098–D1103.
Gayraud, F., Klußmann, M., & Neundorf, I. (2021). Recent advances and trends in chemical CPP-drug conjugation techniques. Molecules, 26, 1591.
Bhadoria, P., Gupta, G., & Agarwal, A. (2021). Viral pandemics in the past two decades: An overview. Journal of Family Medicine and Primary Care, 10, 2745.
Tompa, D. R., Immanuel, A., Srikanth, S., & Kadhirvel, S. (2021). Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs. International Journal of Biological Macromolecules, 172, 524–541.
Morens, D. M., Folkers, G. K., & Fauci, A. S. (2004). The challenge of emerging and re-emerging infectious diseases. Nature, 430, 242–249.
Peter, A. P., Wayne, C. K., Show, P. L., & Ling, T. C. (2020). Potential pathway that could treat Coronaviruses (COVID-19). Curr Biochem Eng, 6, 3–4.
Lanh, P. T., Nguyen, H. M., Duong, B. T. T., Hoa, N. T., Thom, L. T., Tam, L. T., Thu, H. T., Nha, V. V., Hong, D. D., Mouradov, A., Koyande, A. K., Show, P. L., & Quyen, D. V. (2021). Generation of microalga Chlamydomonas reinhardtii expressing VP28 protein as oral vaccine candidate for shrimps against white spot syndrome virus (WSSV) infection. Aquaculture, 540, 736737.
Khairkhah, N., Bolhassani, A., & Najafipour R. (2022). Current and future direction in treatment of HPV-related cervical disease. Journal of Molecular Medicine, 1-17
Hwang, K. A., Ahn, J. H., & Nam, J. H. (2018). Diagnosis of viral infection using real-time polymerase chain reaction. Journal of Bacteriology and Virology, 48, 1–13.
Low, S. S., Loh, H. S., Boey, J. S., Khiew, P. S., Chiu, W. S., & Tan, M. T. T. (2017). Sensitivity enhancement of graphene/zinc oxide nanocomposite-based electrochemical impedance genosensor for single stranded RNA detection. Biosensors and Bioelectronics, 94, 365–373.
Lou, Z., Sun, Y., & Rao, Z. (2014). Current progress in antiviral strategies. Trends in Pharmacological Sciences, 35, 86–102.
Delcroix, M., & Riley, L. W. (2010). Cell-penetrating peptides for antiviral drug development. Pharmaceuticals, 3, 448–470.
Tünnemann, G., Martin, R. M., Haupt, S., Patsch, C., Edenhofer, F., & Cardoso, M. C. (2006). Cargo-dependent mode of uptake and bioavailability of TAT-containing proteins and peptides in living cells. The FASEB Journal, 20, 1775–1784.
Jallouk, A. P., Palekar, R. U., Pan, H., Schlesinger, P. H., & Wickline, S. A. (2015). Modifications of natural peptides for nanoparticle and drug design. Advances in Protein Chemistry and Structural Biology, 98, 57–91.
Hariton-Gazal, E., Feder, R., Mor, A., Graessmann, A., Brack-Werner, R., Jans, D., Gilon, C., & Loyter, A. (2002). Targeting of nonkaryophilic cell-permeable peptides into the nuclei of intact cells by covalently attached nuclear localization signals. Biochemistry, 41, 9208–9214.
Trabulo, S., Cardoso, A. L., Mano, M., & De Lima, M. C. P. (2010). Cell-penetrating peptides-mechanisms of cellular uptake and generation of delivery systems. Pharmaceuticals, 3, 961–993.
Futaki, S. (2006). Oligoarginine vectors for intracellular delivery: Design and cellular-uptake mechanisms. Peptide Science: Original Research on Biomolecules, 84, 241–249.
Mitchell, D. J., Steinman, L., Kim, D., Fathman, C., & Rothbard, J. (2000). Polyarginine enters cells more efficiently than other polycationic homopolymers. The Journal of Peptide Research, 56, 318–325.
Verdurmen, W. P., & Brock, R. (2011). Biological responses towards cationic peptides and drug carriers. Trends in Pharmacological Sciences, 32, 116–124.
Milletti, F. (2012). Cell-penetrating peptides: Classes, origin, and current landscape. Drug Discovery Today, 17, 850–860.
Johansson, H. J., El-Andaloussi, S., Holm, T., Mäe, M., Jänes, J., Maimets, T., & Langel, U. (2008). Characterization of a novel cytotoxic cell-penetrating peptide derived from p14ARF protein. Molecular Therapy, 16, 115–123.
Magzoub, M., Sandgren, S., Lundberg, P., Oglęcka, K., Lilja, J., Wittrup, A., Eriksson, L. E. G., Langel, U., Belting, M., & Graslund, A. (2006). N-terminal peptides from unprocessed prion proteins enter cells by macropinocytosis. Biochemical and Biophysical Research Communications, 348, 379–385.
Lundberg, P., Magzoub, M., Lindberg, M., Hällbrink, M., Jarvet, J., Eriksson, L., Langel, U., & Graslund, A. (2002). Cell membrane translocation of the N-terminal (1–28) part of the prion protein. Biochemical and Biophysical Research Communications, 299, 85–90.
Madani, F., Lindberg, S., Langel, Ü., Futaki, S., & Gräslund, A. (2011). Mechanisms of cellular uptake of cell-penetrating peptides. Journal of Biophysics, 2011, 414729.
Magzoub, M., Eriksson, L. G., & Gräslund, A. (2002). Conformational states of the cell-penetrating peptide penetratin when interacting with phospholipid vesicles: Effects of surface charge and peptide concentration. Biochimica Et Biophysica Acta (BBA)-Biomembranes, 1563, 53–63.
Langel, Ü. (2021). Cell-penetrating peptides and transportan. Pharmaceutics, 13, 987.
Moiola, M., Memeo, M. G., & Quadrelli, P. (2019). Stapled peptides-a useful improvement for peptide-based drugs. Molecules, 24, 3654.
Wollack, J. W., Zeliadt, N. A., Ochocki, J. D., Mullen, D. G., Barany, G., Wattenberg, E. V., & Distefano, M. D. (2010). Investigation of the sequence and length dependence for cell-penetrating prenylated peptides. Bioorganic & Medicinal Chemistry Letters, 20, 161–163.
Zhang, P., Covic, L., & Kuliopulos, A. (2015). Pepducins and other lipidated peptides as mechanistic probes and therapeutics (pp. 191–203). Springer.
Gao, S., Simon, M. J., Hue, C. D., Morrison, B., & Banta, S. (2011). An unusual cell penetrating peptide identified using a plasmid display-based functional selection platform. ACS Chemical Biology, 6, 484–491.
Gao, C., Mao, S., Ditzel, H. J., Farnaes, L., Wirsching, P., Lerner, R. A., & Janda, K. D. (2002). A cell-penetrating peptide from a novel pVII–pIX phage-displayed random peptide library. Bioorganic & Medicinal Chemistry, 10, 4057–4065.
Nakayama, F., Yasuda, T., Umeda, S., Asada, M., Imamura, T., Meineke, V., & Akashi, M. (2011). Fibroblast growth factor-12 (FGF12) translocation into intestinal epithelial cells is dependent on a novel cell-penetrating peptide domain: Involvement of internalization in the in vivo role of exogenous FGF12. Journal of Biological Chemistry, 286, 25823–25834.
Pujals, S., Fernández-Carneado, J., López-Iglesias, C., Kogan, M. J., & Giralt, E. (2006). Mechanistic aspects of CPP-mediated intracellular drug delivery: relevance of CPP self-assembly. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1758, 264–279.
El-Andaloussi, S., Holm, T., & Langel, U. (2005). Cell-penetrating peptides: Mechanisms and applications. Current Pharmaceutical Design, 11, 3597–3611.
Mäe, M., Andaloussi, S. E., Lundin, P., Oskolkov, N., Johansson, H. J., Guterstam, P., & Langel, U. (2009). A stearylated CPP for delivery of splice correcting oligonucleotides using a non-covalent co-incubation strategy. Journal of Controlled Release, 134, 221–227.
Gros, E., Deshayes, S., Morris, M. C., Aldrian-Herrada, G., Depollier, J., Heitz, F., & Divita, G. (2006). A non-covalent peptide-based strategy for protein and peptide nucleic acid transduction. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1758, 384–393.
Vives, E., Brodin, P., & Lebleu, B. (1997). A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. Journal of Biological Chemistry, 272, 16010–16017.
Elmquist, A., Lindgren, M., Bartfai, T., & Langel, Ü. (2001). VE-cadherin-derived cell-penetrating peptide, pVEC, with carrier functions. Experimental Cell Research, 269, 237–244.
Derossi, D., Chassaing, G., & Prochiantz, A. (1998). Trojan peptides: The penetratin system for intracellular delivery. Trends in Cell Biology, 8, 84–87.
Elliott, G., & O’Hare, P. (1997). Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell, 88, 223–233.
Pooga, M., Hällbrink, M., Zorko, M., & Langel, Ü. (1998). Cell penetration by transportan. The FASEB Journal, 12, 67–77.
Morris, M. C., Depollier, J., Mery, J., Heitz, F., & Divita, G. (2001). A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nature Biotechnology, 19, 1173–1176.
Crombez, L., Aldrian-Herrada, G., Konate, K., Nguyen, Q. N., McMaster, G. K., Brasseur, R., Heitz, F., & Divita, G. (2009). A new potent secondary amphipathic cell-penetrating peptide for siRNA delivery into mammalian cells. Molecular Therapy, 17, 95–103.
Ivanova, G. D., Arzumanov, A., Abes, R., Yin, H., Wood, M. J., Lebleu, B., & Gait, M. J. (2008). Improved cell-penetrating peptide–PNA conjugates for splicing redirection in HeLa cells and exon skipping in mdx mouse muscle. Nucleic Acids Research, 36, 6418–6428.
Oehlke, J., Beyermann, M., Wiesner, B., Melzig, M., Berger, H., Krause, E., & Bienert, M. (1997). Evidence for extensive and non-specific translocation of oligopeptides across plasma membranes of mammalian cells. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1330, 50–60.
Scheller, A., Wiesner, B., Melzig, M., Bienert, M., & Oehlke, J. (2000). Evidence for an amphipathicity independent cellular uptake of amphipathic cell-penetrating peptides. European Journal of Biochemistry, 267, 6043–6050.
Gong, Z., Doolin, M. T., Adhikari, S., Stroka, K. M., & Karlsson, A. J. (2019). Role of charge and hydrophobicity in translocation of cell-penetrating peptides into Candida albicans cells. AIChE Journal, 65, e16768.
Anko, M., Majhenc, J., Kogej, K., Sillard, R., Langel, Ü., Anderluh, G., & Zorko, M. (2012). Influence of stearyl and trifluoromethylquinoline modifications of the cell penetrating peptide TP10 on its interaction with a lipid membrane. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1818, 915–924.
Oehlke, J., Scheller, A., Wiesner, B., Krause, E., Beyermann, M., Klauschenz, E., Melzig, M., & Bienert, M. (1998). Cellular uptake of an α-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1414, 127–139.
Gerbal-Chaloin, S., Gondeau, C., Aldrian-Herrada, G., Heitz, F., Gauthier-Rouvière, C., & Divita, G. (2007). First step of the cell-penetrating peptide mechanism involves Rac1 GTPase-dependent actin-network remodelling. Biology of the Cell, 99, 223–238.
Park, C. B., Kim, M. S., & Kim, S. C. (1996). A novel antimicrobial peptide frombufo bufo gargarizans. Biochemical and Biophysical Research Communications, 218, 408–413.
Rousselle, C., Smirnova, M., Clair, P., Lefauconnier, J. M., Chavanieu, A., Calas, B., Scherrmann, J. M., & Temsamani, J. (2001). Enhanced delivery of doxorubicin into the brain via a peptide-vector-mediated strategy: Saturation kinetics and specificity. Journal of Pharmacology and Experimental Therapeutics, 296, 124–131.
Arukuusk, P., Pärnaste, L., Hällbrink, M., & Langel, Ü. (2015). PepFects and NickFects for the intracellular delivery of nucleic acids (pp. 303–315). Springer.
Oehlke, J., Krause, E., Wiesner, B., Beyermann, M., & Bienert, M. (1997). Extensive cellular uptake into endothelial cells of an amphipathic β-sheet forming peptide. FEBS Letters, 415, 196–199.
Wyman, T. B., Nicol, F., Zelphati, O., Scaria, P., Plank, C., & Szoka, F. C. (1997). Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry, 36, 3008–3017.
Lin, Y. Z., Yao, S., Veach, R. A., Torgerson, T. R., & Hawiger, J. (1995). Inhibition of nuclear translocation of transcription factor NF-κB by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. Journal of Biological Chemistry, 270, 14255–14258.
Rhee, M., & Davis, P. (2006). Mechanism of uptake of C105Y, a novel cell-penetrating peptide. Journal of Biological Chemistry, 281, 1233–1240.
Hsiao, Y. C., Lee, C. Y., Lin, Y. J., Tsai, S. H., Jeng, K. C. G., Chao, W. T., & Lung, F. D. T. (2013). Design, synthesis, and evaluation of fluorescent cell-penetrating peptidic antagonists of Grb2-SH2 for targeting MCF-7 breast cancer cells. Medicinal Chemistry Research, 22, 5337–5343.
Gomez, J., Gama, V., Yoshida, T., Sun, W., Hayes, P., Leskov, K., Boothman, D., & Matsuyama, S. (2007). Bax-inhibiting peptides derived from Ku70 and cell-penetrating pentapeptides. Biochemical Society Transactions, 35, 797–801.
Lai, P. K., & Kaznessis, Y. N. (2018). Insights into membrane translocation of protegrin antimicrobial peptides by multistep molecular dynamics simulations. ACS Omega, 3, 6056–6065.
Duchardt, F., Ruttekolk, I. R., Verdurmen, W. P., Lortat-Jacob, H., Bürck, J., Hufnagel, H., Fischer, R., van den Heuvel, M., Lowik, D. W. P. M., Vuister, G. W., Ulrich, A., Waard, M., & Brock, R. (2009). A cell-penetrating peptide derived from human lactoferrin with conformation-dependent uptake efficiency. Journal of Biological Chemistry, 284, 36099–36108.
Zhang, X., Oglęcka, K., Sandgren, S., Belting, M., Esbjörner, E. K., Nordén, B., & Graslund, A. (2010). Dual functions of the human antimicrobial peptide LL-37-target membrane perturbation and host cell cargo delivery. Biochimica et Biophysica Acta (BBA)- Biomembranes, 1798, 2201–2208.
Sadler, K., Eom, K. D., Yang, J. L., Dimitrova, Y., & Tam, J. P. (2002). Translocating proline-rich peptides from the antimicrobial peptide bactenecin 7. Biochemistry, 41, 14150–14157.
Veldhuizen, E. J., Schneider, V. A., Agustiandari, H., Van Dijk, A., Tjeerdsma-van Bokhoven, J. L., Bikker, F. J., & Haagsman, H. P. (2014). Antimicrobial and immunomodulatory activities of PR-39 derived peptides. PLoS ONE, 9, e95939.
Franz, J., Lelle, M., Peneva, K., Bonn, M., & Weidner, T. (2016). SAP (E)-A cell-penetrating polyproline helix at lipid interfaces. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1858, 2028–2034.
Wyatt, L.C., Moshnikova, A., Crawford, T., Engelman, D.M., Andreev, O.A., & Reshetnyak, Y.K. (2018). Peptides of pHLIP family for targeted intracellular and extracellular delivery of cargo molecules to tumors. Proceedings of the National Academy of Sciences, 115, E2811-E2818
Li, W., Nicol, F., & Szoka, F. C., Jr. (2004). GALA: A designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Advanced Drug Delivery Reviews, 56, 967–985.
Namvar, A., Bolhassani, A., Khairkhah, N., & Motevalli, F. (2015). Physicochemical properties of polymers: An important system to overcome the cell barriers in gene transfection. Biopolymers, 103, 363–375.
de Souza, G. A. P., Salvador, E. A., de Oliveira, F. R., Malaquias, L. C. C., Abrahão, J. S., & Coelho, L. F. L. (2020). An in silico integrative protocol for identifying key genes and pathways useful to understand emerging virus disease pathogenesis. Virus Research, 284, 197986.
Kardani, K., Milani, A., Shabani, H., & S., & Bolhassani, A. (2019). Cell penetrating peptides: The potent multi-cargo intracellular carriers. Expert Opinion on Drug Delivery, 16, 1227–1258.
Fonseca, S. B., Pereira, M. P., & Kelley, S. O. (2009). Recent advances in the use of cell-penetrating peptides for medical and biological applications. Advanced Drug Delivery Reviews, 61, 953–964.
Szunerits, S., Barras, A., Khanal, M., Pagneux, Q., & Boukherroub, R. (2015). Nanostructures for the inhibition of viral infections. Molecules, 20, 14051–14081.
Bolhassani, A., Jafarzade, B. S., & Mardani, G. (2017). In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides, 87, 50–63.
Wang, L., Liang, R., Gao, Y., Li, Y., Deng, X., Xiang, R., Zhang, Y., Ying, T., Jiang, S., & Yu, F. (2019). Development of small-molecule inhibitors against Zika virus infection. Frontiers in Microbiology, 10, 2725.
Yang, N.J., & Hinner, M.J. (2015). Getting across the cell membrane: an overview for small molecules, peptides, and proteins. Site-specific Protein Labeling, pp: 29–53
Barbu, E., Molnàr, É., Tsibouklis, J., & Górecki, D. C. (2009). The potential for nanoparticle-based drug delivery to the brain: Overcoming the blood-brain barrier. Expert Opinion on Drug Delivery, 6, 553–565.
Torchilin, V. P. (2008). Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Peptide Science, 90, 604–610.
Lim, S., Koo, J. H., & Choi, J. M. (2016). Use of cell-penetrating peptides in dendritic cell-based vaccination. Immune Network, 16, 33–43.
Jiang, Y., Li, M., Zhang, Z., Gong, T., & Sun, X. (2014). Cell-penetrating peptides as delivery enhancers for vaccine. Current Pharmaceutical Biotechnology, 15, 256–266.
Brooks, N., Esparon, S., Pouniotis, D., & Pietersz, G. A. (2015). Comparative immunogenicity of a cytotoxic T cell epitope delivered by penetratin and TAT cell penetrating peptides. Molecules, 20, 14033–14050.
Kristensen, M., Birch, D., & Mørck Nielsen, H. (2016). Applications and challenges for use of cell-penetrating peptides as delivery vectors for peptide and protein cargos. International Journal of Molecular Sciences, 17, 185.
Lu, R. M., Hwang, Y. C., Liu, I. J., Lee, C. C., Tsai, H. Z., Li, H. J., & Wu, H. C. (2020). Development of therapeutic antibodies for the treatment of diseases. Journal of Biomedical Science, 27, 1–30.
Hu, M., Chen, P., Wang, J., Scollard, D. A., Vallis, K. A., & Reilly, R. M. (2007). 123I-labeled HIV-1 tat peptide radioimmunoconjugates are imported into the nucleus of human breast cancer cells and functionally interact in vitro and in vivo with the cyclin-dependent kinase inhibitor, p21WAF-1/Cip-1. European Journal of Nuclear Medicine and Molecular Imaging, 34, 368–377.
Ali, S. A., Teow, S. Y., Omar, T. C., Khoo, A. S. B., Choon, T. S., & Yusoff, N. M. (2016). A cell internalizing antibody targeting capsid protein (p24) inhibits the replication of HIV-1 in T cells lines and PBMCS: A proof of concept study. PLoS ONE, 11, e0145986.
Zhang, J. F., Xiong, H. L., Cao, J. L., Wang, S. J., Guo, X. R., Lin, B. Y., Zhang, Y., Zhao, J. H., Wang, Y. B., Zhang, T. Y., Yuan, Q., Zhang, J., & Xia, N. S. (2018). A cell-penetrating whole molecule antibody targeting intracellular HBx suppresses hepatitis B virus via TRIM21-dependent pathway. Theranostics, 8, 549.
Saurabh, S., Vidyarthi, A. S., & Prasad, D. (2014). RNA interference: Concept to reality in crop improvement. Planta, 239, 543–564.
Martin, M. E., & Rice, K. G. (2007). Peptide-guided gene delivery. The AAPS Journal, 9, E18–E29.
Kleemann, E., Neu, M., Jekel, N., Fink, L., Schmehl, T., Gessler, T., Seeget, W., & Kissel, T. (2005). Nano-carriers for DNA delivery to the lung based upon a TAT-derived peptide covalently coupled to PEG–PEI. Journal of Controlled Release, 109, 299–316.
Hu, B., Zhong, L., Weng, Y., Peng, L., Huang, Y., Zhao, Y., & Liang, X. J. (2020). Therapeutic siRNA: State of the art. Signal Transduction and Targeted Therapy, 5, 1–25.
Wang, J., Chen, G., Liu, N., Han, X., Zhao, F., Zhang, L., & Chen, P. (2022). Strategies for improving the safety and RNAi efficacy of noncovalent peptide/siRNA nanocomplexes. Advances in Colloid and Interface Science, 302, 102638.
Singh, T., Murthy, A. S., Yang, H. J., & Im, J. (2018). Versatility of cell-penetrating peptides for intracellular delivery of siRNA. Drug Delivery, 25, 1996–2006.
Crombez, L., Charnet, A., Morris, M. C., Aldrian-Herrada, G., Heitz, F., & Divita, G. (2007). A non-covalent peptide-based strategy for siRNA delivery. Biochemical Society Transactions, 35, 44–46.
Stone, J. K., Rijnbrand, R., Stein, D. A., Ma, Y., Yang, Y., Iversen, P. L., & Andino, R. (2008). A morpholino oligomer targeting highly conserved internal ribosome entry site sequence is able to inhibit multiple species of picornavirus. Antimicrobial Agents and Chemotherapy, 52, 1970–1981.
Swenson, D. L., Warfield, K. L., Warren, T. K., Lovejoy, C., Hassinger, J. N., Ruthel, G., Blouch, R. E., Moulton, H. M., Weller, D. D., Iversen, P. L., & Bavari, S. (2009). Chemical modifications of antisense morpholino oligomers enhance their efficacy against Ebola virus infection. Antimicrobial Agents and Chemotherapy, 53, 2089–2099.
Neuman, B. W., Stein, D. A., Kroeker, A. D., Churchill, M. J., Kim, A. M., Kuhn, P., Dawson, P., Moulton, H. M., Bestwick, R. K., Iversen, P. L., & Buchmeier, M. J. (2005). Inhibition, escape, and attenuated growth of severe acute respiratory syndrome coronavirus treated with antisense morpholino oligomers. Journal of Virology, 79, 9665–9676.
Moerdyk-Schauwecker, M., Stein, D. A., Eide, K., Blouch, R. E., Bildfell, R., Iversen, P., & Jin, L. (2009). Inhibition of HSV-1 ocular infection with morpholino oligomers targeting ICP0 and ICP27. Antiviral Research, 84, 131–141.
Tripathi, S., Chaubey, B., Barton, B. E., & Pandey, V. N. (2007). Anti HIV-1 virucidal activity of polyamide nucleic acid-membrane transducing peptide conjugates targeted to primer binding site of HIV-1 genome. Virology, 363, 91–103.
Ahn, D. G., Shim, S. B., Moon, J. E., Kim, J. H., Kim, S. J., & Oh, J. W. (2011). Interference of hepatitis C virus replication in cell culture by antisense peptide nucleic acids targeting the X-RNA. Journal of Viral Hepatitis, 18, e298–e306.
Sleeman, K., Stein, D. A., Tamin, A., Reddish, M., Iversen, P. L., & Rota, P. A. (2009). Inhibition of measles virus infections in cell cultures by peptide-conjugated morpholino oligomers. Virus Research, 140, 49–56.
Yoo, J. S., Kim, C. M., Kim, J. H., Kim, J. Y., & Oh, J. W. (2009). Inhibition of Japanese encephalitis virus replication by peptide nucleic acids targeting cis-acting elements on the plus-and minus-strands of viral RNA. Antiviral Research, 82, 122–133.
Gabriel, G., Nordmann, A., Stein, D. A., Iversen, P. L., & Klenk, H. D. (2008). Morpholino oligomers targeting the PB1 and NP genes enhance the survival of mice infected with highly pathogenic influenza A H7N7 virus. Journal of General Virology, 89, 939–948.
Shiraishi, T., Pankratova, S., & Nielsen, P. E. (2005). Calcium ions effectively enhance the effect of antisense peptide nucleic acids conjugated to cationic tat and oligoarginine peptides. Chemistry and Biology, 12, 923–929.
Wolf, Y., Pritz, S., Abes, S., Bienert, M., Lebleu, B., & Oehlke, J. (2006). Structural requirements for cellular uptake and antisense activity of peptide nucleic acids conjugated with various peptides. Biochemistry, 45, 14944–14954.
Endoh, T., & Ohtsuki, T. (2009). Cellular siRNA delivery using cell-penetrating peptides modified for endosomal escape. Advanced Drug Delivery Reviews, 61, 704–709.
Deshayes, S., Morris, M., Heitz, F., & Divita, G. (2008). Delivery of proteins and nucleic acids using a non-covalent peptide-based strategy. Advanced Drug Delivery Reviews, 60, 537–547.
Sung, W. S., Park, Y., Choi, C. H., Hahm, K. S., & Lee, D. G. (2007). Mode of antibacterial action of a signal peptide, Pep27 from Streptococcus pneumoniae. Biochemical and Biophysical Research Communications, 363, 806–810.
Kang, H. K., Park, J., Seo, C. H., & Park, Y. (2021). PEP27-2, a potent antimicrobial cell-penetrating peptide, reduces skin abscess formation during Staphylococcus aureus infections in mouse when used in combination with antibiotics. ACS Infectious Diseases, 7, 2620–2636.
Keogan, S., Passic, S., & Krebs, F. C. (2012). Infection by CXCR4-tropic human immunodeficiency virus type 1 is inhibited by the cationic cell-penetrating peptide derived from HIV-1 tat. International Journal of Peptides, 2012, 349427.
Ruzsics, Z., Hoffmann, K., Riedl, A., Krawczyk, A., Widera, M., Sertznig, H., Schipper, L., Kapper-Falcone, V., Debreczeny, M., Ernst, W., Grabherr, R., Hengel, H., & Harant, H. (2020). A novel, broad-acting peptide inhibitor of double-stranded DNA virus gene expression and replication. Frontiers in Microbiology, 11, 601555.
Chaubey, B., Tripathi, S., Ganguly, S., Harris, D., Casale, R. A., & Pandey, V. N. (2005). A PNA-transportan conjugate targeted to the TAR region of the HIV-1 genome exhibits both antiviral and virucidal properties. Virology, 331, 418–428.
Pan, X. B., Wei, L., Han, J. C., Ma, H., Deng, K., & Cong, X. (2011). Artificial recombinant cell-penetrating peptides interfere with envelopment of hepatitis B virus nucleocapsid and viral production. Antiviral Research, 89, 109–114.
Zhang, P., Moreno, R., Lambert, P. F., & DiMaio, D. (2020). Cell-penetrating peptide inhibits retromer-mediated human papillomavirus trafficking during virus entry. Proceedings of the National Academy of Sciences, 117, 6121–6128.
Vocero-Akbani, A. M., Heyden, N. V., Lissy, N. A., Ratner, L., & Dowdy, S. F. (1999). Killing HIV-infected cells by transduction with an HIV protease-activated caspase-3 protein. Nature Medicine, 5, 29–33.
Chu, X., Wu, B., Fan, H., Hou, J., Hao, J., Hu, J., Wang, B., Liu, G., Li, C., & Meng, S. (2016). PTD-fused p53 as a potential antiviral agent directly suppresses HBV transcription and expression. Antiviral Research, 127, 41–49.
Darbinian, N., Popov, Y., Khalili, K., & Amini, S. (2008). Creation of a bi-directional protein transduction system for suppression of HIV-1 expression by p27SJ. Antiviral Research, 79, 136–141.
Zhang, X. M., He, D. N., Zhou, B., Pang, R., Liu, K., Zhao, J., & Chen, P. (2013). In vitro inhibition of vesicular stomatitis virus replication by purified porcine Mx1 protein fused to HIV-1 Tat protein transduction domain (PTD). Antiviral Research, 99, 149–157.
Mino, T., Mori, T., Aoyama, Y., & Sera, T. (2008). Cell-permeable artificial zinc-finger proteins as potent antiviral drugs for human papillomaviruses. Archives of Virology, 153, 1291–1298.
Theisen, D. M., Pongratz, C., Wiegmann, K., Rivero, F., Krut, O., & Krönke, M. (2006). Targeting of HIV-1 Tat traffic and function by transduction-competent single chain antibodies. Vaccine, 24, 3127–3136.
Wang, Y., Li, Y., Li, N., Zhu, Q., Hui, L., Liu, X., Han, Q., Lv, Y., Wang, Q., Yang, G., Zhou, Z., & Liu, Z. (2015). Transbody against hepatitis B virus core protein inhibits hepatitis B virus replication in vitro. International Immunopharmacology, 25, 363–369.
Glab-Ampai, K., Chulanetra, M., Malik, A. A., Juntadech, T., Thanongsaksrikul, J., Srimanote, P., & Chaicumpa, W. (2017). Human single chain-transbodies that bound to domain-I of non-structural protein 5A (NS5A) of hepatitis C virus. Scientific Reports, 7, 1–18.
Glab-Ampai, K., Malik, A. A., Chulanetra, M., Thanongsaksrikul, J., Thueng-In, K., Srimanote, P., Tongtawe, P., & Chaicumpa, W. (2016). Inhibition of HCV replication by humanized-single domain transbodies to NS4B. Biochemical and Biophysical Research Communications, 476, 654–664.
Poungpair, O., Pootong, A., Maneewatch, S., Srimanote, P., Tongtawe, P., Songserm, T., Tapchaisri, P., & Chaicumpa, W. (2010). A human single chain transbody specific to matrix protein (M1) interferes with the replication of influenza A virus. Bioconjugate Chemistry, 21, 1134–1141.
Teimoori, S., Seesuay, W., Jittavisutthikul, S., Chaisri, U., Sookrung, N., Densumite, J., Saelim, N., Chulanetra, M., Maneewatch, S., & Chaicumpa, W. (2016). Human transbodies to VP40 inhibit cellular egress of Ebola virus-like particles. Biochemical and Biophysical Research Communications, 479, 245–252.
Bivalkar-Mehla, S., Mehla, R., & Chauhan, A. (2017). Chimeric peptide-mediated siRNA transduction to inhibit HIV-1 infection. Journal of Drug Targeting, 25, 307–319.
Zhang, C., Ren, W., Liu, Q., Tan, Z., Li, J., & Tong, C. (2019). Transportan-derived cell-penetrating peptide delivers siRNA to inhibit replication of influenza virus in vivo. Drug Design, Development and Therapy, 13, 1059.
Meng, S., Wei, B., Xu, R., Zhang, K., Wang, L., Zhang, R., & Li, J. (2009). TAT peptides mediated small interfering RNA delivery to Huh-7 cells and efficiently inhibited hepatitis C virus RNA replication. Intervirology, 52, 135–140.
Zeng, Z., Han, S., Hong, W., Lang, Y., Li, F., Liu, Y., Li, Z., Wu, Y., Li, W., Zhang, X., & Cao, Z. (2016). A Tat-conjugated peptide nucleic acid Tat-PNA-DR inhibits hepatitis B virus replication in vitro and in vivo by targeting LTR direct repeats of HBV RNA. Molecular Therapy-Nucleic Acids, 5, e295.
Turner, J. J., Ivanova, G. D., Verbeure, B., Williams, D., Arzumanov, A. A., Abes, S., Lebleu, B., & Gait, M. J. (2005). Cell-penetrating peptide conjugates of peptide nucleic acids (PNA) as inhibitors of HIV-1 Tat-dependent trans-activation in cells. Nucleic Acids Research, 33, 6837–6849.
Ahn, D. G., Lee, W., Choi, J. K., Kim, S. J., Plant, E. P., Almazán, F., Taylor, D. R., Enjuanes, L., & Oh, J. W. (2011). Interference of ribosomal frameshifting by antisense peptide nucleic acids suppresses SARS coronavirus replication. Antiviral Research, 91, 1–10.
Enterlein, S., Warfield, K. L., Swenson, D. L., Stein, D. A., Smith, J. L., Gamble, C. S., Kroeker, A. D., Iversen, P. L., Bavari, S., & Muhlberger, E. (2006). VP35 knockdown inhibits Ebola virus amplification and protects against lethal infection in mice. Antimicrobial Agents and Chemotherapy, 50, 984–993.
Ge, Q., Pastey, M., Kobasa, D., Puthavathana, P., Lupfer, C., Bestwick, R. K., Iversen, P. L., Chen, J., & Stein, D. A. (2006). Inhibition of multiple subtypes of influenza A virus in cell cultures with morpholino oligomers. Antimicrobial Agents and Chemotherapy, 50, 3724–3733.
Duchardt, F., Fotin-Mleczek, M., Schwarz, H., Fischer, R., & Brock, R. (2007). A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic, 8, 848–866.
Silva, S., Almeida, A. J., & Vale, N. (2019). Combination of cell-penetrating peptides with nanoparticles for therapeutic application: A review. Biomolecules, 9, 22.
Alves, I. D., Jiao, C. Y., Aubry, S., Aussedat, B., Burlina, F., Chassaing, G., & Sagan, S. (2010). Cell biology meets biophysics to unveil the different mechanisms of penetratin internalization in cells. Biochimica et Biophysica Acta (BBA)-Biomembranes., 1798, 2231–2239.
Bechara, C., & Sagan, S. (2013). Cell-penetrating peptides: 20 years later, where do we stand? FEBS Letters, 587, 1693–1702.
Deshayes, S., Heitz, A., Morris, M. C., Charnet, P., Divita, G., & Heitz, F. (2004). Insight into the mechanism of internalization of the cell-penetrating carrier peptide Pep-1 through conformational analysis. Biochemistry, 43, 1449–1457.
Chassaing, G., Sagan, S., Lequin, O., Lamaziere, A., Ayala-Sanmartin, J., & Trugnan, G. (2005). Six inverted lipid models: A pathway for peptide internalization? Half Title, 89
Lim, J. P., & Gleeson, P. A. (2011). Macropinocytosis: An endocytic pathway for internalising large gulps. Immunology and Cell Biology, 89, 836–843.
El-Sayed, A., & Harashima, H. (2013). Endocytosis of gene delivery vectors: From clathrin-dependent to lipid raft-mediated endocytosis. Molecular Therapy, 21, 1118–1130.
Pujals, S., & Giralt, E. (2008). Proline-rich, amphipathic cell-penetrating peptides. Advanced Drug Delivery Reviews, 60, 473–484.
Rejman, J., Oberle, V., Zuhorn, I. S., & Hoekstra, D. (2004). Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochemical Journal, 377, 159–169.
Futaki, S., Suzuki, T., Ohashi, W., Yagami, T., Tanaka, S., Ueda, K., & Sugiura, Y. (2001). Arginine-rich peptides: An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. Journal of Biological Chemistry, 276, 5836–5840.
Yang, S. T., Zaitseva, E., Chernomordik, L. V., & Melikov, K. (2010). Cell-penetrating peptide induces leaky fusion of liposomes containing late endosome-specific anionic lipid. Biophysical Journal, 99, 2525–2533.
El-Sayed, A., Futaki, S., & Harashima, H. (2009). Delivery of macromolecules using arginine-rich cell-penetrating peptides: Ways to overcome endosomal entrapment. The AAPS Journal, 11, 13–22.
Wadia, J. S., Stan, R. V., & Dowdy, S. F. (2004). Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nature Medicine, 10, 310–315.
Wender, P. A., Mitchell, D. J., Pattabiraman, K., Pelkey, E. T., Steinman, L., & Rothbard, J. B. (2000). The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proceedings of the National Academy of Sciences, 97, 13003–13008.
Jiang, Y., Ruta, V., Chen, J., Lee, A., & MacKinnon, R. (2003). The principle of gating charge movement in a voltage-dependent K+ channel. Nature, 423, 42–48.
Åmand, H. L., Fant, K., Nordén, B., & Esbjörner, E. K. (2008). Stimulated endocytosis in penetratin uptake: Effect of arginine and lysine. Biochemical and Biophysical Research Communications, 371, 621–625.
Jobin, M. L., Blanchet, M., Henry, S., Chaignepain, S., Manigand, C., Castano, S., Lecomte, S., Burlina, F., Sagan, S., & Alves, I. D. (2015). The role of tryptophans on the cellular uptake and membrane interaction of arginine-rich cell penetrating peptides. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1848, 593–602.
Kurzawa, L., Pellerano, M., & Morris, M. C. (2010). PEP and CADY-mediated delivery of fluorescent peptides and proteins into living cells. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1798, 2274–2285.
Kamide, K., Nakakubo, H., Uno, S., & Fukamizu, A. (2010). Isolation of novel cell-penetrating peptides from a random peptide library using in vitro virus and their modifications. International Journal of Molecular Medicine, 25, 41–51.
Henriques, S. T., & Castanho, M. A. (2008). Translocation or membrane disintegration? Implication of peptide–membrane interactions in pep-1 activity. Journal of Peptide Science: An Official Publication of the European Peptide Society, 14, 482–487.
Meng, Z., Luan, L., Kang, Z., Feng, S., Meng, Q., & Liu, K. (2017). Histidine-enriched multifunctional peptide vectors with enhanced cellular uptake and endosomal escape for gene delivery. Journal of Materials Chemistry B, 5, 74–84.
Váňová, J., Číhařová, B., Hejtmánková, A., Epperla, C. P., Škvára, P., Forstová, J., Kalbacova, M. H., & Spanielova, H. (2022). VirPorters: Insights into the action of cationic and histidine-rich cell-penetrating peptides. International Journal of Pharmaceutics, 611, 121308.
Munyendo, W. L., Lv, H., Benza-Ingoula, H., Baraza, L. D., & Zhou, J. (2012). Cell penetrating peptides in the delivery of biopharmaceuticals. Biomolecules, 2, 187–202.
Gessner, I., & Neundorf, I. (2020). Nanoparticles modified with cell-penetrating peptides: Conjugation mechanisms, physicochemical properties, and application in cancer diagnosis and therapy. International Journal of Molecular Sciences, 21, 2536.
Brooks, N. A., Pouniotis, D. S., Tang, C. K., Apostolopoulos, V., & Pietersz, G. A. (2010). Cell-penetrating peptides: application in vaccine delivery. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1805, 25–34.
Sadeghian, I., Heidari, R., Sadeghian, S., Raee, M. J., & Negahdaripour, M. (2021). Potential of cell-penetrating peptides (CPPs) in delivery of antiviral therapeutics and vaccines. European Journal of Pharmaceutical Sciences, 169, 106094.
Kapoor, P., Singh, H., Gautam, A., Chaudhary, K., Kumar, R., & Raghava, G. P. (2012). TumorHoPe: A database of tumor homing peptides. PLoS ONE, 7, e35187.
Meng, F., Sun, Y., Lee, R. J., Wang, G., Zheng, X., Zhang, H., Fu, Y., Yan, G., Wang, Y., Deng, W., Parks, E., Kim, B. Y. S., Yang, Z., Jiang, W., & Teng, L. (2019). Folate receptor-targeted albumin nanoparticles based on microfluidic technology to deliver cabazitaxel. Cancers, 11, 1571.
Yang, X., Yang, S., Chai, H., Yang, Z., Lee, R. J., Liao, W., & Teng, L. (2015). A novel isoquinoline derivative anticancer agent and its targeted delivery to tumor cells using transferrin-conjugated liposomes. PLoS ONE, 10, e0136649.
Ye, J., Shin, M. C., Liang, Q., He, H., & Yang, V. C. (2015). 15 years of ATTEMPTS: A macromolecular drug delivery system based on the CPP-mediated intracellular drug delivery and antibody targeting. Journal of Controlled Release, 205, 58–69.
Xie, J., Bi, Y., Zhang, H., Dong, S., Teng, L., Lee, R. J., & Yang, Z. (2020). Cell-penetrating peptides in diagnosis and treatment of human diseases: From preclinical research to clinical application. Frontiers in Pharmacology, 11, 697.
Järver, P., Mäger, I., & Langel, Ü. (2010). In vivo biodistribution and efficacy of peptide mediated delivery. Trends in Pharmacological Sciences, 31, 528–535.
Saar, K., Lindgren, M., Hansen, M., Eiriksdottir, E., Jiang, Y., Rosenthal-Aizman, K., Sassian, M., & Langel, U. (2005). Cell-penetrating peptides: A comparative membrane toxicity study. Analytical Biochemistry, 345, 55–65.
Erazo-Oliveras, A., Muthukrishnan, N., Baker, R., Wang, T. Y., & Pellois, J. P. (2012). Improving the endosomal escape of cell-penetrating peptides and their cargos: Strategies and challenges. Pharmaceuticals, 5, 1177–1209.
Kersemans, V., Kersemans, K., & Cornelissen, B. (2008). Cell penetrating peptides for in vivo molecular imaging applications. Current Pharmaceutical Design, 14, 2415–2427.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. NK., AN., and AB. performed literature search, draft writing, and manuscript revisions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical Approval
This article does not contain any studies with human participants or animals performed by author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khairkhah, N., Namvar, A. & Bolhassani, A. Application of Cell Penetrating Peptides as a Promising Drug Carrier to Combat Viral Infections. Mol Biotechnol 65, 1387–1402 (2023). https://doi.org/10.1007/s12033-023-00679-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00679-1