Abstract
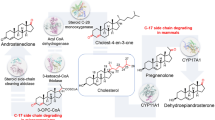
The cholesterol hydroxylase/lyase (CHL) system, located in the mitochondria of the mammalian adrenal cortex cells, consists of cytochrome P450scc (CYP11A1), adrenodoxin (Adx), and adrenodoxin reductase (AdR) and performs the first stage of the steroidogenesis: AdR and Adx enable the electron transfer between NADPH and cytochrome P450scc, and P450scc catalyzes the conversion of cholesterol into pregnenolone. CHL system was reconstructed in Escherichia coli using the polycistronic plasmid pTrc99A/CHL. In E. coli cells, the recombinant proteins form the catalytically active system. CHL activity towards 22R-hydroxycholesterol was 4.0 ± 1.3 nmol pregnenolone/h per 1 mg homogenate protein. The alteration of the order of heterologous cDNAs in the expression cassette from AdR–Adx–P450scc to P450scc–Adx–AdR results in alteration of stoichiometric ratio P450scc/Adx/AdR from 1:1.45:4.2 to 1:1.67:0.98; the former ratio is more optimal for the functioning of the cytochrome P450scc. The application of modified cDNA of Adx (AdxS112W) does not increase the CHL activity; however, the introduction of the second copy of AdxS112W gene into the expression cassette increases both the expression level of АdxS112W and the CHL activity in comparison with P450scc/АdxS112W/AdR system. In vivo activity of the CHL system in bacteria is limited by the substrate uptake by bacterial cells: it varied in the range of 0.05–0.62 mg pregnenolone/l resting cell suspension per 1-day cultivation, depending on the type and concentration of permeabilizing agents in the medium. The obtained results contribute to the knowledge of CHL system functioning in living bacteria.






Similar content being viewed by others
References
Bernhard, R. (2006). Cytochromes P450 as versatile biocatalysts. Journal of Biotechnology, 124, 128–145. https://doi.org/10.1016/j.jbiotec.2006.01.026.
Miller, W. L. (2008). Steroidogenic enzymes. Endocrine Development, 13, 1–18. https://doi.org/10.1159/000134751.
Urlacher, V. B., & Girhard, M. (2012). Cytochrome P450 monooxygenases: An update on perspectives for synthetic application. Trends in Biotechnology, 30, 26–36. https://doi.org/10.1016/j.tibtech.2011.06.012.
Nelson, D. R., Kamataki, T., Waxman, D. J., Guengerich, F. P., Estabrook, R. W., Feyereisen, R., Gonzalez, F. J., Coon, M. J., Gunsalus, I. C., Gotoh, O., et al. (1993). The P450 superfamily: Update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cellular Biology, 12, 1–51. https://doi.org/10.1089/dna.1993.12.1.
Hanlon, S. P., Friedberg, T., Wolf, C. R., Chisalba, O., & Kittelmann, M. (2012). Recombinant yeast and bacteria that express human P450s: Bioreactors, for drug discovery, development, and biotechnology. In R. D. Schmid & V. B. Urlacher (Eds.), Modern Biooxidation: Enzymes, Reactions and Applications (pp. 233–252). New York: Wiley.
Wada, A., Mathew, P. A., Barnes, H. J., Sanders, D., Estabrook, R. W., & Waterman, M. R. (1991). Expression of functional bovine cholesterol side chain cleavage cytochrome P450 (P450scc) in Escherichia coli. Archives of Biochemistry and Biophysics, 290, 376–380.
Sagara, Y., Barnes, H., & Waterman, M. R. (1993). Expression in Escherichia coli of functional cytochrome P450c17 lacking its hydrophobic amino-terminal signal anchor. Archives of Biochemistry and Biophysics, 304, 272–278. https://doi.org/10.1006/abbi.1993.1349.
Barnes, H. J., Arlotto, M. P., & Waterman, M. R. (1991) Expression and enzymatic activity of recombinant cytochrome P450 17 alpha-hydroxylase in Escherichia coli. Proceedings of National Academy Science United states of America, 88, 5597–5601.
Hu, M. C., & Chung, B. C. (1990). Expression of human 21-hydroxylase (P450c21) in bacterial and mammalian cells: A system to characterize normal and mutant enzymes. Molecular Endocrinology, 4, 893–898. https://doi.org/10.1210/mend-4-6-893.
Osawa, Y., Higashiyama, T., Toma, Y., & Yarborough, C. (1997). Diverse function of aromatase and the N-terminal sequence deleted form. Journal of Steroid Biochemistry and Molecular Biology, 61, 117–126.
Nonaka, Y., Fujii, T., Kagava, N., Waterman, M. R., Takemori, H., & Okamoto, M. (1998). Structure/function relationship of CYP11B1 associated with Dahl’s salt-resistant rats—expression of rat CYP11B1 and CYP11B2 in Escherichia coli. European Journal of Biochemistry, 258, 869–878.
Novikova, L. A., Faletrov, Y. V., Kovaleva, I. E., Mauersberger, S., Luzikov, V. N., & Shkumatov, V. M. (2009). From structure and functions of steroidogenic enzymes to new technologies of gene engineering. Biochemistry, 74, 1482–1504.
Shet, M. S., Fisher, C. W., & Estabrook, R. W. (1997). The function of recombinant cytochrome P450s in intact Escherichia coli cells: The 17 alpha-hydroxylation of progesterone and pregnenolone by P450c17. Archives of Biochemistry and Biophysics, 339, 218–225. https://doi.org/10.1006/abbi.1996.9868.
Ehmer, P. B., Jose, J., & Hartmann, R. W. (2000). Development of a simple and rapid assay for the evaluation of inhibitors of human 17alpha-hydroxylase-C(17,20)-lyase (P450cl7) by coexpression of P450cl7 with NADPH-cytochrome-P450-reductase in Escherichia coli. The Journal of Steroid Biochemistry and Molecular Biology, 75, 57–63.
Shkumatov, V. M., Radyuk, V. G., Falertov, Y. V., Vinogradova, A. A., Luzikov, V. N., & Novikova, L. A. (2006). Expression of cytochrome P450scc in Escherichia coli cells: Intracellular location and interaction with bacterial redox proteins. Biochemistry, 71, 884–892.
Shet, M. S., Fisher, C. W., Arlotto, M. P., Shackleton, C. H., Holmans, P. L., Martin-Wixtrom, C. A., Saeki, Y., & Estabrook, R. W. (1994). Purification and enzymatic properties of a recombinant fusion protein expressed in Escherichia coli containing the domains of bovine P450 17A and rat NADPH-P450 reductase. Archives of Biochemistry and Biophysics, 311, 402–417.
Nazarov, P. A., Drutsa, V. L., Miller, W. L., Shkumatov, V. M., Luzikov, V. N., & Novikova, L. A. (2003). Formation and functioning of fused cholesterol side-chain cleavage enzymes. DNA Cellular Biology, 22, 243–252. https://doi.org/10.1089/104454903321908638.
Shashkova, T. V., Luzikov, V. N., & Novikova, L. A. (2006). Coexpression of all constituents of the cholesterol hydroxylase/lyase system in Escherichia coli cells. Biochemistry, 71, 810–814.
Makeeva, D. S., Dovbnya, D. V., Donova, M. V., & Novikova, L. A. (2013). Functional reconstruction of bovine P450scc steroidogenic system in Escherichia coli. American Journal of Molecular Biology, 3, 173–182. https://doi.org/10.4236/ajmb.2013.34023.
Brixius-Anderko, S., Schiffer, L., Hannemann, F., Janocha, B., & Bernhardt, R. (2015). A CYP21A2 based whole-cell system in Escherichia coli for the biotechnological production of premedrol. Microbiology Cell Fact, 14, 135. https://doi.org/10.1186/s12934-015-0333-2.
Schiffer, L., Anderko, S., Hobler, A., Hannemann, F., Kagawa, N., & Bernhardt, R. (2015). A recombinant CYP11B1 dependent Escherichia coli biocatalyst for selective cortisol production and optimization towards a preparative scale. Microbiology Cell Fact, 14, 25. https://doi.org/10.1186/s12934-015-0209-5.
Schiffer, L., Müller, A.-R., Hobler, A., Brixius-Anderko, S., Zapp, J., Hannemann, F., & Bernhardt, R. (2016). Biotransformation of the mineralocorticoid receptor antagonists spironolactone and canrenone by human CYP11B1 and CYP11B2: Characterization of the products and their influence on mineralocorticoid receptor transactivation. Journal of Steroid Biochemistry and Molecular Biology, 163, 68–76. https://doi.org/10.1016/j.jsbmb.2016.04.004.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual (2nd edn.). Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
Akhrem, A. A., Lapko, V. N., Lapko, A. G., Shkumatov, V. M., & Chashchin, V. L. (1979). Isolation, structural organization and mechanism of action of mitochondrial steroid hydroxylating systems. Acta Biology Medicine of Germany, 38, 257–273.
Chashchin, V. L., Vasilevsky, V. I., Shkumatov, V. M., Lapko, V. N., Adamovich, T. B., Berikbaeva, T. M., & Akhrem, A. A. (1984). The domain structure of the cholesterol side-chain cleavage cytochrome P-450 from bovine adrenocortical mitochondria. Localization of haem group and domains in the polypeptide chain. Biochim Biophysics Acta, 791, 375–383.
Hannemann, F., Virus, C., & Bernhardt, R. (2006). Design of an Escherichia coli system for whole cell mediated steroid synthesis and molecular evolution of steroid hydroxylases. Journal of Biotechnology, 124, 172–181. https://doi.org/10.1016/j.jbiotec.2006.01.009.
Efimova, V. S., Isaeva, L. V., Makeeva, D. S., Rubtsov, M. A., & Novikova, L. A. (2017). Expression of cholesterol hydroxylase/lyase system proteins in yeast s. cerevisiae cells as a self-processing polyprotein. Molecular Biotechnology, 59, 394–406. https://doi.org/10.1007/s12033-017-0028-5.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Towbin, H., Staehelin, T., & Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceeding of National Academy Science United States of America. 76, 4350–4354.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–270.
Vinogradova, A. A., Luzikov, V. N., & Novikova, L. A. (2007). Comparative study of topogenesis of cytochrome P450scc (CYP11A1) and its hybrids with adrenodoxin expressed in Escherichia coli cells. Biochemistry, 72, 208–214.
Parikh, A., Gillam, E. M., & Guengerich, F. P. (1997). Drug metabolism by Escherichia coli expressing human cytochromes P450. Nature Biotechnology, 15, 784–788. https://doi.org/10.1038/nbt0897-784.
Sawada, N., Sakaki, T., Kitanaka, S., Takeyama, K., Kato, S., & Inouye, K. (1999). Enzymatic properties of human 25-hydroxyvitamin D3 1α-hydroxylase. European Journal of Biochemistry, 265, 959–956.
Hanukoglu, I., & Hanukoglu, Z. (1986). Stoichiometry of mitochondrial cytochromes P-450, adrenodoxin and adrenodoxin reductase in adrenal cortex and corpus luteum. Implications for membrane organization and gene regulation. European Journal of Biochemistry, 157, 27–31.
Usanov, S. A., Chernogolov, A. A., Petrashin, A. I., Akhrem, A. A., & Chashchin, V. L. (1987). Immunochemical study of the cholesterol hydroxylating system topology and stoichiometry of the electron transport chain components in mitochondrial membrane. Biological Membranes, 4, 1102–1116.
Uhlmann, H., Kraft, R., & Bernhardt, R. (1994). C-terminal region of adrenodoxin affects its structural integrity and determines differences in its electron transfer function to cytochrome P-450. Journal of Biological Chemistry, 269, 22557–22564.
Schiffler, B., Kiefer, M., Wilken, A., Hannemann, F., Adolph, H. W., & Bernhardt, R. (2001). The interaction of bovine adrenodoxin with CYP11A1 (cytochrome P450scc) and CYP11B1 (cytochrome P45011beta). Acceleration of reduction and substrate conversion by site-directed mutagenesis of adrenodoxin. Journal of Biological Chemistry, 276, 36225–36232. https://doi.org/10.1074/jbc.M102320200.
Ringle, M., Khatri, Y., Zapp, J., Hannemann, F., & Bernhardt, R. (2013). Application of a new versatile electron transfer system for cytochrome P450-based Escherichia coli whole-cell bioconversions. Applied Microbiology and Biotechnology, 97, 7741–7754. https://doi.org/10.1007/s00253-012-4612-0.
Janocha, S., & Bernhardt, R. (2013). Design and characterization of an efficient CYP105A1-based whole-cell biocatalyst for the conversion of resin acid diterpenoids in permeabilized Escherichia coli. Applied Microbiology and Biotechnology, 97, 7639–7649. https://doi.org/10.1007/s00253-013-5008-5.
Donova, M. V., & Egorova, O. V. (2012). Microbial steroid transformations: Current state and prospects. Applied Microbiology and Biotechnology, 94, 1423–1447. https://doi.org/10.1007/s00253-012-4078-0.
Wallimann, P., Marti, T., Furer, A., & Diederich, F. (1997). Steroids in molecular recognition. Chemical Reviews, 97, 1567–1608.
Hesselink, P. G. M., van Vliet, S., de Vries, H., & Witholt, B. (1989). Optimization of steroid side-chain cleavage by Mycobacterium sp. in the presence of cyclodextrins. Enzyme Microb. Technol, 11, 398–404. https://doi.org/10.1016/0141-0229(89)90133-6.
Suzuki, C. K., Rep, M., van Dijl, J. M., Suda, K., Grivell, L. A., & Schatz, G. (1997). ATP-dependent proteases that also chaperone protein biogenesis. Trends in Biochemical Sciences, 22, 118–123.
Guengerich, F. P., Gillam, E. M. J., Ohmori, S., Sandhu, P., Brian, W. R., Sari, M.–A., & Iwasaki, M. (1993). Expression of human cytochrome P450 enzymes in yeast and bacteria and relevance to studies on catalytic specificity. Toxicology, 1993, 82, 21–37.
Acknowledgements
The authors would like to thank M. S. Serebryakova for conducting the mass spectrometry experiments, and V. N. Tashlitsky for conducting the experiments using the HPLC.
Funding
This research was supported by Russian Foundation for Basic Research (Grant No. 16-54-00139).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors state that they have no Conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Efimova, V.S., Isaeva, L.V., Rubtsov, M.A. et al. Analysis of In Vivo Activity of the Bovine Cholesterol Hydroxylase/Lyase System Proteins Expressed in Escherichia coli. Mol Biotechnol 61, 261–273 (2019). https://doi.org/10.1007/s12033-019-00158-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-019-00158-6