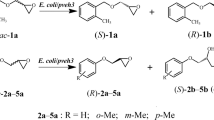
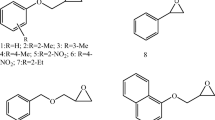
Abstract
Epoxide hydrolases (EHs; 3.3.2.x) catalyze the enantioselective ring opening of racemic epoxides to the corresponding enantiopure vicinal diols and remaining equivalent unreacted epoxides. These epoxides and diols are used for the synthesis of chiral drug intermediates. With an upsurge in the methods for identification of novel microbial EHs, a lot of EHs have been discovered and utilized for kinetic resolution of racemic epoxides. However, there is still a constraint on the account of limited EHs being successfully applied on the preparative scale for industrial biotransformations. This limitation has to be overcome before application of identified functional EHs on large scale. Many strategies such as optimizing reaction media, immobilizing EHs and laboratory-scale directed evolution of EHs have been adopted for enhancing the industrial potential of EHs. In this review, these approaches have been highlighted which can serve as a pathway for the enrichment of already identified EHs for their application on an industrial scale in future studies.





Similar content being viewed by others
Abbreviations
- EHs:
-
Epoxide hydrolase
- ILs:
-
Ionic liquids
- DESs :
-
Deep eutectic solvents
- DMSO:
-
Dimethylsulfoxide
- HBDs:
-
Hydrogen bond donors
- EDA:
-
Ethylenediamine
- IDA:
-
Iminodiacetic acid
- CPG:
-
Calcium pectate gelled
- SA:
-
Sodium alginate
- CS:
-
Cellulose sulfate
- PMCG:
-
Poly (methylene-co-guanidine)
- Fmoc-FF:
-
Fluorenylmethoxycarbonyl diphenylalanine
- PEI:
-
Polymer, polyethyleneimine
- CLEAS:
-
Cross-linked enzyme aggregates
- epPCR:
-
Error-prone polymerase chain reaction
- PCR:
-
Polymerase chain reaction
- ISM:
-
Iterative saturation mutagenesis
- CAST:
-
Combinatorial Active Site Saturation Test
- B-FIT:
-
B-Factor Iterative Test
- ASRA:
-
Adaptive substituent reordering algorithm
- FRESCO:
-
Framework for Rapid Enzyme Stabilization by Computational Libraries
References
Lin, H., Liu, J.-Y., Wang, H.-B., Ahmed, A. A. Q., & Wu, Z.-L. (2011). Biocatalysis as an alternative for the production of chiral epoxides: a comparative review. Journal of Molecular Catalysis. B, Enzymatic, 72(3–4), 77–89.
Breuer, M., Ditrich, K., Habicher, T., Hauer, B., Keßeler, M., Stürmer, R., et al. (2004). Industrial methods for the production of optically active intermediates. Angewandte Chemie Int Ed, 43(7), 788–824.
Wohlgemuth, R. (2010). Biocatalysis-key to sustainable industrial chemistry. Current Opinion in Biotechnology, 21(6), 713–724.
Lin, H., Liu, Y., & Wu, Z.-L. (2011). Asymmetric epoxidation of styrene derivatives by styrene monooxygenase from Pseudomonas sp. LQ26: effects of α- and β-substituents. Tetrahedron Asymmetry, 22(2), 134–137.
Xue, F., Liu, Z.-Q., Zou, S.-P., Wan, N.-W., Zhu, W.-Y., Zhu, Q., et al. (2014). A novel enantioselective epoxide hydrolase from Agromyces mediolanus ZJB120203: cloning, characterization and application. Process Biochemistry, 49(3), 409–417.
Jimenez, D. J., Dini-Andreote, F., Ottoni, J. R., de Oliveira, V. M., van Elsas, J. D., & Andreote, F. D. (2015). Compositional profile of α/β-hydrolase fold proteins in mangrove soil metagenomes: prevalence of epoxide hydrolases and haloalkane dehalogenases in oil-contaminated sites. Microbial Biotechnology, 8(3), 604–613.
Saini, P., Wani, S. I., Kumar, R., Chhabra, R., Chimni, S. S., & Sareen, D. (2014). Trigger factor assisted folding of the recombinant epoxide hydrolases identified from C. pelagibacter and S. nassauensis. Protein Expression and Purification, 104C, 71–84.
van Loo, B., Kingma, J., Arand, M., Wubbolts, M. G., & Janssen, D. B. (2006). Diversity and biocatalytic potential of epoxide hydrolases identified by genome analysis. Applied and Environmental Microbiology, 72(4), 2905–2917.
Lee, E. Y., & Shuler, M. L. (2007). Molecular engineering of epoxide hydrolase and its application to asymmetric and enantioconvergent hydrolysis. Biotechnology and Bioengineering, 98(2), 318–327.
de Vries, E. J., & Janssen, D. B. (2003). Biocatalytic conversion of epoxides. Current Opinion in Biotechnology, 14(4), 414–420.
Choi, W. J., & Choi, C. Y. (2005). Production of chiral epoxides: epoxide hydrolase-catalyzed enantioselective hydrolysis. Biotechnology and Bioprocess Engineering, 10(3), 167–179.
Dauvrin, T., Couthuin, Deslee, A., & Braives. (2002). Epoxide Hydrolase, Patent Publication No. US 6,379,938 B1. 30 April, 2002.
Arand, M., Archelas, A. R., Baratti, J., & Furstoss, R. (2006). Epoxide hydrolase of Aspergillus origin, Patent Publication No. US 7,060,477 B2. 13 June, 2006.
Zocher, F., Enzelberger, M., Schmid, R. D., Wohleben, W., & Hauer, B. (2004). Epoxide hydrolase from Streptomyces, Patent Publication No. US 6,828,115 B1. 7 December, 2004.
Zhao, L., Mathur, E. J., Weiner, D., Richardson, T., Milan, A., Burk, M. J., … Short, J. M. (2005). Epoxide hydrolases, nucleic acids encoding them and methods of making and using them. patent Publication No. US 6,979,733 B2. 27 December, 2005.
Chartrain, M. M., Senanayake, C. H., Rosazza, J. P. N., & Zhang, J. (1998). Resolution of racemic indene oxide to yield (1S,2R)-indene oxide using Diplodia gossipina. Patent Publication No. US 5,849,568 A. 15 December, 1998.
Spelberg, J. H. L., Rink, R., Haren, R. M. K., & Roden, D. B. J. (2002). Enantioselective epoxide hydrolases and genes encoding these. Patent Publication No. US 6,387,668 B1. 14 May, 2002.
Kim, S.-J., Kang, S.-G., Hwang, Y.-O., Woo, J.-H., Cho, J.-C., Kang, J.-H., & Kwon, K.-K. (2011). Enantioselective epoxide hydrolase and method for preparing and enantiopure epoxide using the same, Patent Publication No. US 8,030,048 B2. 4 October, 2011.
Kamal, A., Khanna, R., Kumar, G., Shaik, A. B., & Kumar, M. S. (2015). Novel bacterial strain of Achromobacter sp. MTCC 5605 and a highly enantioselective epoxide hydrolase isolated therefrom, Patent Publication No. US 9,150,840 B2. 10 June, 2015.
Jinyou, Z., Reddy, J., Senanayake, C., & Chartrain, M. (1995). Chiral bio-resolution of racemic indene oxide by fungal epoxide hydrolases. Journal of Fermentation and Bioengineering, 80(3), 244–246.
Cleij, M., Archelas, A., & Furstoss, R. (1999). Microbiological transformations 43. Epoxide hydrolases as tools for the synthesis of enantiopure-methylstyrene oxides: a new and efficient synthesis of (S)-Ibuprofen. Journal of Organic Chemistry, 64(14), 5029–5035.
Orru, R. V. A., Osprian, I., Kroutil, W., & Faber, K. (1998). An efficient large-scale synthesis of (R)-(–)-mevalonolactone using simple biological and chemical catalysts. Synthesis, 9, 1259–1263.
Manoj, K. M., Archelas, A., Baratti, J., & Furstoss, R. (2001). Microbiological transformations. Part 45: a green chemistry preparative scale synthesis of enantiopure building blocks of Eliprodil: elaboration of a high substrate concentration epoxide hydrolase-catalyzed hydrolytic kinetic resolution process. Tetrahedron, 57(4), 695–701.
Morisseau, C., Baratti, J., Zylber, J., Archelas, A., & Furstoss, R. (1997). Microbiological transformations 37. An enantioconvergent synthesis of the β-blocker (R) -Nifenalol® using a combined chemoenzymatic approach. Tetrahedron, 53(28), 9707–9714.
Li, C., Liu, Q., Ding, D., Cui, H., Ji, A., & Qu, Y. (2003). Epoxide hydrolase-catalyzed resolution of ethyl 3-phenylglycidate using whole cells of Pseudomonas sp. BZS21. Biotechnology Letters, 25(24), 2113–2116.
Choi, W. J., Puah, S. M., Tan, L. L., & Ng, S. S. (2008). Production of (R)-ethyl-3,4-epoxybutyrate by newly isolated Acinetobacter baumannii containing epoxide hydrolase. Applied Microbiology and Biotechnology, 79(1), 61–67.
Chen, X.-J., Archelas, A., & Furstoss, R. (1993). Microbiological Transformations. 27. the first examples for preparative-scale enantioselective or diastereoselective epoxide hydrolyses using microorganisms. an unequivocal access to all four bisabolol stereoisomers. Journal of Organic Chemistry, 58(20), 5528–5532.
Jia, X., Wang, Z., & Li, Z. (2008). Preparation of (S)-2-,3-, and 4-chlorostyrene oxides with the epoxide hydrolase from Sphingomonas sp. HXN-200. Tetrahedron Asymmetry, 19(4), 407–415.
Chen, L., Shen, H., Wei, C., & Zhu, Q. (2013). Bioresolution of (R)-glycidyl azide by Aspergillus niger ZJUTZQ208: a new and concise synthon for chiral vicinal amino alcohols. Applied Microbiology and Biotechnology, 97(6), 2609–2616.
Kong, X.-D., Yuan, S., Li, L., Chen, S., Xu, J.-H., & Zhou, J. (2014). Engineering of an epoxide hydrolase for efficient bioresolution of bulky pharmaco substrates. Proceedings of the National Academy of Sciences of the United States of America, 111(44), 15717–15722.
Choi, W. J. (2009). Biotechnological production of enantiopure epoxides by enzymatic kinetic resolution. Applied Microbiology and Biotechnology, 84(2), 239–247.
Krishna, S. H. (2002). Developments and trends in enzyme catalysis in nonconventional media. Biotechnology Advances, 20(3–4), 239–267.
Adamczak, M., & Krishna, S. H. (2004). Strategies for improving enzymes for efficient biocatalysis. Food Technol. Biotechnol., 42(4), 251–264.
Laane, C., Boeren, S., Vos, K., & Veeger, C. (1987). Rules for optimization of biocatalysis in organic solvents. Biotechnology and Bioengineering, 30(1), 81–87.
Nellaiah, H., Morisseau, C., Archelas, A., Furstoss, R., & Baratti, J. C. (1996). Enantioselective hydrolysis of p-nitrostyrene oxide by an epoxide hydrolase preparation from Aspergillus niger. Biotechnology and Bioengineering, 49(1), 70–77.
Choi, W. J., Choi, C. Y., De Bont, J. A. M., & Weijers, C. A. G. M. (1999). Resolution of 1,2-epoxyhexane by Rhodotorula glutinis using a two-phase membrane bioreactor. Applied Microbiology and Biotechnology, 53(1), 7–11.
Beloti, L. L., Costa, B. Z., Toledo, M. A. S., Santos, C. A., Crucello, A., Fávaro, M. T. P., et al. (2013). A novel and enantioselective epoxide hydrolase from Aspergillus brasiliensis CCT 1435: Purification and characterization. Protein Expression and Purification, 91(2), 175–183.
Filho, M. V., Stillger, T., Muller, M., Liese, A., & Wandrey, C. (2003). Is logP a convenient criterion to guide the choice of solvents for biphasic enzymatic reactions? Angewandte Chemie Int Ed, 42(26), 2993–2996.
Wenjing, C., Wenyong, L., Xiaoting, W., & Minhua, Z. (2011). Asymmetric hydrolysis of styrene oxide catalyzed by Mung bean epoxide hydrolase in organic solvent/buffer biphasic system. Chinese Journal of Catalysis, 32(9), 1557–1563.
Zaks, A., & Klibanov, M. (1988). Enzymatic catalysis in nonaqueous solvents. Journal of Biological Chemistry, 263(7), 3194–3201.
Lee, E. Y. (2007). Enantioselective hydrolysis of epichlorohydrin in organic solvents using recombinant epoxide hydrolase. Journal of Industrial and Engineering Chemistry, 13(1), 159–162.
Karboune, S., Archelas, A., & Baratti, J. (2006). Properties of epoxide hydrolase from Aspergillus niger for the hydrolytic kinetic resolution of epoxides in pure organic media. Enzym. Microb. Technol., 39(2), 318–324.
Lotter, J., Botes, A. L., van Dyk, M. S., & Breytenbach, J. C. (2004). Correlation between the physicochemical properties of organic solvents and their biocompatibility toward epoxide hydrolase activity in whole-cells of a yeast. Rhodotorula sp. Biotechnol. Lett., 26(15), 1191–1195.
Hwang, S., Choi, C. Y., & Lee, E. Y. (2008). Enantioconvergent bioconversion of p-chlorostyrene oxide to (R)-p-chlorophenyl-1,2-ethandiol by the bacterial epoxide hydrolase of Caulobacter crescentus. Biotechnology Letters, 30(7), 1219–1225.
Karboune, S., Archelas, A., & Baratti, J. C. (2010). Free and immobilized Aspergillus niger epoxide hydrolase-catalyzed hydrolytic kinetic resolution of racemic p-chlorostyrene oxide in a neat organic solvent medium. Process Biochemistry, 45(2), 210–216.
Jin, H.-X., Hu, Z.-C., & Zheng, Y.-G. (2012). Enantioselective hydrolysis of epichlorohydrin using whole Aspergillus niger ZJB-09173 cells in organic solvents. Journal of Biosciences, 37(4), 695–702.
Zhao, W., Kotik, M., Iacazio, G., & Archelas, A. (2015). Enantioselective bio-hydrolysis of various racemic and meso aromatic epoxides using the recombinant epoxide hydrolase Kau2. Advanced Synthesis & Catalysis, 357(8), 1895–1908.
Baldascini, H., & Janssen, D. B. (2005). Interfacial inactivation of epoxide hydrolase in a two-liquid-phase system. Enyzme and Microbial Technology, 36(2–3), 285–293.
Baldascini, H., Ganzeveld, K. J., Janssen, D. B., & Beenackers, A. A. C. M. (2001). Effect of mass transfer limitations on the enzymatic kinetic resolution of epoxides in a two-liquid-phase system. Biotechnology and Bioengineering, 73(1), 44–54.
Simeó, Y., & Faber, K. (2006). Selectivity enhancement of enantio- and stereo-complementary epoxide hydrolases and chemo-enzymatic deracemization of (±)-2-methylglycidyl benzyl ether. Tetrahedron Asymmetry, 17(3), 402–409.
Kim, H. S., Lee, O. K., Hwang, S., Kim, B. J., & Lee, E. Y. (2008). Biosynthesis of (R)-phenyl-1,2-ethanediol from racemic styrene oxide by using bacterial and marine fish epoxide hydrolases. Biotechnology Letters, 30(1), 127–133.
Salameh, M. A., & Wiegel, J. (2010). Effects of detergents on activity, thermostability and aggregation of two alkalithermophilic lipases from Thermosyntropha lipolytica. Open Biochemistry Journal, 4, 22–28.
Mogensen, J. E., Sehgal, P., & Otzen, D. E. (2005). Activation, inhibition, and destabilization of Thermomyces lanuginosus lipase by detergents. Biochemistry, 44(5), 1719–1730.
Gong, P., Xu, J., Tang, Y., & Wu, H. (2003). Improved catalytic performance of Bacillus megaterium epoxide hydrolase in a medium containing Tween-80. Biotechnology Progress, 19(2), 652–654.
Kroutil, W., Genzel, Y., Pietzsch, M., Syldatk, C., & Faber, K. (1998). Purification and characterization of a highly selective epoxide hydrolase from Nocardia sp. EH1. Journal of Biotechnology, 61(1–2), 143–150.
van Rantwijk, F., & Sheldon, R. A. (2007). Biocatalysis in ionic liquids. Chemical Reviews, 107(6), 2757–2785.
Chiappea, C., Leandria, E., Hammock, B. D., & Morisseau, C. (2007). Effect of ionic liquids on epoxide hydrolase-catalyzed synthesis of chiral 1,2-diols. Green Chemistry, 9(2), 162–168.
Gorke, J., Srienc, F., & Kazlauskas, R. (2010). Toward advanced ionic liquids. Polar, enzyme-friendly solvents for biocatalysis. Biotechnol. Bioprocess Engineering, 15(1), 40–53.
Dominguez de Maria, P., & Maugeri, Z. (2011). Ionic liquids in biotransformations: from proof-of-concept to emerging deep-eutectic-solvents. Current Opinion in Chemical Biology, 15(2), 220–225.
Dominguez de Maria, P. (2008). “Nonsolvent” applications of ionic liquids in biotransformations and organocatalysis. Angewandte Chemie Int Ed, 47(37), 6960–6968.
Park, S., & Kazlauskas, R. J. (2003). Biocatalysis in ionic liquids- advantages beyond green technology. Current Opinion in Biotechnology, 14(4), 432–437.
Kaar, J. L., Jesionowski, A. M., Berberich, J. A., Moulton, R., & Russell, A. J. (2003). Impact of ionic liquid physical properties on lipase activity and stability. Journal of the American Chemical Society, 125(14), 4125–4131.
Wells, A. S., & Coombe, V. T. (2006). On the freshwater ecotoxicity and biodegradation properties of some common ionic liquids. Organic Process Research & Development, 10(4), 794–798.
Chiappe, C., Leandri, E., Lucchesi, S., Pieraccini, D., Hammock, B. D., & Morisseau, C. (2004). Biocatalysis in ionic liquids: the stereoconvergent hydrolysis of trans-β-methylstyrene oxide catalyzed by soluble epoxide hydrolase. Journal of Molecular Catalysis. B, Enzymatic, 27(4–6), 243–248.
Chen, W., Lou, W., Yu, C., Wu, H., Zong, M., & Smith, T. J. (2012). Use of hydrophilic ionic liquids in a two-phase system to improve Mung bean epoxide hydrolases-mediated asymmetric hydrolysis of styrene oxide. Journal of Biotechnology, 162(2–3), 183–190.
Chen, W.-J., Lou, W.-Y., & Zong, M.-H. (2012). Efficient asymmetric hydrolysis of styrene oxide catalyzed by Mung bean epoxide hydrolases in ionic liquid-based biphasic systems. Bioresource technology, 115, 58–62.
Matsumoto, M., Sugimoto, T., Ishiguro, Y., Yamaguchi, H., & Kondo, K. (2014). Effect of organic solvents and ionic liquids on resolution of 2-epoxyhexane by whole cells of Rhodotorula glutinis in a two-liquid phase system. Journal of Chemical Technology and Biotechnology, 89(4), 522–527.
Xu, P., Zheng, G.-W., Du, P.-X., Zong, M.-H., & Lou, W.-Y. (2015). Whole-cell biocatalytic processes with ionic liquids. ACS Sustainable Chemistry & Engineering, 4(2), 371–386.
Zhao, H. (2010). Methods for stabilizing and activating enzymes in ionic liquids-a review. Journal of Chemical Technology and Biotechnology, 85(7), 891–907.
Yu, C., Wei, P., Li, X., Zong, M., & Lou, W. (2014). Using ionic liquid in a biphasic system to improve asymmetric hydrolysis of styrene oxide catalyzed by cross-linked enzyme aggregates (CLEAs) of Mung bean epoxide. hydrolases. Industrial and Engineering Chemistry Research, 53(19), 7923–7930.
Yang, Z., & Pan, W. (2005). Ionic liquids: green solvents for nonaqueous biocatalysis. Enyzme and Microbial Technology, 37(1), 19–28.
Naushad, M., Alothman, Z. A., Khan, A. B., & Ali, M. (2012). Effect of ionic liquid on activity, stability, and structure of enzymes: a review. International Journal of Biological Macromolecules, 51(4), 555–560.
Patel, R., Kumari, M., & Khan, A. B. (2014). Recent advances in the applications of ionic liquids in protein stability and activity: a review. Applied Biochemistry and Biotechnology, 172(8), 3701–3720.
Clouthier, C. M., & Pelletier, J. N. (2012). Expanding the organic toolbox: a guide to integrating biocatalysis in synthesis. Chemical Society Reviews, 41(4), 1585–1605.
Abbott, A. P., Harris, R. C., & Ryder, K. S. (2007). Application of hole theory to define ionic liquids by their transport properties. J. Phys. Chem. B, 111(18), 4910–4913.
Seddon, K. R., Stark, A., & Torres, M. (2000). Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure and Applied Chemistry, 72(12), 2275–2287.
Huang, Z.-L., Wu, B.-P., Wen, Q., Yang, T.-X., & Yang, Z. (2014). Deep eutectic solvents can be viable enzyme activators and stabilizers. Journal of Chemical Technology and Biotechnology, 89(12), 1975–1981.
Lindberg, D., de la Fuente Revenga, M., & Widersten, M. (2010). Deep eutectic solvents (DESs) are viable cosolvents for enzyme-catalyzed epoxide hydrolysis. Journal of Biotechnology, 147(3–4), 169–1671.
Gorke, J. T., Srienc, F., & Kazlauskas, R. J. (2008). Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chemical Communications, 14(10), 1235–1237.
Monhemi, H., Housaindokht, R., & Moosavi-movahedi, A. (2014). How a protein can remain stable in a solvent with high content of urea: insights from molecular dynamics simulation of Candida antarctica lipase B in urea: choline chloride deep eutectic solvent. Physical Chemistry Chemical Physics: PCCP, 16(28), 14882–14893.
Gutiérrez, M. C., Ferrer, M. L., Yuste, L., Rojo, F., & del Monte, F. (2010). Bacteria incorporation in deep-eutectic solvents through freeze- drying. Angewandte Chemie Int Ed, 49(12), 2158–2162.
Hayyan, M., Hashim, M. A., Hayyan, A., Al-Saadi, M. A., AlNashef, I. M., Mirghani, M. E. S., et al. (2013). Are deep eutectic solvents benign or toxic? Chemosphere, 90(7), 2193–2195.
Hayyan, M., Looi, C. Y., Hayyan, A., Wong, W. F., & Hashim, M. A. (2015). In vitro and in vivo toxicity profiling of ammonium-based deep eutectic solvents. PLoS ONE, 10(2), e0117934.
Smith, E. L., Abbott, A. P., & Ryder, K. S. (2014). Deep eutectic solvents (DESs) and their applications. Chemical Reviews, 114(21), 11060–11082.
Rodrigues, R. C., Ortiz, C., & Ferna, R. (2013). Modifying enzyme activity and selectivity by immobilization. Chemical Society Reviews, 42(15), 6290–6307.
Mateo, C., Palomo, J. M., Fernandez-lorente, G., Guisan, J. M., & Fernandez-lafuente, R. (2007). Improvement of enzyme activity, stability and selectivity via immobilisation techniques. Enyzme and Microbial Technology, 40(6), 1451–1463.
Hanefeld, U., & Magner, E. (2009). Understanding enzyme immobilisation. Chemical Society Reviews, 38(2), 453–468.
Garcia-Galan, C., Berenguer-Murcia, Á., Fernandez-Lafuente, R., & Rodrigues, R. C. (2011). Potential of different enzyme immobilization strategies to improve enzyme performance. Advanced Synthesis & Catalysis, 353(16), 2885–2904.
Zhang, D.-H., Yuwen, L.-X., & Peng, L.-J. (2013). Parameters affecting the performance of immobilized enzyme. J. Chem., 7.
Mateo, C., Fernandez-Lafuente, R., Archelas, A., Guisan, J. M., & Furstoss, R. (2007). Preparation of a very stable immobilized Solanum tuberosum epoxide hydrolase. Tetrahedron Asymmetry, 18(10), 1233–1238.
Bao, W., Pan, H., Zhang, Z., Cheng, Y., Xie, Z., & Zhang, J. (2015). Isolation of the stable strain Labrys sp. BK-8 for L (+)-tartaric acid production. Journal of Bioscience and Bioengineering, 119(1), 538–542.
Yildirim, D., Tukel, S. S., Alptekin, O., & Alagoz, D. (2013). Immobilized Aspergillus niger epoxide hydrolases: cost-effective biocatalysts for the preparation of enantiopure styrene oxide, propylene oxide and epichlorohydrin. Journal of Molecular Catalysis. B, Enzymatic, 88, 84–90.
Jin, H.-X., Liu, Z.-Q., Hu, Z.-C., & Zheng, Y.-G. (2013). Production of (R)-epichlorohydrin from 1,3-dichloro-2-propanol by two-step biocatalysis using haloalcohol dehalogenase and epoxide hydrolase in two-phase system. Biochemical Engineering Journal, 74, 1–7.
Kroutil, W., Orru, R. V. A., & Faber, K. (1998). Stabilization of Nocardia EH1 epoxide hydrolase by immobilization. Biotechnology Letters, 20(4), 373–377.
Karboune, S., Amourache, L., Nellaiah, H., Morisseau, C., & Baratti, J. (2001). Immobilization of the epoxide hydrolase from Aspergillus niger. Biotechnology Letters, 23(19), 1633–1639.
Mateo, C., Archelas, A., Fernandez-lafuente, R., Guisan, M., Furstoss, R., Eda, E. C., et al. (2003). Enzymatic transformations. Immobilized A. niger epoxide hydrolase as a novel biocatalytic tool for repeated-batch hydrolytic kinetic resolution of epoxides. Organic & Biomolecular Chemistry, 1(15), 2739–2743.
Karboune, S., Archelas, A., Furstoss, R., & Baratti, J. (2005). Immobilization of epoxide hydrolase from Aspergillus niger onto DEAE-cellulose: enzymatic properties and application for the enantioselective resolution of a racemic epoxide. Journal of Molecular Catalysis. B, Enzymatic, 32(5–6), 175–183.
Petri, A., Marconcini, P., & Salvadori, P. (2005). Efficient immobilization of epoxide hydrolase onto silica gel and use in the enantioselective hydrolysis of racemic para-nitrostyrene oxide. Journal of Molecular Catalysis. B, Enzymatic, 32(5–6), 219–224.
Yildirim, D., Tükel, S. S., Alagöz, D., & Alptekin, O. (2011). Preparative-scale kinetic resolution of racemic styrene oxide by immobilized epoxide hydrolase. Enyzme and Microbial Technology, 49(6–7), 555–559.
Katchalski-katzir, E., & Kraemer, D. M. (2000). Eupergit® C, a carrier for immobilization of enzymes of industrial potential. Journal of Molecular Catalysis. B, Enzymatic, 10(1–3), 157–176.
Bu, M., Vikartovsk, A., Lac, I., Koll, G., & Brygin, M. (2005). Immobilization of a whole-cell epoxide-hydrolyzing biocatalyst in sodium alginate − cellulose sulfate − poly(methylene-co-guanidine) capsules using a controlled encapsulation process. Enyzme and Microbial Technology, 36(1), 118–126.
Huang, R., Wu, M., Goldman, M. J., & Li, Z. (2015). Encapsulation of enzyme via one-step template-free formation of stable organic-inorganic capsules: a simple and efficient method for immobilizing enzyme with high activity and recyclability. Biotechnology and Bioengineering, 112(6), 1092–1101.
Kim, J., Grate, J. W., & Wang, P. (2008). Nanobiocatalysis and its potential applications. Trends in Biotechnology, 26(11), 639–646.
Lee, K. S., Woo, M. H., Kim, H. S., Lee, E. Y., & Lee, I. S. (2009). Synthesis of hybrid Fe(3)O(4)-silica-NiO superstructures and their application as magnetically separable high-performance biocatalysts. Chemical Communications, 25, 3780–3782.
Hyun, Y., Lee, I., Hee, S., Kyung, O., Shim, J., Lee, J., et al. (2013). Enhanced stability and reusability of marine epoxide hydrolase using ship-in-a-bottle approach with magnetically-separable mesoporous silica. Journal of Molecular Catalysis. B, Enzymatic, 89, 48–51.
Choi, S. H., Kim, H. S., Lee, I. S., & Lee, E. Y. (2010). Functional expression and magnetic nanoparticle-based Immobilization of a protein-engineered marine fish epoxide hydrolase of Mugil cephalus for enantioselective hydrolysis of racemic styrene oxide. Biotechnology Letters, 32(11), 1685–1691.
Wang, W., Wang, D. I. C., & Li, Z. (2011). Facile fabrication of recyclable and active nanobiocatalyst: purification and immobilization of enzyme in one pot with Ni-NTA functionalized magnetic nanoparticle. Chemical Communications, 47(28), 8115–8117.
Kim, Y., Lee, I., Choi, S., Lee, O., Kim, J., & Lee, E. (2013). Nanoimmobilization of marine epoxide hydrolase of Mugil cephalus for repetitive enantioselective resolution of racemic styrene oxide in aqueous buffer. Journal of Nanoscience and Nanotechnology, 13(3), 2266–2271.
Trevan, M. D. (1988). Enzyme immobilization by entrapment- new protein techniques. Methods in Molecular Biology, 3, 491–494.
Maritz, J., Krieg, H. M., Yeates, C. A., Botes, A. L., & Breytenbach, J. C. (2003). Calcium alginate entrapment of the yeast Rhodosporidium toruloides for the kinetic resolution of 1,2-epoxyoctane. Biotechnology Letters, 25(20), 1775–1781.
Mateo, C., Palomo, M., van Langen, L. M., van Rantwijk, F., & Sheldon, R. A. (2004). A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates. Biotechnology and Bioengineering, 86(3), 273–276.
Yu, C.-Y., Li, X.-F., Lou, W.-Y., & Zong, M.-H. (2013). Cross-linked enzyme aggregates of Mung bean epoxide hydrolases: a highly active, stable and recyclable biocatalyst for asymmetric hydrolysis of epoxides. Journal of Biotechnology, 166(1–2), 12–19.
Reetz, M. T., Kahakeaw, D., & Lohmer, R. (2008). Addressing the numbers problem in directed evolution. ChemBioChem, 9(11), 1797–1804.
Turner, N. J. (2009). Directed evolution drives the next generation of biocatalysts. Nature Chemical Biology, 5(8), 567–573.
Reetz, M. T., & Carballeira, J. D. (2007). Iterative saturation mutagenesis (ISM) for rapid directed evolution of functional enzymes. Nature Protocols, 2(4), 891–903.
Fujii, R., Kitaoka, M., & Hayashi, K. (2004). One-step random mutagenesis by error-prone rolling circle amplification. Nucleic Acids Research, 32(19), e145.
Reetz, M. T., Torre, C., Eipper, A., Lohmer, R., Hermes, M., Brunner, B., et al. (2004). Enhancing the enantioselectivity of an epoxide hydrolase by directed evolution. Organic Letters, 6(2), 177–180.
Yuan, L., Kurek, I., English, J., & Keenan, R. (2005). Laboratory-directed protein evolution. Microbiology and Molecular Biology Reviews, 69(3), 373–392.
van Loo, B., Spelberg, J. H. L., Kingma, J., Sonke, T., Wubbolts, M. G., Janssen, D. B., et al. (2004). Directed evolution of epoxide hydrolase from A. radiobacter toward higher enantioselectivity by error-prone PCR and DNA shuffling. Chemistry & Biology, 11(7), 981–990.
Arnold, K., Bordoli, L., Kopp, J., & Schwede, T. (2006). The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics, 22(2), 195–201.
Zheng, L., Baumann, U., & Reymond, J.-L. (2004). An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Research, 32(14), e115.
van Loo, B., Kingma, J., Heyman, G., Wittenaar, A., Spelberg, J. H. L., Sonke, T., et al. (2009). Improved enantioselective conversion of styrene epoxides and meso-epoxides through epoxide hydrolases with a mutated nucleophile-flanking residue. Enzy. Microb. Technol., 44(3), 145–153.
Xuea, F., Liua, Z.-Q., Wana, N.-W., Zhua, H.-Q., & Zhenga, Yu-Guo. (2015). Engineering the epoxide hydrolase from Agromyces mediolanus for enhanced enantioselectivity and activity in the kinetic resolution of racemic epichlorohydrin. RSC Adv., 5, 31525–31532.
Xue, F., Liu, Z., Wang, Y., Zhu, H., Wan, N., & Zheng, Y. (2015). Efficient synthesis of (S) -epichlorohydrin in high yield by cascade biocatalysis with halohydrin dehalogenase and epoxide hydrolase mutants. Catalysis Communications, 72, 147–149.
Kotik, M., Zhao, W., Iacazio, G., & Archelas, A. (2013). Directed evolution of metagenome-derived epoxide hydrolase for improved enantioselectivity and enantioconvergence. Journal of Molecular Catalysis. B, Enzymatic, 91, 44–51.
Reetz, M. T., & Zheng, H. (2011). Manipulating the expression rate and enantioselectivity of an epoxide hydrolase by using directed evolution. ChemBioChem, 12(10), 1529–1535.
Reetz, M. T., Wang, L.-W., & Bocola, M. (2006). Directed evolution of enantioselective enzymes: iterative cycles of CASTing for probing protein-sequence space. Angewandte Chemie Int Ed, 45(8), 1236–1241.
Feng, X., Sanchis, J., Reetz, M. T., & Rabitz, H. (2012). Enhancing the efficiency of directed evolution in focused enzyme libraries by the adaptive substituent reordering algorithm. Chemistry--A European Journal, 18(18), 5646–5654.
Kong, X.-D., Ma, Q., Zhou, J., Zeng, B.-B., & Xu, J.-H. (2014). A smart library of epoxide hydrolase variants and the top hits for synthesis of (S)-β-blocker precursors. Angewandte Chemie Int Ed, 53(26), 6641–6644.
Sun, Z., Lonsdale, R., Kong, X.-D., Xu, J.-H., Zhou, J., & Reetz, M. T. (2015). Reshaping an enzyme binding pocket for enhanced and inverted stereoselectivity: use of smallest amino acid alphabets in directed evolution. Angewandte Chemie Int Ed, 54(42), 12410–12415.
Gumulya, Y., & Reetz, M. T. (2011). Enhancing the thermal robustness of an enzyme by directed evolution: least favorable starting points and inferior mutants can map superior evolutionary pathways. ChemBioChem, 12(16), 2502–2510.
Reetz, M. T., Carballeira, J. D., & Vogel, A. (2006). Iterative saturation mutagenesis on the basis of B factors as a strategy for increasing protein thermostability. Angewandte Chemie Int Ed, 45(46), 7745–7751.
Wijma, H. J., Floor, R. J., Jekel, P. A., Baker, D., Marrink, S. J., & Janssen, D. B. (2014). Computationally designed libraries for rapid enzyme stabilization. Protein Engineering, Design & Selection, 27(2), 49–58.
Goldsmith, M., & Tawfik, D. S. (2012). Directed enzyme evolution: beyond the low-hanging fruit. Current Opinion in Structural Biology, 22(4), 406–412.
Reetz, M. T. (2009). Directed evolution of enantioselective enzymes: an unconventional approach to asymmetric catalysis in organic chemistry. Journal of Organic Chemistry, 74(16), 5767–5778.
Wang, M., Si, T., & Zhao, H. (2012). Biocatalyst development by directed evolution. Bioresource technology, 115, 117–125.
Reetz, M. T. (2011). Laboratory evolution of stereoselective enzymes: a prolific source of catalysts for asymmetric reactions. Angewandte Chemie Int Ed, 50(1), 138–174.
Bornscheuer, U. T., Huisman, G. W., Kazlauskas, R. J., Lutz, S., Moore, J. C., & Robins, K. (2012). Engineering the third wave of biocatalysis. Nature, 485(7397), 185–194.
Widersten, M. (2014). Protein engineering for development of new hydrolytic biocatalysts. Current Opinion in Chemical Biology, 21, 42–47.
Widersten, M., Gurell, A., & Lindberg, D. (2010). Structure-function relationships of epoxide hydrolases and their potential use in biocatalysis. Biochimica et Biophysica Acta, 1800(3), 316–326.
Jochens, H., Stiba, K., Savile, C., Fujii, R., Yu, J.-G., Gerassenkov, T., et al. (2009). Converting an esterase into an epoxide hydrolase. Angewandte Chemie Int Ed, 48(19), 3532–3535.
Acknowledgements
This work was financially supported by Department of Biotechnology (DBT) via Grant No. BT/PR/4694/PID/6/633/2012, Government of India, New Delhi. PS gratefully acknowledge DBT for the SRF. The financial assistance received from Department of Science and Technology-Promotion of University Research and Scientific Excellence (DST-PURSE) and University Grants Commission-Special Assistance Programme (UGC-SAP) (DRS Phase-I) is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saini, P., Sareen, D. An Overview on the Enhancement of Enantioselectivity and Stability of Microbial Epoxide Hydrolases. Mol Biotechnol 59, 98–116 (2017). https://doi.org/10.1007/s12033-017-9996-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-017-9996-8