Abstract
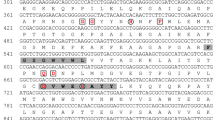
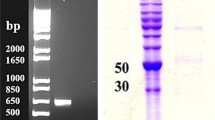
Superoxide dismutase (SOD) detoxifies cell-toxic superoxide radicals and constitutes an important component of antioxidant machinery in aerobic organisms, including cyanobacteria. The iron-containing SOD (SodB) is one of the most abundant soluble proteins in the cytosol of the nitrogen-fixing cyanobacterium Nostoc punctiforme ATCC 29133, and therefore, we investigated its biochemical properties and response to oxidative stress. The putative SodB-encoding open reading frame Npun_R6491 was cloned and overexpressed in Escherichia coli as a C-terminally hexahistidine-tagged protein. The purified recombinant protein had a SodB specific activity of 2560 ± 48 U/mg protein at pH 7.8 and was highly thermostable. The presence of a characteristic iron absorption peak at 350 nm, and its sensitivity to H2O2 and azide, confirmed that the SodB is an iron-containing SOD. Transcript level of SodB in nitrogen-fixing cultures of N. punctiforme decreased considerably (threefold) after exposure to an oxidative stress-generating herbicide methyl viologen for 4 h. Furthermore, in-gel SOD activity analysis of such cultures grown at increasing concentrations of methyl viologen also showed a loss of SodB activity. These results suggest that SodB is not the primary scavenger of superoxide radicals induced by methyl viologen in N. punctiforme.



Similar content being viewed by others
References
Latifi, A., Ruiz, M., & Zhang, C. C. (2009). Oxidative stress in cyanobacteria. FEMS Microbiology Reviews, 33, 258–278.
Simon, F. W., Grzebyk, D., & Schofield, O. (2005). The role and evolution of superoxide dismutases in algae. Journal of Phycology, 41, 453–465.
Pilon, M., Ravet, K., & Tapken, W. (2011). The biogenesis and physiological function of chloroplast superoxide dismutases. Biochimica and Biophyica Acta, 1807, 980–998.
Lancaster, V. L., LoBrutto, R., Selvaraj, F. M., & Blankenship, R. E. (2004). A cambialistic superoxide dismutase in the thermophilic photosynthetic bacterium Chloroflexus aurantiacus. Journal of Bacteriology, 186, 3408–3414.
Priya, B., Premanandh, J., Dhanalakshmi, R. T., Seethalakshmi, T., Uma, L., Prabaharan, D., et al. (2007). Comparative analysis of cyanobacterial superoxide dismutases to discriminate canonical form. BMC Genomics, 8, 435.
Kim, J. H., & Suh, K. H. (2005). Light-dependent expression of superoxide dismutase from cyanobacterium Synechocystis sp. strain PCC 6803. Archives of Microbiology, 183, 218–223.
Ke, W. T., Dai, G. Z., Jiang, H. B., Zhang, R., & Qiu, B. S. (2014). Essential roles of iron superoxide dismutase in photoautotrophic growth of Synechocystis sp. PCC 6803 and heterogeneous expression of marine Synechococcus sp. CC9311 copper/zinc superoxide dismutase within its sodB knockdown mutant. Microbiology, 160, 228–241.
Regelsberger, G., Laaha, U., Dietmann, D., Ruker, F., Caninin, A., Caiola, M. G., et al. (2004). The iron superoxide dismutase from the filamentous cyanobacterium Nostoc PCC 7120: Localization, overexpression, and biochemical characterization. Journal of Biological Chemistry, 279, 44384–44393.
Li, T., Huang, X., Zhou, R., Liu, Y., Li, B., Nomura, C., et al. (2002). Differential expression and localization of Mn and Fe superoxide dismutases in the heterocystous cyanobacterium Anabaena PCC 7120. Journal of Bacteriology, 184, 5096–5103.
Bhattacharya, J., GhoshDastidar, K., Chatterjee, A., Majee, M., & Lahiri Majumder, A. (2004). Synechocystis Fe superoxide dismutase gene confers oxidative stress tolerance to Escherichia coli. Biochemical and Biophysical Research Communication, 316, 540–544.
Moirangthem, L. D., Bhattacharya, S., Stensjö, K., Lindblad, P., & Bhattacharya, J. (2014). A high constitutive catalase activity confers resistance to methyl viologen-promoted oxidative stress in a mutant of the cyanobacterium Nostoc punctiforme ATCC 29133. Applied Microbiology and Biotechnology, 98, 3809–3818.
Ow, S. Y., Noirel, J., Cardona, T., Taton, A., Lindblad, P., Stensjö, K., et al. (2009). Quantitative overview of N2 fixation in Nostoc punctiforme ATCC 29133 through cellular enrichments and iTRAQ shotgun proteomics. Journal of Proteome Research, 8, 187–189.
Raghavan, P. S., Rajaram, H., & Apte, S. K. (2011). Nitrogen status dependent oxidative stress tolerance conferred by overexpression of MnSOD and FeSOD proteins in Anabaena sp. strain PCC 7120. Plant Molecular Biology, 77, 407–417.
Thomas, D. J., Avenson, T. J., Thomas, J. B., & Herbert, S. K. (1998). A cyanobacterium lacking iron superoxide dismutase is sensitized to oxidative stress induced with methyl viologen but is not sensitized to oxidative stress induced with norflurazon. Plant Physiology, 116, 1593–1602.
Ushimaru, T., Nishiyama, Y., Hayashi, H., & Murata, N. (2002). No coordinated transcriptional regulation of the Sod-Kat antioxidative system in Synechocystis sp. PCC 6803. Journal of Plant Physiology, 159, 805–807.
Shirkey, B., Kovarcik, D. P., Wright, D. J., Wilmoth, G., Prickett, T. F., Helm, R. F., et al. (2000). Active Fe-containing superoxide dismutase and abundant sodF mRNA in Nostoc commune (cyanobacteria) after years of dessication. Journal of Bacteriology, 182, 189–197.
Ismaiel, M. M. S., El-Ayouty, Y. M., Loewen, P. C., & Piercey-Normore, M. D. (2014). Characterization of the iron-containing superoxide dismutase and its response to stress in cyanobacterium Spirulina (Arthrospira) platensis. Journal of Applied Phycology, 26, 1649–1658.
Hunsucker, S. W., Klage, K., Saughter, S. M., Potts, M., & Helm, R. F. (2004). A preliminary investigation of the Nostoc punctiforme proteome. Biochemical and Biophysical Research Communication, 317, 1121–1127.
Rippka, R., Deruelles, J., Waterbury, J. B., Herdman, M., & Stanier, R. Y. (1979). Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Journal of General Microbiology, 111, 1–61.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
Rice, P., Longden, I., & Bleasby, A. (2000). EMBOSS: The European Molecular Biology Open Software Suite. Trends in Genetics, 16, 76–277.
Mazur, B. J., Rice, D., & Haselkorn, R. (1980). Identification of blue green algal nitrogen fixation genes by using heterologous DNA hybridization probes. Proceedings of National Academy of Sciences USA, 77, 186–190.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry, 44, 276–287.
Agervald, Å., Stensjö, K., Holmqvist, M., & Lindblad, P. (2008). Transcription of the extended hyp-operon in Nostoc sp. strain PCC 7120. BMC Microbiology, 8, 69.
Campbell, W. S., & Laudenbach, D. E. (1995). Characterization of four superoxide dismutase genes from a filamentous cyanobacterium. Journal of Bacteriology, 177, 964–972.
Babbs, C. F., Pham, J. A., & Coolbaugh, R. C. (1989). Lethal hydroxyl radical production in paraquat-treated plants. Plant Physiology, 90, 1267–1270.
Agervald, Å., Baebprasert, W., Zhang, X., Incharoensakdi, A., Lindblad, P., & Stensjö, K. (2010). The CyAbrB transcription factor CalA regulates the iron superoxide dismutase in Nostoc sp. strain PCC 7120. Environmental Microbiology, 12, 2826–2837.
Ekman, M., Sandh, G., Nenninger, A., Oliveira, P., & Stensjö, K. (2014). Cellular and functional specificity among ferritin-like proteins in the multicellular cyanobacterium Nostoc punctiforme. Environmental Microbiology, 16, 829–844.
Acknowledgments
MLD, RV and KSI acknowledge the financial support from Department of Science and Technology (DST), University Grant Commission (UGC) and Department of Biotechnology (DBT), Government of India, respectively. KS and PL acknowledge the financial support by the Swedish Energy Agency and KA Wallenberg Foundation. JB is grateful to Prof. Conrad W. Mullineaux, Queen Mary University of London, for providing pET-26b plasmid and Dr. Sudeshna Bhattacharya for her technical support. The authors also acknowledge DST for providing financial support in the form of a project, and DBT for Bioinformatics centre and research facility in the form of State Biotech hub.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moirangthem, L.D., Ibrahim, K.S., Vanlalsangi, R. et al. Molecular Cloning and Biochemical Characterization of the Iron Superoxide Dismutase from the Cyanobacterium Nostoc punctiforme ATCC 29133 and Its Response to Methyl Viologen-Induced Oxidative Stress. Mol Biotechnol 57, 1003–1009 (2015). https://doi.org/10.1007/s12033-015-9894-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-015-9894-x