Abstract
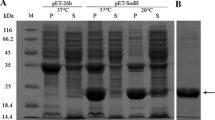
The FeSOD isoforms of Pseudochlorella pringsheimii were identified, a preliminary characterization of the enzyme was conducted, and the relationship among the FeSOD gene from P. pringsheimii and that of other organisms was examined. The FeSOD has an open reading frame of 612 bp that encodes 203 deduced amino acids with a molecular mass of 23 kDa. Expression of the recombinant FeSOD gene was done successfully in Escherichia coli. The purified FeSOD has a specific enzyme activity that reached 688 U mg−1 protein (in vitro assay). Alkaline conditions showed the highest activity for the recombinant FeSOD. Moreover, it showed a relative thermostability up to 50 °C, while at 50 and 70 °C, the activity was reduced by 32 and 68%, respectively, after 1 h as compared to the maximum. Phylogenetic analysis revealed three main clusters i.e., the prokaryotic Cyanophyta, bacteria, and the eukaryotic Chlorophyta intermingled with plant species and a dinoflagellate. P. pringsheimii was closely grouped with Chlorella pyrenoidosa, however, other species showed a relative disparity. Alignment of FeSOD gene sequences of the different species showed many conserved regions which could be used for FeSOD sequences among unexplored species and may be useful for the taxonomy of the revised coccoid Chlorella species.




Similar content being viewed by others
References
Almansa MS, Palma JM, Yáñez J, Del Río LA, Sevilla F (1991) Purification of an iron-containing superoxide dismutase from a citrus plant, Citrus limonum R. Free Radic Res Commun 12:319–328
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Armbrust E, Berges J, Bowler C, Green B, Martinez D, Putnam N, Zhou S, Allen A, Apt K, Bechner M, Brzezinski M, Chaal B, Chiovitti A, Davis A, Goodstein D, Hadi M, Hellsten U, Hildebrand M, Jenkins B, Jurka J, Kapitonov V, Kroger N, Lau W, Lane T, Larimer F, Lippmeier J, Lucas S, Medina M, Montsant A, Obornik M, Parker MS, Palenik B, Pazour G, Richardson P, Rynearson T, Saito M, Schwartz D, Thamatrakoln K, Valentin K, Vardi A, Wilkerson F, Rokhsar D (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306:79–86
Asada K, Yoshikawa K, Takahashi M, Maeda Y, Enmani K (1975) Superoxide dismutases from blue-green alga, Plectonema boryanum. J Biol Chem 250:2801–2807
Asada K, Kanematsu S, Okada S, Hayakawa T (1980) Phylogenic distribution of three types of superoxide dismutase in organisms and in cell organelles. In: Bannister JV, Hill HAO (eds) Chemical and biochemical aspects of superoxide and superoxide dismutase. Elsevier, Amsterdam, pp 136–153
Bafana A, Dutt S, Kumar A, Kumar S, Ahuja PS (2011) The basic and applied aspects of superoxide dismutase. J Mol Catal B Enzym 68:129–138
Bannister JV, Bannister WH, Rotilio G (1987) Aspects of the structure, function and applications of superoxide dismutase. CRC Crit Rev Biochem 22:111–180
Bannister WH, Bannister JV, Barra D, Bond J, Bossa F (1991) Evolutionary aspects of superoxide dismutase: the copper/zinc enzyme. Free Radic Res Commun 12–13:349–361
Baytut Ö, Gürkanli CT, Gönülol A, Özkoç I (2014) Molecular phylogeny of Chlorella-related chlorophytes (Chlorophyta) from Anatolian freshwaters of Turkey. Turk J Bot 38:600–607
Bischoff HW, Bold HC (1963) Phycological studies. IV. Some soil algae from enchanted rock and related algal species. University of Texas Publication No. 6318, pp 32–36
Bowler C, Van Montagu M, Inzé D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Biol 43:83–116
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bridges SM, Salin ML (1981) Distribution of iron-containing superoxide dismutase in vascular plants. Plant Physiol 68:275–278
Bueno P, Varela J, Gimenez-Gallego G, del Rio LA (1995) Peroxisomal copper, zinc superoxide dismutase (characterization of the isoenzyme from watermelon cotyledons). Plant Physiol 108:1151–1160
Campana F, Zervoudis S, Perdereau B, Gez E, Fourquet A, Badiu C, Tsakiris G, Koulaloglou S (2004) Topical superoxide dismutase reduces post-irradiation breast cancer fibrosis. J Cell Mol Med 8:109–116
Darienko T, Gustavs L, Mudimu O, Menendez CR, Schumann R, Karsten U, Friedl T, Pröschold T (2010) Chloroidium, a common terrestrial coccoid green alga previously assigned to Chlorella (Trebouxiophyceae, Chlorophyta). Eur J Phycol 45:79–95
Doering M, Piercey-Normore MD (2009) Genetically divergent algae an epiphytic lichen community on Jack Pine in Manitoba. The Lichenologist 41:69–80
Dos Santos WG, Pacheco I, Liu MY, Teixeira M, Xavier AV, LeGall J (2000) Purification and characterization of an iron superoxide dismutase and a catalase from the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol 182:769–804
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112
Görs M, Schumann R, Gustavs L, Karsten U (2010) The potential of ergosterol as chemotaxonomic marker to differentiate between “Chlorella” species (Chlorophyta). J Phycol 46:1296–1300
Grace SC (1990) Phylogenetic distribution of superoxide dismutase supports an endosymbiotic origin for chloroplasts and mitochondria. Life Sci 47:1875–1886
He N, Li Q, Sun D, Ling X (2008) Isolation, purification and characterization of superoxide dismutase from garlic. Biochem Eng J 38:33–38
Heeg JS, Wolf M (2015) ITS2 and 18S rDNA sequence-structure phylogeny of Chlorella and allies (Chlorophyta, Trebouxiophyceae, Chlorellaceae). Plant Gene 4:20–28
Hirooka S, Higuchi S, Uzuka A, Nozaki H, Miyagishima SY (2014) Acidophilic green alga Pseudochlorella sp. YKT1 accumulates high amount of lipid droplets under a nitrogen-depleted condition at a low-pH. PLoS ONE 9:e107702
Ismaiel MMS, El-Ayouty YM, Loewen PC, Piercey-Normore MD (2014) Characterization of the iron-containing superoxide dismutase and its response to stress in cyanobacterium Spirulina (Arthrospira) platensis. J Appl Phycol 26:1649–1658
Kaminaka H, Morita S, Tokumoto M, Yokoyama H, Masumura T, Tanaka K (1999) Molecular cloning and characterization of a cDNA for an iron-superoxide dismutase in rice (Oryza sativa L.). Biosci Biotechnol Biochem 63:302–308
Kanematsu S, Asada K (1990) Characteristic amino acid sequences of chloroplast and cytosol isozymes of Cu–Zn superoxide dismutase in spinach, rice and horsetail. Plant Cell Physiol 31:99–112
Kessler E (1976) Comparative physiology, biochemistry, and the taxonomy of Chlorella (Chlorophyceae). Plant Syst Evol 125:129–138
Kim EJ, Kim HP, Hah YC, Roe JH (1996) Differential expression of superoxide dismutases containing Ni and Fe/Zn in Streptomyces coelicolor. Eur J Biochem 241:178–185
Kitayama K, Kitayama M, Osafune T, Togasaki RK (1999) Subcellular localization of iron and manganese superoxide dismutase in Chlamydomonas reinhardtii (Chlorophyceae). J Phycol 35:136–142
Kliebenstein DJ, Monde RA, Last RL (1998) Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant Physiol 118:637–650
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li T, Huang X, Zhou R, Liu Y, Li B, Nomura C, Zhao J (2002) Differential expression and localization of Mn and Fe superoxide dismutases in the heterocystous cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol 184:5096–5103
Lin AU, Lin MT, Chen YT, Shaw JF (1995) Subunit interaction enhances enzyme activity and stability of sweet potato Cu/Zn-superoxide dismutase purified by His-tagged recombinant protein method. Plant Mol Biol 28:303–311
Mallick N (2004) Copper-induced oxidative stress in the chlorophycean microalga Chlorella vulgaris: response of the antioxidant system. J Plant Physiol 161:591–597
Matsuzaki M, Misumi O, Shin-I T, Maruyama S, Takahara M, Miyagishima S-Y, Mori T, Nishida K, Yagisawa F, Nishida K, Yoshida Y, Nishimura Y, Nakao S, Kobayashi T, Momoyama Y, Higashiyama T, Minoda A, Sano M, Nomoto H, Oishi K, Hayashi H, Ohta F, Nishizaka S, Haga S, Miura S, Morishita T, Kabeya Y, Terasawa K, Suzuki Y, Ishii Y, Asakawa S, Takano H, Ohta N, Kuroiwa H, Tanaka K, Shimizu N, Sugano S, Sato N, Nozaki H, Ogasawara N, Kohara Y, Kuroiwa T (2004) Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 428:653–657
Miller AF (2012) Superoxide dismutases: ancient enzymes and new insights. FEBS Lett 586:585–595
Miszalski Z, Ślesak I, Niewiadomska E, Baczek-Kwinta R, Lüttge U, Ratajczak R (1998) Subcellular localization and stress responses of superoxide dismutase isoforms from leaves in the C3-CAM intermediate halophyte Mesembryanthemum crystallinum L. Plant Cell Environ 21:169–179
Pokora W, Reszka J, Tukaj Z (2003) Activities of superoxide dismutase (SOD) isoforms during growth of Scenedesmus (Chlorophyta) species and strains grown in batch-cultures. Acta Physiol Plant 25:375–384
Puget K, Michelson AM (1974) Iron containing superoxide dismutases from luminous bacteria. Biochimie 56:1255–1267
Rubio MC, Ramos J, Webb KJ, Minchin FR, González E, Arrese-Igor C, Becana M (2001) Expression studies of superoxide dismutases in nodules and leaves of transgenic alfalfa reveal abundance of iron-containing isozymes, posttranslational regulation, and compensation of isozyme activities. Mol Plant Microbe Interact 14:1178–1188
Sakamoto A, Nosaka Y, Tanaka K (1993) Cloning and sequencing analysis of a complementary DNA for manganese-superoxide dismutase from rice (Oryza sativa L.). Plant Physiol 103:1477–1478
Sakurai H, Kusumoto N, Kitayama K, Togasaki RK (1993) Isozymes of superoxide dismutase in Chlamydomonas one of the major isozymes containing Fe. Plant Cell Physiol 34:1133–1137
Salin ML, Bridges SM (1982) Isolation and characterization of an iron-containing superoxide dismutase from water lily, Nuphar luteum. Plant Physiol 69:161–165
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Sandalio LM, del Río LA (1987) Localization of superoxide dismutase in glyoxysomes from Citrullus vulgaris. Functional implications in cellular metabolism. J Plant Physiol 127:395–409
Sarsour EH, Goswami M, Kalen AL, Goswami PC (2010) MnSOD activity protects mitochondrial morphology of quiescent fibroblasts from age associated abnormalities. Mitochondrion 10:342–349
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101:7–12
Shihira I, Krauss RW (1965) Chlorella: physiology and taxonomy of forty one isolates. University of Maryland, College Park, pp 1–97
Shirkey B, Kovarcik DP, Wright DJ, Wilmoth G, Prickett TF, Helm RF, Gregory EM, Potts M (2000) Active Fe containing superoxide dismutase and abundant sodF mRNA in Nostoc commune (Cyanobacteria) after years of desiccation. J Bacteriol 182:89–197
Stallings WC, Pattridge KA, Strong RK, Ludwig ML (1984) Manganese and iron superoxide dismutases are structural homologs. J Biol Chem 259:10695–10699
Steinman H (1982) Superoxide dismutases: protein chemistry and structure–function relationships. In: Oberley LW (ed) Superoxide dismutase. CRC Press, Boca Raton, pp 11–68
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Van Camp W, Bowler C, Villarroel R, Tsang EW, Van Montagu M, Inzé D (1990) Characterization of iron superoxide dismutase cDNAs from plants obtained by genetic complementation in Escherichia coli. Proc Natl Acad Sci 87:9903–9907
Wang X, Yang H, Ruan L, Liu X, Li F, Xu X (2008) Cloning and characterization of a thermostable superoxide dismutase from the thermophilic bacterium Rhodothermus sp. XMH10. J Ind Microbiol Biotechnol 35:133–139
Wang J, Sommerfeld M, Qiang H (2011) Cloning and expression of isoenzymes of superoxide dismutase in Haematococcus pluvialis (Chlorophyceae) under oxidative stress. J Appl Phycol 23:995–1003
Wolfe-Simon F, Grzebyk D, Schofield O, Falkowski PG (2005) The role and evolution of superoxide dismutases in algae. J Phycol 41:453–465
Acknowledgements
The authors thank Drs. G. Hausner and M.H. Abdelfattah (University of Manitoba, MB, Canada), for technical assistance. The first author would like to thank the Ministry of Higher Education and Scientific Research (MHESR, Egypt) for the financial support (through a post doctoral fellowship). This work was financially assisted by a Natural Sciences and Engineering Research Council (NSERC) grant (to Dr. Piercey-Normore).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ismaiel, M.M.S., Piercey-Normore, M.D. Molecular characterization and expression analysis of iron superoxide dismutase gene from Pseudochlorella pringsheimii (Trebouxiophyceae, Chlorophyta). Physiol Mol Biol Plants 25, 221–228 (2019). https://doi.org/10.1007/s12298-018-0569-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-018-0569-5