Abstract
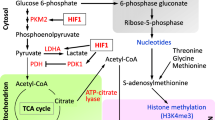
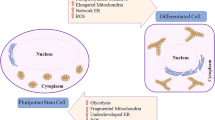
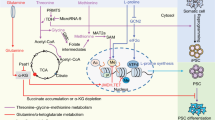
The availability of glucose and oxygen are important regulatory elements that help directing stem cell fate. In the undifferentiated state, stem cells, and their artificially reprogrammed equivalent-induced pluripotent stem cells (iPS) are characterized by limited oxidative capacity and active anaerobic glycolysis. Recent studies have shown that pluripotency—a characteristic of staminality—is associated with a poorly developed mitochondrial patrimony, while differentiation is accompanied by an activation of mitochondrial biogenesis. Besides being an important energy source in hypoxia, high glucose level results in hyperosmotic stress. The identification of specific metabolic pathways and biophysical factors that regulate stem cell fate, including high glucose in the extracellular medium, may therefore facilitate reprogramming efficiency and control the differentiation and fate of iPS cells, which are increasingly being explored as therapeutic tools. In this article, we review recent knowledge of the role of glucose metabolism and high glucose level as major anaerobic energy source, and a determinant of osmolarity as possible tools for reprogramming therapies in clinical applications. As in the diabetic setting hyperglycemia negatively affect the stem/progenitor cell fate and likely somatic reprogramming, we also discuss the in vivo potential transferability of the available in vitro findings.

Similar content being viewed by others
References
Almeida, A., Bolanos, J. P., et al. (2010). E3 ubiquitin ligase APC/C-Cdh1 accounts for the Warburg effect by linking glycolysis to cell proliferation. Proceedings of the National Academy of Sciences of the United States of America, 107(2), 738–741.
Ambasudhan, R., Talantova, M., et al. (2011). Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell, 9(2), 113–118.
Angele, P., Yoo, J. U., et al. (2003). Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. Journal of Orthopaedic Research, 21(3), 451–457.
Armstrong, L., Tilgner, K., et al. (2011). Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells, 28(4), 661–673.
Awad, H. A., Wickham, M. Q., et al. (2004). Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials, 25(16), 3211–3222.
Balaban, N. Q., Schwarz, U. S., et al. (2001). Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nature Cell Biology, 3(5), 466–472.
Bar-Even, A., Flamholz, A., et al. (2010). Rethinking glycolysis: On the biochemical logic of metabolic pathways. Nature Chemical Biology, 8(6), 509–517.
Batten, B. E., Albertini, D. F., et al. (1987). Patterns of organelle distribution in mouse embryos during preimplantation development. American Journal of Anatomy, 178(2), 204–213.
Beningo, K. A., Dembo, M., et al. (2001). Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. Journal of Cell Biology, 153(4), 881–888.
Blundell, M. P., Demaison, C., et al. (1999). Quality of repopulation in nonobese diabetic severe combined immunodeficient mice engrafted with expanded cord blood CD34+ cells. Blood, 94(9), 3269–3270.
Bogan, J. S. (2012). Regulation of glucose transporter translocation in health and diabetes. Annual Review of Biochemistry, 81, 507–532.
Bolanos, J. P., Almeida, A., et al. (2010). Glycolysis: A bioenergetic or a survival pathway? Trends in Biochemical Sciences, 35(3), 145–149.
Caiazzo, M., Dell’anno, M. T., et al. (2011). Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature, 476(7359), 224–227.
Chen, C. S., Alonso, J. L., et al. (2003). Cell shape provides global control of focal adhesion assembly. Biochemical and Biophysical Research Communications, 307(2), 355–361.
Chen, Y. H., Lin, S. J., et al. (2007). High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes, 56(6), 1559–1568.
Chicurel, M. E., Chen, C. S., et al. (1998). Cellular control lies in the balance of forces. Current Opinion in Cell Biology, 10(2), 232–239.
Covello, K. L., Kehler, J., et al. (2006). HIF-2alpha regulates Oct-4: Effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes & Development, 20(5), 557–570.
Cubbon, R. M., Kahn, M. B., et al. (2009). Effects of insulin resistance on endothelial progenitor cells and vascular repair. Clinical Science (London), 117(5), 173–190.
Denker, H. W. (2006). Potentiality of embryonic stem cells: An ethical problem even with alternative stem cell sources. Journal of Medical Ethics, 32(11), 665–671.
Deschepper, M., Oudina, K., et al. (2011). Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. Journal of Cellular and Molecular Medicine, 15(7), 1505–1514.
Dike, L. E., Chen, C. S., et al. (1999). Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cellular and Developmental Biology, 35(8), 441–448.
Egan, C. G., Lavery, R., et al. (2008). Generalised reduction of putative endothelial progenitors and CXCR4-positive peripheral blood cells in type 2 diabetes. Diabetologia, 51(7), 1296–1305.
Facucho-Oliveira, J. M., & St John, J. C. (2009). The relationship between pluripotency and mitochondrial DNA proliferation during early embryo development and embryonic stem cell differentiation. Stem Cell Review, 5(2), 140–158.
Fadini, G. P., Miorin, M., et al. (2005). Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. Journal of the American College of Cardiology, 45(9), 1449–1457.
Fadini, G. P., Pucci, L., et al. (2007). Glucose tolerance is negatively associated with circulating progenitor cell levels. Diabetologia, 50(10), 2156–2163.
Felice, F., Lucchesi, D., et al. (2010). Oxidative stress in response to high glucose levels in endothelial cells and in endothelial progenitor cells: Evidence for differential glutathione peroxidase-1 expression. Microvascular Research, 80(3), 332–338.
Folkman, J., & Moscona, A. (1978). Role of cell shape in growth control. Nature, 273(5661), 345–349.
Folmes, C. D., Nelson, T. J., et al. (2011). Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metabolism, 14(2), 264–271.
Fu, J., Tay, S. S., et al. (2006). High glucose alters the expression of genes involved in proliferation and cell-fate specification of embryonic neural stem cells. Diabetologia, 49(5), 1027–1038.
Gallagher, K. A., Liu, Z. J., et al. (2007). Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. Journal of Clinical Investigation, 117(5), 1249–1259.
Gong, Z., & Niklason, L. E. (2008). Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB Journal, 22(6), 1635–1648.
Görbe, A., Varga, Z. V., et al. (2013). Cytoprotection by the NO-donor SNAP against ischemia/reoxygenation injury in mouse embryonic stem cell-derived cardiomyocytes. Int J Immunopathol Pharmacol in press.
Gordan, J. D., Bertout, J. A., et al. (2007). HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell, 11(4), 335–347.
Gordan, J. D., Thompson, C. B., et al. (2007). HIF and c-Myc: Sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell, 12(2), 108–113.
Guilak, F., Cohen, D. M., et al. (2009). Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell, 5(1), 17–26.
Han, J. K., Lee, H. S., et al. (2008). Peroxisome proliferator-activated receptor-delta agonist enhances vasculogenesis by regulating endothelial progenitor cells through genomic and nongenomic activations of the phosphatidylinositol 3-kinase/Akt pathway. Circulation, 118(10), 1021–1033.
Hoben, G. M., Koay, E. J., et al. (2008). Fibrochondrogenesis in two embryonic stem cell lines: Effects of differentiation timelines. Stem Cells, 26(2), 422–430.
Huang, C. Y., Hagar, K. L., et al. (2004). Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells, 22(3), 313–323.
Hwang, N. S., Varghese, S., et al. (2007). Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. Journal of Cellular Physiology, 212(2), 281–284.
Hwang, N. S., Varghese, S., et al. (2006). Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Engineering, 12(9), 2695–2706.
Ieda, M., Fu, J. D., et al. (2010). Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell, 142(3), 375–386.
Ingber, D. (1991). Extracellular matrix and cell shape: Potential control points for inhibition of angiogenesis. Journal of Cellular Biochemistry, 47(3), 236–241.
Ingber, D. E. (2006). Cellular mechanotransduction: Putting all the pieces together again. FASEB Journal, 20(7), 811–827.
Jang, Y. Y., & Sharkis, S. J. (2007). A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood, 110(8), 3056–3063.
Johnson, C. P., Tang, H. Y., et al. (2007). Forced unfolding of proteins within cells. Science, 317(5838), 663–666.
Kawamura, T., Suzuki, J., et al. (2009). Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature, 460(7259), 1140–1144.
Kloxin, A. M., Kasko, A. M., et al. (2009). Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science, 324(5923), 59–63.
Krankel, N., Adams, V., et al. (2005). Hyperglycemia reduces survival and impairs function of circulating blood-derived progenitor cells. Arteriosclerosis Thrombosis and Vascular Biology, 25(4), 698–703.
Le Belle, J. E., Orozco, N. M., et al. (2011). Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell, 8(1), 59–71.
Leeper, N. J., Hunter, A. L., et al. (2012). Stem cell therapy for vascular regeneration: Adult, embryonic, and induced pluripotent stem cells. Circulation, 122(5), 517–526.
Lehninger, A. L. (1971). Biochemistry and human welfare: Where now and whither? Federation Proceedings, 30(4), 1397–1402.
Leto, D., & Saltiel, A. R. (2012). Regulation of glucose transport by insulin: Traffic control of GLUT4. Nature Reviews Molecular Cell Biology, 13(6), 383–396.
Loomans, C. J., de Koning, E. J., et al. (2004). Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes, 53(1), 195–199.
Lum, J. J., Bui, T., et al. (2007). The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes & Development, 21(9), 1037–1049.
Lunt, S. Y., & Vander Heiden, M. G. (2011). Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annual Review of Cell and Developmental Biology, 27, 441–464.
Ly, D. L., Waheed, F., et al. (2013). Hyperosmotic stress regulates the distribution and stability of myocardin-related transcription factor, a key modulator of the cytoskeleton. American Journal of Physiology, 304(2), C115–C127.
Madonna, R. (2012). Human-induced pluripotent stem cells: In quest of clinical applications. Molecular Biotechnology, 52(2), 193–203.
Madonna, R., Geng, Y. J., et al. (2009). Adipose tissue-derived stem cells: Characterization and potential for cardiovascular repair. Arteriosclerosis Thrombosis and Vascular Biology, 29(11), 1723–1729.
Madonna, R., Shelat, H., et al. (2011). Aquaporin-1 is required for vascular development of human induced pluripotent stem cells following exposure to glucose-induced hyperosmolarity. Circulation AOS.701.01 (Abstract Oral Session).
Manasek, F. J., Burnside, M. B., et al. (1972). Myocardial cell shape change as a mechanism of embryonic heart looping. Developmental Biology, 29(4), 349–371.
Mauck, R. L., Yuan, X., et al. (2006). Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage, 14(2), 179–189.
McBride, H. M., Neuspiel, M., et al. (2006). Mitochondria: More than just a powerhouse. Current Biology, 16(14), R551–R560.
McBride, S. H., Falls, T., et al. (2008). Modulation of stem cell shape and fate B: Mechanical modulation of cell shape and gene expression. Tissue Engineering Part A, 14(9), 1573–1580.
Mylotte, L. A., Duffy, A. M., et al. (2008). Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells, 26(5), 1325–1336.
Nelson, C. M., Jean, R. P., et al. (2005). Emergent patterns of growth controlled by multicellular form and mechanics. Proceedings of the National Academy of Sciences of the United States of America, 102(33), 11594–11599.
Niklason, L. E., Gao, J., et al. (1999). Functional arteries grown in vitro. Science, 284(5413), 489–493.
O’Cearbhaill, E. D., Punchard, M. A., et al. (2008). Response of mesenchymal stem cells to the biomechanical environment of the endothelium on a flexible tubular silicone substrate. Biomaterials, 29(11), 1610–1619.
Panopoulos, A. D., Yanes, O., et al. (2011). The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Research, 22(1), 168–177.
Prigione, A., & Adjaye, J. (2011). Modulation of mitochondrial biogenesis and bioenergetic metabolism upon in vitro and in vivo differentiation of human ES and iPS cells. International Journal of Developmental Biology, 54(11–12), 1729–1741.
Rafalski, V. A., Mancini, E., et al. (2012). Energy metabolism and energy-sensing pathways in mammalian embryonic and adult stem cell fate. Journal of Cell Science, 125(Pt 23), 5597–5608.
Rao, M., & Condic, M. L. (2008). Alternative sources of pluripotent stem cells: Scientific solutions to an ethical dilemma. Stem Cells and Development, 17(1), 1–10.
Raya, A., Rodriguez-Piza, I., et al. (2009). Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature, 460(7251), 53–59.
Riveline, D., Zamir, E., et al. (2001). Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. Journal of Cell Biology, 153(6), 1175–1186.
Saitoh, S., Takahashi, I., et al. (2000). Compressive force promotes chondrogenic differentiation and hypertrophy in midpalatal suture cartilage in growing rats. Anatomical Record, 260(4), 392–401.
Sawada, Y., Tamada, M., et al. (2006). Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell, 127(5), 1015–1026.
Schupp, M., & Lazar, M. A. (2010). Fingered for a fat fate. Cell Metabolism, 11(4), 244–245.
Seeger, F. H., Haendeler, J., et al. (2005). p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation, 111(9), 1184–1191.
Sniadecki, N. J., Anguelouch, A., et al. (2007). Magnetic microposts as an approach to apply forces to living cells. Proceedings of the National Academy of Sciences of the United States of America, 104(37), 14553–14558.
Sordella, R., Jiang, W., et al. (2003). Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell, 113(2), 147–158.
Spinetti, G., Kraenkel, N., et al. (2008). Diabetes and vessel wall remodelling: From mechanistic insights to regenerative therapies. Cardiovascular Research, 78(2), 265–273.
Su, Y., Liu, X. M., et al. (2008). Endothelial dysfunction in impaired fasting glycemia, impaired glucose tolerance, and type 2 diabetes mellitus. American Journal of Cardiology, 102(4), 497–498.
Sukharev, S., & Corey, D. P. (2004). Mechanosensitive channels: Multiplicity of families and gating paradigms. Science’s STKE, 2004(219), re4.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676.
Tan, J. L., Tien, J., et al. (2003). Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proceedings of the National Academy of Sciences of the United States of America, 100(4), 1484–1489.
Tepper, O. M., Galiano, R. D., et al. (2002). Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation, 106(22), 2781–2786.
Varum, S., Rodrigues, A. S., et al. (2011). Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE, 6(6), e20914.
Vats, A., Bielby, R. C., et al. (2005). Stem cells. Lancet, 366(9485), 592–602.
Wang, H., Riha, G. M., et al. (2005). Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arteriosclerosis Thrombosis and Vascular Biology, 25(9), 1817–1823.
Westfall, S. D., Sachdev, S., et al. (2008). Identification of oxygen-sensitive transcriptional programs in human embryonic stem cells. Stem Cells and Development, 17(5), 869–881.
Yamamoto, K., Sokabe, T., et al. (2005). Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. American Journal of Physiology, 288(4), H1915–H1924.
Yang, Y., Relan, N. K., et al. (1999). Embryonic mesenchymal cells share the potential for smooth muscle differentiation: Myogenesis is controlled by the cell’s shape. Development, 126(13), 3027–3033.
Ye, Z., Zhan, H., et al. (2009). Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood, 114(27), 5473–5480.
Yoshida, Y., Takahashi, K., et al. (2009). Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell, 5(3), 237–241.
Zheng, H., Dai, T., et al. (2008). SDF-1alpha/CXCR4 decreases endothelial progenitor cells apoptosis under serum deprivation by PI3K/Akt/eNOS pathway. Atherosclerosis, 201(1), 36–42.
Zheng, H., Shen, C. J., et al. (2010). Stromal cell-derived factor 1alpha reduces senescence of endothelial progenitor subpopulation in lectin-binding and DiLDL-uptaking cell through telomerase activation and telomere elongation. Journal of Cellular Physiology, 223(3), 757–763.
Zhu, S., Li, W., et al. (2010). Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell, 7(6), 651–655.
Acknowledgments
This study was supported by grants from the Istituto Nazionale Ricerche Cardiovascolari (INRC) and CARIPLO.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madonna, R., Görbe, A., Ferdinandy, P. et al. Glucose Metabolism, Hyperosmotic Stress, and Reprogramming of Somatic Cells. Mol Biotechnol 55, 169–178 (2013). https://doi.org/10.1007/s12033-013-9668-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-013-9668-2