Abstract
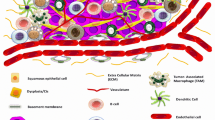
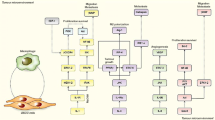
Head and neck cancers (HNC) continues to dominate major cancers contributing to mortality worldwide. Squamous cell carcinoma is the major type of HNC. Oral Squamous Cell Carcinoma grouped under HNC is a malignant tumor occurring in the oral cavity. The primary risk factors of OSCC are tobacco, alcohol consumption, etc. This review focuses on modulations, mechanisms, growth and differentiation of oral squamous cell carcinoma. Cancer cell surrounds itself with a group of elements forming a favorable environment known as tumor microenvironment (TME). It consists of numerous cells which includes immune cells, blood cells and acellular components that are responsible for the progression, immunosuppression, metastasis and angiogenesis of cancer. This review highlights the most important tissue biomarkers (mTOR, CAF, FOXp3, CD163, CD33, CD34) that are associated with TME cells. mTOR remains as the primary regulator responsible in cancer and its importance towards immune-suppression is highlighted. Tumor-associated macrophages associated with cancer development and its relationship with immunomodulatory mechanism and Tregs, which are potential blockers of immune response and its mechanism and aberrations are discussed. Cancer-associated fibroblasts that are a part of TME and their role in evading the immune response and myeloid derived suppressor cells that have slight control over the immune response and their mechanism in the tumor progression is further explained. These markers have been emphasised as therapeutic targets and are currently in different stages of clinical trials.

Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- HNC:
-
Head and neck cancer
- EGFR:
-
Epidermal growth factor receptor
- VEGF:
-
Vascular endothelial growth factor
- OSCC:
-
Oral squamous cell carcinoma
- HPV:
-
Human papilloma virus
- TME:
-
Tumor microenvironment
- ECM:
-
Extracellular matrix
- mTOR:
-
Mammalian target of rapamycin
- mTORC1 & 2:
-
Mammalian target of rapamycin complex 1 & 2
- MDSCs:
-
Myeloid-derived suppressor cells
- HSC:
-
Hematopoietic stem cells
- TAM:
-
Tumor-associated macrophages
- FOXp3::
-
Forkhead Box p3
- Tregs:
-
Regulatory T cells
- CAF:
-
Cancer-associated fibroblasts
- RTK:
-
Receptor tyrosine kinase
- GPCR:
-
G-protein coupled receptor
- PIP2 & 3:
-
Phosphatidylinositol P 2 & 3
- AKT:
-
Protein kinase B
- PTEN:
-
Phosphatase and tensin
- Rheb:
-
RAS homolog enriched in brain
- TSC1 & 2:
-
Tuberous sclerosis protein 1 & 2
- RAPTOR:
-
Regulatory associated protein of mTOR
- TH :
-
T helper cells
- DC:
-
Dendritic cells
- NK:
-
Natural killer cells
- APC:
-
Antigen presenting cells
- IL:
-
Interleukin
- TGF-β:
-
Tumor growth factor-β
- MAPK:
-
Mitogen activated protein kinase
- PDL1:
-
Programmed death ligand-1
- PKD 3:
-
Protein kinase D3
- LLSCC:
-
Lower lip squamous cell carcinoma
- CXCL12:
-
CXC motif chemokine ligand 12
- myCAF:
-
Myofibroblasts CAF
- iCAF:
-
Inflammatory CAF
- apCAF:
-
Antigen presenting CAF
- EMT:
-
Epithelial mesenchymal transition
References
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020. https://doi.org/10.1038/s41572-020-00224-3.
Gormley M, Creaney G, Schache A, Ingarfield K, Conway DI. Reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors. Br Dent J. 2022;233(9):780–6. https://doi.org/10.1038/s41415-022-5166-x.
UMDECDE Los. Cancer.net. Head and neck cancer accounts, and neck cancer in 2020. https://www.cancer.net/cancer-types/head-and-neck-cancer/statistics.
Patterson RH, et al. Global burden of head and neck cancer: economic consequences, health, and the role of surgery. Otolaryngol Head Neck Surg. 2020;162(3):296–303. https://doi.org/10.1177/0194599819897265.
Kennel T, Garrel R, Costes V, Boisselier P, Crampette L, Favier V. Head and neck carcinoma of unknown primary. Eur Ann Otorhinolaryngol Head Neck Dis. 2019;136(3):185–92. https://doi.org/10.1016/j.anorl.2019.04.002.
Diering D, Dowd EC, Frank MJ, Collins A, Goldd JM, Barch DM. Head and neck cancer. Physiol Behav. 2017;176(12):139–48. https://doi.org/10.1016/S0140-6736(08)60728-X.Head.
Szyfter K. Genetics and molecular biology of head and neck cancer. Biomolecules. 2021;11(9):10–1. https://doi.org/10.3390/biom11091293.
Wipt P, George KM. The molecular pathogenesis of head and neck cancer. Bone. 2008;23(1):1–7.
Mody MD, Rocco JW, Yom SS, Haddad RI, Saba NF. Head and neck cancer. Lancet. 2021;398(10318):2289–99. https://doi.org/10.1016/S0140-6736(21)01550-6.
Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(3):386–96. https://doi.org/10.1016/j.mayocp.2015.12.017.
Chamoli A, et al. Overview of oral cavity squamous cell carcinoma: risk factors, mechanisms, and diagnostics. Oral Oncol. 2021;121:105451. https://doi.org/10.1016/j.oraloncology.2021.105451.
Venugopal R, Bavle RM, Konda P, Muniswamappa S, Makarla S. Familial cancers of head and neck region. J Clin Diagn Res. 2017;11(6):1–6. https://doi.org/10.7860/JCDR/2017/25920.9967.
Zhang Y, He J, He B, Huang R, Li M. Effect of tobacco on periodontal disease and oral cancer. Tob Induc Dis. 2019;17:1–15. https://doi.org/10.18332/tid/106187.
Santacroce L, et al. Focus on hpv infection and the molecular mechanisms of oral carcinogenesis. Viruses. 2021;13(4):1–11. https://doi.org/10.3390/v13040559.
Blatt S, et al. Biomarkers in diagnosis and therapy of oral squamous cell carcinoma: a review of the literature. J Cranio-Maxillofac Surg. 2017;45(5):722–30. https://doi.org/10.1016/j.jcms.2017.01.033.
Vivek R. Role of ayurveda in dental practice. Am J Oral Med Radiol. 2015;2(4):246–8.
Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol. 2016;40:41–8. https://doi.org/10.1016/j.copbio.2016.02.007.
Lebleu VS. Imaging the tumor microenvironment. Cancer J. 2015;21(3):174–8. https://doi.org/10.1097/PPO.0000000000000118.
de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41(3):374–403. https://doi.org/10.1016/j.ccell.2023.02.016.
Wang G, et al. Tumor microenvironment in head and neck squamous cell carcinoma: functions and regulatory mechanisms. Cancer Lett. 2021;507(2021):55–69. https://doi.org/10.1016/j.canlet.2021.03.009.
Arneth B. Tumor microenvironment. Medicina. 2019;56:1. https://doi.org/10.3390/medicina56010015.
Huang S. mTOR signaling in metabolism and cancer. Cells. 2020;9(10):2–5. https://doi.org/10.3390/cells9102278.
Moaaz M, Lotfy H, Elsherbini B, Motawea MA, Fadali G. TGF-p enhances the anti-inflammatory effect of tumor-infiltrating CD33+11b+HLA-DR myeloid-derived suppressor cells in gastric cancer: a possible relation to microRNA-494. Asian Pac J Cancer Prev. 2020;21(11):3393–403. https://doi.org/10.31557/APJCP.2020.21.11.3393.
Calegari F, Waskow C. CD34—structure, functions and relationship with cancer stem cells. Princ Regen Med. 2013;2:109–13. https://doi.org/10.1016/B978-0-12-809880-6.00008-4.
Ma S, et al. CD163 as a potential biomarker in colorectal cancer for tumor microenvironment and cancer prognosis: a Swedish study from tissue microarrays to big data analyses. Cancers. 2022;14(24):1–15. https://doi.org/10.3390/cancers14246166.
Jia H, et al. The expression of FOXP3 and its role in human cancers. Biochim Biophys Acta Rev Cancer. 2019;1871(1):170–8. https://doi.org/10.1016/j.bbcan.2018.12.004.
Tao L, Huang G, Song H, Chen Y, Chen L. Cancer associated fibroblasts: an essential role in the tumor microenvironment (review). Oncol Lett. 2017;14(3):2611–20. https://doi.org/10.3892/ol.2017.6497.
Conciatori F, et al. Role of mTOR signaling in tumor microenvironment: an overview. Int J Mol Sci. 2018;19(8):1–19. https://doi.org/10.3390/ijms19082453.
Tian T, Li X, Zhang J. mTOR signaling in cancer and mtor inhibitors in solid tumor targeting therapy. Int J Mol Sci. 2019;20(3):1–34. https://doi.org/10.3390/ijms20030755.
Harachi M, Masui K, Okamura Y, Tsukui R, Mischel PS, Shibata N. mTOR complexes as a nutrient sensor for driving cancer progression. Int J Mol Sci. 2018;19:10. https://doi.org/10.3390/ijms19103267.
Lui VWY, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013;3(7):761–9. https://doi.org/10.1158/2159-8290.CD-13-0103.
Umemura S, et al. Therapeutic priority of the PI3K/AKT/mTOR pathway in small cell lung cancers as revealed by a comprehensive genomic analysis. J Thorac Oncol. 2014;9(9):1324–31. https://doi.org/10.1097/JTO.0000000000000250.
Lee JH, Kang KW, Lee HW. Expression of phosphorylated mTOR and its clinical significances in small cell lung cancer. Int J Clin Exp Pathol. 2015;8(3):2987–93.
Cooper WA, Lam DCL, O’Toole SA, Minna JD. Molecular biology of lung cancer. J Thorac Dis. 2013;5(Suppl 5):S479–90. https://doi.org/10.3978/j.issn.2072-1439.2013.08.03.
Riquelme I, et al. The gene expression status of the PI3K/AKT/mTOR pathway in gastric cancer tissues and cell lines. Pathol Oncol Res. 2016;22(4):797–805. https://doi.org/10.1007/s12253-016-0066-5.
De Roock W, De Vriendt V, Normanno N, Ciardiello F, Tejpar S. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011;12(6):594–603. https://doi.org/10.1016/S1470-2045(10)70209-6.
Zhang J, Roberts TM, Shivdasani RA. Targeting PI3K signaling as a therapeutic approach for colorectal cancer. Gastroenterology. 2011;141(1):50–61. https://doi.org/10.1053/j.gastro.2011.05.010.
Sun M, et al. AKT1/PKBalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol. 2001;159(2):431–7. https://doi.org/10.1016/s0002-9440(10)61714-2.
Lawrence MS, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505(7484):495–501. https://doi.org/10.1038/nature12912.
Audenet F, Attalla K, Sfakianos JP. The evolution of bladder cancer genomics: what have we learned and how can we use it? Urol Oncol. 2018;36(7):313–20. https://doi.org/10.1016/j.urolonc.2018.02.017.
Houédé N, Pourquier P. Targeting the genetic alterations of the PI3K-AKT-mTOR pathway: its potential use in the treatment of bladder cancers. Pharmacol Ther. 2015;145:1–18. https://doi.org/10.1016/j.pharmthera.2014.06.004.
Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 2009;9(2):237–49. https://doi.org/10.2174/156800909787580999.
Whiteside TL. Regulatory T cell subsets in human cancer: are they regulating for or against tumor progression? Cancer Immunol Immunother. 2014;63(1):67–72. https://doi.org/10.1007/s00262-013-1490-y.
Mafi S, et al. mTOR-mediated regulation of immune responses in cancer and tumor microenvironment. Front Immunol. 2022;12:1–19. https://doi.org/10.3389/fimmu.2021.774103.
Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9(5):324–37. https://doi.org/10.1038/nri2546.
Yang K, et al. Metabolic signaling directs the reciprocal lineage decisions of and T cells. Sci Immunol. 2018;3(25):1–13. https://doi.org/10.1126/sciimmunol.aas9818.
Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12(4):295–303. https://doi.org/10.1038/ni.2005.
Lee K, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32(6):743–53. https://doi.org/10.1016/j.immuni.2010.06.002.
Wang F, et al. Crosstalks between mTORC1 and mTORC2 variagate cytokine signaling to control NK maturation and effector function. Nat Commun. 2018;9(1):1–17. https://doi.org/10.1038/s41467-018-07277-9.
Chen YL, et al. mTOR inhibitors can enhance the anti-tumor effects of DNA vaccines through modulating dendritic cell function in the tumor microenvironment. Cancers. 2019;11:5. https://doi.org/10.3390/cancers11050617.
Vander Broek R, Mohan S, Eytan DF, Chen Z, Van Waes C. The PI3K/Akt/mTOR axis in head and neck cancer: functions, aberrations, cross-talk, and therapies. Oral Dis. 2015;21(7):815–25. https://doi.org/10.1111/odi.12206.
Marques AEM, et al. mTOR pathway protein immunoexpression as a prognostic factor for survival in head and neck cancer patients: a systematic review and meta-analysis. J Oral Pathol Med. 2016;45(5):319–28. https://doi.org/10.1111/jop.12390.
Harsha C, Banik K, Ang HL, Girisa S, Vikkurthi R. Targeting AKT/mTOR in oral cancer. Mech Adv Clin Trials. 2022;1:1–26.
Ferreira DM, Neves TJ, Lima LGCA, Alves FA, Begnami MD. Prognostic implications of the phosphatidylinositol 3-kinase/Akt signaling pathway in oral squamous cell carcinoma: overexpression of p-mTOR indicates an adverse prognosis. Appl Cancer Res. 2017;37(1):1–8. https://doi.org/10.1186/s41241-017-0046-4.
Lakshminarayana S, et al. Molecular pathways of oral cancer that predict prognosis and survival: a systematic review. J Carcinog. 2019;17(1):1–12. https://doi.org/10.4103/jcar.JCar_17_18.
Chen D, Zhang X, Li Z, Zhu B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics. 2020;11(3):1016–30. https://doi.org/10.7150/THNO.51777.
Anupama Mukherjee AD, Spadigam A. Tumor-associated macrophages: Harbingers of aggressiveness in oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2021;21(3):244–51. https://doi.org/10.4103/jomfp.JOMFP.
Xue Y, Song X, Fan S, Deng R. The role of tumor-associated macrophages in oral squamous cell carcinoma. Front Physiol. 2022;13:1–8. https://doi.org/10.3389/fphys.2022.959747.
Suárez-Sánchez FJ, Lequerica-Fernández P, Suárez-Canto J, Rodrigo JP, Rodriguez-Santamarta T, Domínguez-Iglesias F, García-Pedrero JM, de Vicente JC. Macrophages in oral carcinomas: relationship with cancer stem cell markers and PD-L1 expression. Cancers. 2020;12(7):1764.
Chen W, Xiao M, Zhang J, Chen W. M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J Exp Clin Cancer Res. 2018;37(1):1–15. https://doi.org/10.1186/s13046-018-0815-2.
Cui B, et al. Protein kinase D3 regulates the expression of the immunosuppressive protein, PD-L1, through STAT1/STAT3 signaling. Int J Oncol. 2020;56(4):909–20. https://doi.org/10.3892/ijo.2020.4974.
Aggarwal S, Sharma SC, Das SN. Dynamics of regulatory T cells (Tregs) in patients with oral squamous cell carcinoma. J Surg Oncol. 2017;116(8):1103–13. https://doi.org/10.1002/jso.24782.
da Cunha Filho FAP, et al. Immunohistochemical analysis of FoxP3+ regulatory T cells in lower lip squamous cell carcinomas. Braz Oral Res. 2016;30(1):1–8. https://doi.org/10.1590/1807-3107BOR-2016.VOL30.0130.
Norouzian M, Mehdipour F, Ashraf MJ, Khademi B, Ghaderi A. Regulatory and effector T cell subsets in tumor-draining lymph nodes of patients with squamous cell carcinoma of head and neck. BMC Immunol. 2022;23(1):1–19. https://doi.org/10.1186/s12865-022-00530-3.
Zhou X, Su YX, Lao XM, Liang YJ, Liao GQ. CD19+IL-10+ regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4+ T cells to CD4+Foxp3+ regulatory T cells. Oral Oncol. 2016;53(2016):27–35. https://doi.org/10.1016/j.oraloncology.2015.11.003.
Koike K, et al. Prognostic value of FoxP3 and CTLA-4 expression in patients with oral squamous cell carcinoma. PLoS ONE. 2020;15(8):1–17. https://doi.org/10.1371/journal.pone.0237465.
Schipmann S, Wermker K, Schulze HJ, Kleinheinz J, Brunner G. Cutaneous and oral squamous cell carcinoma-dual immunosuppression via recruitment of FOXP3+ regulatory T cells and endogenous tumour FOXP3 expression? J Cranio-Maxillofac Surg. 2014;42(8):1827–33. https://doi.org/10.1016/j.jcms.2014.06.022.
Zhang B, et al. CXCL12 is associated with FoxP3+ tumor-infiltrating lymphocytes and affects the survival of patients with oral squamous cell carcinoma. Oncol Lett. 2019;18(2):1099–106. https://doi.org/10.3892/ol.2019.10415.
Attias M, Al-Aubodah T, Piccirillo CA. Mechanisms of human FoxP3+ Treg cell development and function in health and disease. Clin Exp Immunol. 2019;197(1):36–51. https://doi.org/10.1111/cei.13290.
Ziani L, Chouaib S, Thiery J. Alteration of the antitumor immune response by cancer-associated fibroblasts. Front Immunol. 2018. https://doi.org/10.3389/fimmu.2018.00414.
Hu C, Zhang Y, Wu C, Huang Q. Heterogeneity of cancer-associated fibroblasts in head and neck squamous cell carcinoma: opportunities and challenges. Cell Death Discov. 2023;9(1):1–10. https://doi.org/10.1038/s41420-023-01428-8.
Knops AM, et al. Cancer-associated fibroblast density, prognostic characteristics, and recurrence in head and neck squamous cell carcinoma: a meta-analysis. Front Oncol. 2020;10:1–11. https://doi.org/10.3389/fonc.2020.565306.
Takahashi H, et al. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget. 2017;8(5):8633–47. https://doi.org/10.18632/oncotarget.14374.
Bienkowska KJ, Hanley CJ, Thomas GJ. Cancer-associated fibroblasts in oral cancer: a current perspective on function and potential for therapeutic targeting. Front Oral Health. 2021;2:1–11. https://doi.org/10.3389/froh.2021.686337.
Takahashi H, et al. Immunosuppressive activity of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancer Immunol Immunother. 2015;64(11):1407–17. https://doi.org/10.1007/s00262-015-1742-0.
Wu F, et al. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6(1):1–35. https://doi.org/10.1038/s41392-021-00641-0.
Kouketsu A, et al. Myeloid-derived suppressor cells and plasmacytoid dendritic cells are associated with oncogenesis of oral squamous cell carcinoma. J Oral Pathol Med. 2023;52(1):9–19. https://doi.org/10.1111/jop.13386.
Pang X, et al. Myeloid derived suppressor cells contribute to the malignant progression of oral squamous cell carcinoma. PLoS ONE. 2020;15(2):e0229089. https://doi.org/10.1371/journal.pone.0229089.
Nagatsuka H, et al. Various immunostaining patterns of CD31, CD34 and endoglin and their relationship with lymph node metastasis in oral squamous cell carcinomas. J Oral Pathol Med. 2005;34(2):70–6. https://doi.org/10.1111/j.1600-0714.2004.00227.x.
Shahsavari F, Farhadi S, Sadri D, Sedehi M. Evaluation of microvascularity by CD34 expression in esophagus and oral squamous cell carcinoma. J Contemp Dent Pract. 2015;16(6):458–62. https://doi.org/10.5005/jp-journals-10024-1706.
Chu M, et al. Myeloid-derived suppressor cells contribute to oral cancer progression in 4NQO-treated mice. Oral Dis. 2012;18(1):67–73. https://doi.org/10.1111/j.1601-0825.2011.01846.x.
Harsha C, et al. Targeting AKT/mTOR in oral cancer: mechanisms and advances in clinical trials. Int J Mol Sci. 2020;21:9. https://doi.org/10.3390/ijms21093285.
Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6(1):75. https://doi.org/10.1038/s41392-021-00484-9.
Sharma A, Rudra D. Regulatory T cells as therapeutic targets and mediators. Int Rev Immunol. 2019;38(5):183–203. https://doi.org/10.1080/08830185.2019.1621310.
Salimifard S, et al. Cancer associated fibroblasts as novel promising therapeutic targets in breast cancer. Pathol Res Pract. 2020;216(5):152915. https://doi.org/10.1016/j.prp.2020.152915.
Acknowledgements
Not applicable.
Funding
No funding was received for this review.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramalingam, S., Shantha, S., Muralitharan, S. et al. Role of tissue markers associated with tumor microenvironment in the progression and immune suppression of oral squamous cell carcinoma. Med Oncol 40, 303 (2023). https://doi.org/10.1007/s12032-023-02169-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02169-5