Abstract
While healthcare is becoming more patient-centred, evidence-based nutrition interventions are still not accessible to all patients with cancer. As nutrition interventions directly improve clinical and socioeconomic outcomes, patient-centred care is not complete without nutrition care. While awareness of the negative impact of malnutrition on clinical outcomes, quality of life, and functional and emotional wellbeing in cancer is growing, there is relatively poor awareness amongst patients, clinicians, policymakers, and payers that nutrition interventions -particularly those begun in the early stages of the disease course- are an effective method for improving such outcomes. The European Beating Cancer Plan recognises the need for a holistic approach to cancer but lacks actionable recommendations to implement integrated nutrition cancer care at member state level. When considering nutrition care as a human right, the impact on quality of life and functional status must be prioritized, as these may be equally as important to patients, especially in advanced cancer where improvements in clinical outcomes such as survival or tumour burden may not be attainable. We formulate actions needed at the regional and the European level to ensure integrated nutrition care for all patients with cancer. The 4 main Take Home Messages are as follows: 1. The goals of Europe’s Beating Cancer Plan cannot be achieved without integrating nutrition across the cancer care continuum. 2. Malnutrition negatively impacts clinical outcomes and has socioeconomic consequences for patients and healthcare systems. 3. Championing integrating nutrition care into cancer care is therefore the duty and ethical responsibility of clinicians (Hippocratic Oath—primum non nocere) and 4. Nutrition care is a cost effective, evidence-based therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patient-centred care (PCC) requires a shift in focus from the traditional disease-focused, clinician-centric model of care to a system which empowers and enables patients to participate in shared decision-making and self-management [1,2,3,4]. While healthcare professionals (HCPs) generally appreciate the value of PCC, many may overestimate the level of PCC being achieved in practice. Despite strong evidence for improvements in patient-reported and clinical outcomes, nutrition is poorly integrated into multidisciplinary care in many areas of medicine, including cancer care [5,6,7,8,9,10,11,12,13,14,15,16]. Unfortunately, throughout Europe, there is a lack of consistent, coordinated integration of nutrition care throughout the cancer care continuum, and as such, PCC implementation has not been truly achieved. Nonetheless, the concept of PCC is considered so essential in cancer care that the Institute of Medicine listed PCC as the first of six interconnected essential components for the delivery of high-quality cancer care [17]. These recommendations quickly gained traction throughout major medical institutions across the world and were integrated into their guidelines, including those for accreditation by the Organisation of European Cancer Institutes (OECI) [18,19,20]. Recognition of the fact that PCC is not complete, nor as effective without nutrition care is that what differentiates a good physician from a great physician [21].
Suboptimal integration of nutrition into cancer care not only overlooks the value of PCC including nutrition interventions on quality of life (QoL), but also the fact that medical care itself is less effective when the patient is nutritionally depleted. While awareness of the negative impact of malnutrition on clinical outcomes in cancer is growing, there is relatively poor awareness amongst clinicians and patients alike, about the role of nutrition in improving clinical outcomes despite an ever growing body of strong clinical evidence. [5, 22,23,24,25,26,27]. In addition to tangible positive impacts on survival, length of stay and tolerance to treatment, the QoL of people living with, and beyond cancer, is positively impacted by targeted nutrition interventions [28]. The European Beating Cancer Plan calls for a holistic approach to cancer, from prevention and early diagnosis to treatment and quality of life of patients and survivors [29]. PCC, which includes nutrition, is particularly important when patients are faced with a devastating diagnosis and/or a chronic disease such as cancer, and where quality of life is potentially more modifiable than quantity of life. The aim of this paper is therefore to highlight the clinical evidence, the ethical considerations, the economic advantages, and the patient perspectives with respect to nutrition care as an integral part of the cancer care continuum.
Improving nutrition status directly impacts clinical outcomes
High-quality medical care requires full integration of nutrition care into all steps of the cancer care pathway [30, 31]. Consistent evidence derived from randomized controlled trials shows that integrating nutrition care into cancer care positively impacts clinically relevant outcomes including reduction of toxicities, reduced post-operative complications, increased progression free survival and overall survival [32,33,34]. The impact of malnutrition on mortality in cancer has long been recognised and in 1932, an autopsy study of 500 cancer patients at Harvard Medical School found cachexia to be the primary cause of death in 22% of cases, and a complicating factor in many more [35]. Given that the landscape of oncology has dramatically changed since the twentieth century, these statistics are unlikely to accurately represent the natural history of cancer-related malnutrition today, however, plausible biological mechanisms have been proposed which would suggest causality in the cachexia-death relationship [36]. Furthermore, the metabolism of many anti-cancer treatments is negatively impacted by reduced muscle mass, and the resulting side effects compromise quality of life and impair physical performance, or even shorten survival due to dose-limiting toxicities interrupting the intended treatment plan [37, 37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. Therefore, nutrition status is an essential consideration in ensuring optimization of the efficacy of standard medical, radiation and surgical oncology interventions.
Aspects of PCC are components of the internationally recognized quality standards for nutrition care—yet the integration of professionally delivered evidence-based nutrition care into the multidisciplinary team is lacking across the European Union and globally [5,6,7,8,9,10,11,12,13,14,15,16]. In fact, it has been more than 40 years since awareness of the importance of nutrition status in hospital settings was widely acknowledged as the ‘skeleton in the hospital closet’ [64] and a large body of supporting evidence has accumulated. Nonetheless, consistent improvement in nutrition care has not materialised [65]. Relative to astounding contemporary investment in medical oncology research, with 52% of 56,000 molecules in the current drug development pipeline being for cancer [66], there is comparatively little investment in non-pharmacological or complex behavioural interventions. Moreover, when non-pharmacological studies are conducted, they are especially prone to exclusion from meta-analyses, due to high levels of heterogeneity (in methodology, outcomes, interventions and reporting) which is confounded by a lack of consensus on the appropriate methodological approach to evaluating complex interventions [67]. As such, despite a large body of individual studies demonstrating the value of early nutrition care in cancer, the overall body of evidence appears to remain inconclusive due to the relatively low number of studies which can be included at the level of a systematic review or meta-analysis. However, despite a relative lack of investment and a disproportionate focus on the legitimate difficulty in reversing refractory cachexia [68], perhaps the most important point to note is that there is consistent, robust evidence demonstrating the ability of a variety of early nutrition interventions to significantly improve clinical outcomes [28, 60, 69,70,71]. Clinicians, patients, and stakeholders at every level need to understand that evidence-based patient-centred care includes nutrition and as such, its omission is a disservice to people living with and beyond cancer.
Integrating nutrition into cancer care is evidence-based
The impact of nutrition status throughout the care continuum on QoL, functional and emotional well-being, and on clinical outcomes have been underappreciated in favour of quantifiable, measurable end-points of interest to pharmaceutical regulators [72,73,74,75,76,77]. Despite strong recommendations for multimodal management of cancer-related malnutrition, the implementation of such multidisciplinary care is in its infancy [7, 78,79,80,81,82,83,84].
A growing body of evidence reflects that nutrition status has a significant effect on clinical outcomes and QoL. A large majority of patients experience some form of nutrition-related issues during their cancer journey [6, 7, 85, 86]. Moreover, long-term side effects following curative treatment commonly include body composition changes, nutrition impact symptoms and functional limitations which, in turn, impact QoL and are amenable to rehabilitation or supportive care [87]. Physicians and other members of the MDT such as nurses should therefore regularly prescribe nutrition care to cancer patients at all stages of care, whether it be providing first-line advice, or referring to specialists such as oncology dietitians [30, 88,89,90,91].
Integrating nutrition into cancer care is a human right
The WHO Ministerial Conference on Nutrition and Noncommunicable Diseases in the Context of Health 2020 led to the Vienna Declaration which urges the mandating of person-centred nutrition care [92] and the International Working Group for Patients’ Right to Nutritional Care (composed of experts in clinical nutrition and representatives of international nutrition organizations) argue that nutrition care is an emerging human right that lies at the intersection of the existing human right to food and the human right to health, where patients hold the ‘right to be fed’ [93]. A right-holder implies duty-bearers, in this case, the state, policymakers, institutional managers and caregivers, are ethically responsible and must be held accountable. As the performance of these professionals is of critical importance, nutrition education is a priority [94, 95]. However, this can only be achieved by developing an institutional culture that values nutrition care and recognizes the need for a multi-stakeholder approach. Ethical debates such as when to withdraw nutrition and hydration in end-of-life settings [96,97,98] are in fact, only applicable if we introduce nutrition care in the first place.
According to the Europe’s Beating Cancer Plan all cancer inequalities should be reduced across the EU [29]. The inalienable right to high-quality PCC care, regardless of geographic or economic region, to avoid unnecessary deaths and suffering from cancer is supported by the European Charter of Patient Rights [99]. Malnutrition is not a fringe issue, and affects large amounts of cancer patients [100, 101]. Independent of potential sequelae and impact on medical outcomes which might also be improved with nutrition [69], malnutrition has also been documented as its own psychosocial challenge [102, 103].
Integrating nutrition into cancer care is economically advantageous
Cancer care can affect the economic circumstances of patients and their households. QoL, costs of treatment and survival can be significant and lead to further inequalities [104, 105]. Effective cancer control programs and policies should therefore consider economic aspects for all cancer patients, survivors, and their carers [106,107,108,109,110,111]. A poor nutrition status is costly [106, 112, 113] and nutrition interventions contribute to reduced healthcare costs [112, 114,115,116,117]. On the other hand, the economic cost of malnutrition also impacts the healthcare system due to the costs associated with more complications, increased length of hospital stay (LOS), readmissions and increased morbidity [112]. In the UK, it was found that the cost of treating a malnourished patient is up to 3 times higher compared to a non-malnourished patient [112] Data from the Netherlands reveal that managing disease-related malnutrition accounted for 4.9% of total healthcare expenditure [118]. US economic modelling based on international health economics data suggested that widely implementing nutrition support in gastrointestinal cancer alone could account for up to US $242 million in Medicare savings each year [119]. Importantly, these savings have been demonstrated in real-world settings, showing that significant cost savings can be achieved by implementing optimal nutrition care in cancer [114, 115, 120].
Accessible patient-centred nutrition information empowers patients to take action and improves quality of life
Nutrition has been identified as an important and essential factor for empowering patients because it internalises their locus of control (LOC), supporting development of self-efficacy at a time where patients can experience a loss of bodily autonomy and seek self-management strategies which re-embody a sense of control. With these psychological considerations in mind, PCC demands that the right information is provided, in the right way, at the right time to the right patients. Dietitian-led nutrition care aims to achieve exactly this goal and should therefore not be overlooked [121,122,123,124,125,126].
To ensure improved outcomes and prevent recurrence, it is essential that nutrition education addresses patients’ weight management goals during and after treatment, to ensure patients do not fall prey to inappropriate, non-evidence-based nutrition advice which is widespread, easily accessible online, and frequently promoted by unqualified ‘experts’ [127,128,129,130,131,132]. Patient-centred consultations utilizing effective communication strategies are thus essential for increasing awareness about the consequences of cancer diets and encourage informed decision-making. This is especially important as patients consistently report lack of access to nutrition professionals while simultaneously reporting a lack of communication about nutrition on the part of their physicians—even when questions are directly posed [6, 133, 134].
In fact, a European survey including 907 cancer patients and survivors showed that not only is access to nutrition care lacking, but also that when patients are left alone they seek nutrition information elsewhere often finding information that is not evidence-based, possible harmful and sometimes counterproductive [85]. Even information presented on cancer centres websites does not meet the universal health literacy standards [135, 136], Patients with high unmet information needs are more likely to seek out unproven complementary and alternative medicine such as restrictive diets which may potentially negatively impact nutrition status [137, 138].
Unfortunately, psychological analyses have shown that short answers without explanations make patients feel that their needs have not been acknowledged or deemed important. This can lead to patients becoming more cemented in their original, incorrect beliefs [139,140,141,142]. On the other hand, a scientifically sound answers, and more importantly, decisive identification of alternatives encourages good decision-making by patients. Such communication strategies need to be strengthened among physicians—especially with regard to nutrition advice, as many patients will never see a dietitian, and depend on this first-line advice from the clinician with whom they have most contact [143]. Where knowledge on the part of physicians is lacking, the value of a specialised dietitian as part of the multidisciplinary team becomes even more apparent. It is therefore essential that oncologists and members of the multidisciplinary care teams understand their own scope of practice with respect to medical nutrition therapy, and appropriate referral procedures are standard practice [11, 22, 144,145,146,147,148]. In this context, nurses are often the most accessible member of the MDT to patients. They have an essential role in ensuring access to nutrition care, in particular for non-complex cases where they can provide invaluable first-line advice, or in complex cases, where they are responsible for implementing dietitian delivered recommendations. This example demonstrates why all members of the MDT must be able and willing to champion nutrition care, within the scope of their own profession. Moreover, it highlights why nutrition education and training cannot be limited to the nutrition specialist, if our aim is for widely accessible, properly implemented, integrated nutrition care in cancer.
Importantly, patients must be made aware of the importance of good nutrition in cancer and that intervention can positively impact their personal and clinical outcomes. Without this knowledge, patients are not able to advocate for themselves and identify red-flag signs. Additionally, their cancer team should encourage and empower patients to engage in shared decision-making and be confident to request assessment and treatment of nutrition-related concerns as they arise [149, 150].
Eating is a social process and contributes to quality of life [69, 102, 121, 151,152,153,154,155]. Optimal nutrition care is a human right and cannot be delivered in the absence of effective communication strategies [156, 157].
Patient-centred care means listening to patient voices
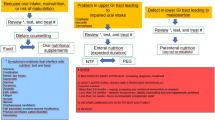
In order to practice patient-centred care, it is essential to listen to and to understand what is important to patients. Many studies have shown that patients are not getting the nutrition care they need, and desire [6, 22, 85, 133, 158]. The European Cancer Patient Coalition (ECPC) recently presented rich qualitative data collected via direct patient interviews which indicate the central role of good nutrition care in cancer, from the patient perspective [159, 160]. Figure 1 includes a selection of quotes from the ECPC booklet showing patients’ points of view in their own words [160]. The patients’ insights highlight the fact that patients recognise the need for nutrition care, but are unfortunately, not consistently receiving the personalised nutrition counselling required to implement meaningful changes in their daily life. The Information Box depicted in Fig. 2 provides guidance to patient-centred communication with regards to nutrition and cancer.
Patient quotes (adapted with permission from [160])
Integrating nutrition into cancer care requires a plan
Scaled integration of these and other emerging nutrition interventions into healthcare would require significant economic investment and continued rigorous research but in the end the cost benefit is proven[17, 114]. To implement integrated nutrition care in cancer, coordination of messaging is needed. To support policy makers, service providers, and patient advocates the following first steps are recommended:
-
Step 1 Widespread advocacy is required to raise awareness amongst clinicians and patients. It should be communicated that nutrition care in cancer has a significant impact on both clinically relevant outcomes and health-related quality of life issues which are central to PCC. This message needs to reach clinicians whose referral practices to dietetics require changes. Patients need to understand this message so they can then self-advocate when they identify that nutrition has become a problem for them. Finally, this message needs to be communicated to policymakers or healthcare management whose buy-in is needed to acquire funding and coordinate human and infrastructural resources to facilitate development and ongoing provision of comprehensive dietetic services in oncology. This call to action should refer to the key aspects of evidence, human rights and economic value outlined above.
-
Step 2 Cancer services should urgently incorporate key performance indicators (KPIs) into their regular quality assurance systems to benchmark and audit adherence to evidence-based nutrition recommendations. These KPIs should be evidence-based or at least based on expert consensus. ESPEN, ESMO, COSA and others have many such guidelines [30, 81, 88, 89, 161] which could be used as starting points to develop a quality assurance standards for Integrated Nutrition Care in Cancer. At an absolute minimum, audits should report on malnutrition screening, oncology specific dietetic staffing and availability of nutrition assessment for all patients with high-risk diagnoses (e.g., head & neck cancers, gastrointestinal cancers, high-dose chemotherapy, radiotherapy to the head & neck or pelvis).
-
Step 3 At the European level, several of the actions of the Beating Cancer Plan should include nutrition, notably, the plan mentions the role of diet and exercise in cancer prevention but does not focus on the specific role of nutrition within the management of cancer. In order to maximise the effect of a number of the flagship initiatives arising from the Beating Cancer Plan [29], ‘National Comprehensive Cancer Centre’ accreditation should be associated with a minimum acceptable level of nutrition and dietetic service provision, in which all cancer patients are nutritionally screened, the dietitian is a core part of the multidisciplinary team, and all members of the MDT receive basic and regular nutrition training. The ‘Knowledge Centre on Cancer’, ‘Inter-Specialty Training Programme’, ‘Cancer Diagnostic and Treatment for All’, ‘Partnership on Personalised Medicine’, ‘Better life for cancer patients’, ‘Cancer Inequalities Registry’, “EU-Network of Comprehensive Cancer Centres” and ‘Guidelines and Quality Assurance’ initiatives must explicitly include nutrition care.
Conclusion
Across the cancer continuum, and especially for people living with incurable disease, the improvement of QoL may be more significant to patients than improvement in traditionally ‘clinically relevant’ outcomes such as tumour burden or overall survival [75]. Thus, when considering nutrition care as a human right, it is only appropriate to think about the outcomes which matter to patients themselves and the impact of malnutrition on QoL and how nutrition and nutrition-related issues affect cancer patients in general.
The pivotal role of nutrition care particularly applies to cancer care and is founded on the basis that anti-cancer treatments can be more effective in patients with a balanced nutrition status, leading to less delays in treatment, and less dose-limiting toxicities [42, 162]. However, across the cancer care continuum, and for the increasing group of people living with and beyond cancer, as well as those who live with the chronic ‘late effects’ of cancer appropriate and timely nutrition care still remains a documented unmet need [163,164,165,166,167,168,169].
Nutrition care in cancer is an essential component of standard cancer care and is a basic right for people living with and beyond cancer. Therefore, these initial recommendations on advocacy, evidence, quality assurance and European actions should function as an essential guide to ensuring that Europe’s Beating Cancer Plan and other health programmes and policies have nutrition care at its centre.
According to Europe’s Beating Cancer Plan, adopted in 2021, the European Commission aims to reduce all cancer inequalities across the EU [29]. Equal access of all cancer patients to high-quality care, regardless of geographic or economic region, to avoid unnecessary deaths and suffering from cancer, is further supported by the European Charter of Patient Rights [99]. However, professionals should not only provide mere access to high-quality care, including nutrition, but take responsibility to educate patients and involve them in decision-making to ensure their individual needs are met.
Data availability
No data were generated in the production of this manuscript.
References
Balogh EP, Ganz PA, Murphy SB, Nass SJ, Ferrell BR, Stovall E. Patient-centered cancer treatment planning: improving the quality of oncology care. Summary of an institute of medicine workshop. Oncologist. 2011;16(12):1800.
Institute of medicine (US) Committee on quality of health care in America. Crossing the quality chasm: a new health system for the 21st century [Internet]. Washington (DC): National Academies Press (US); 2001 [cited 2022 Mar 2]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK222274/
Bardes CL. Defining ‘patient-centered medicine.’ N Engl J Med. 2012;366(9):782–3.
Becker RE, Seeman MV. Patients are our teachers. J Patient Cent Res Rev. 2018;5(2):183–6.
Deftereos I, Kiss N, Brown T, Carey S, Carter VM, Usatoff V, et al. Awareness and perceptions of nutrition support in upper gastrointestinal cancer surgery: a national survey of multidisciplinary clinicians. Clin Nutr ESPEN. 2021;46:343–9.
Sullivan ES, Rice N, Kingston E, Kelly A, Reynolds JV, Feighan J, et al. A national survey of oncology survivors examining nutrition attitudes, problems and behaviours, and access to dietetic care throughout the cancer journey. Clin Nutr ESPEN. 2021;41:331–9.
Caccialanza R, Goldwasser F, Marschal O, Ottery F, Schiefke I, Tilleul P, et al. Unmet needs in clinical nutrition in oncology: a multinational analysis of real-world evidence. Ther Adv Med Oncol. 2020;1(12):1758835919899852.
Levonyak NS, Hodges MP, Haaf N, Mhoon V, Lopez R, Little Jones A, et al. Implementation of a quality improvement project to increase access to dietitian services for patients with gastrointestinal cancer in a safety-net hospital system. JCO Oncol Pract. 2021;17(7):e1048–54.
Sonmez O, Tezcanli E, Bas D, Kazanci HB, Altinok A, Demir A, et al. Identifying knowledge and practices regarding cancer patient malnutrition: a survey study among oncologists. Nutr Cancer. 2021;3:1–8.
Kok A, van der Lugt C, Leermakers-Vermeer MJ, de Roos NM, Speksnijder CM, de Bree R. Nutritional interventions in patients with head and neck cancer undergoing chemoradiotherapy: current practice at the dutch head and neck oncology centers. Clin Nutr ESPEN. 2020;1(40):545.
Lorton CM, Griffin OKH. Late referral of cancer patients with malnutrition to dietitians: a prospective study of clinical practice. Support Care Cancer. 2019;28:2351.
Findlay M, Bauer J, Shaw T, White K, Lai M, Rankin NM. ‘There’s a lot of talent in the room but it’s only really the medical talent that gets heard’: a qualitative exploration of multidisciplinary clinicians’ perspectives of optimal nutrition care of patients with head and neck cancer. Support Care Cancer. 2021;29(11):6399–409.
Lin JX, Chen XW, Chen ZH, Huang XY, Yang JJ, Xing YF, et al. A multidisciplinary team approach for nutritional interventions conducted by specialist nurses in patients with advanced colorectal cancer undergoing chemotherapy: a clinical trial. Medicine (Baltimore). 2017;96(26): e7373.
Winters DA, Soukup T, Sevdalis N, Green JSA, Lamb BW. The cancer multidisciplinary team meeting: in need of change? History, challenges and future perspectives. BJU Int. 2021;128(3):271–9.
Trujillo EB, Claghorn K, Dixon SW, Hill EB, Braun A, Lipinski E, et al. Inadequate nutrition coverage in outpatient cancer centers: results of a national survey. J Oncol. 2019;2019:7462940.
Haut Conseil de la santé publique. Évaluation de 10 ans de politique de lutte contre le cancer 2004–2014, pp. 1–261. 2016. Paris, France. Available from: https://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier=hcspr20160408_evalpolitiqueluttecontrecancer.pdf. Accessed 08 Mar 2022
Nekhlyudov L, Levit L, Hurria A, Ganz PA. Patient-centered, evidence-based, and cost-conscious cancer care across the continuum: translating the institute of medicine report into clinical practice. CA Cancer J Clin. 2014;64(6):408–21.
Oberst S, van Harten W, Sæter G, de Paoli P, Nagy P, Burrion JB, et al. 100 European core quality standards for cancer care and research centres. Lancet Oncol. 2020;21(8):1009–11.
German Medical Association Bundesärtzekammer. Qualitätsmerkmale eines krankheitsorientierten Zentrums [Internet]. [cited 2022 Mar 7]. Available from: https://www.bundesaerztekammer.de/aerzte/qualitaetssicherung/zentren-und-zertifizierung/qualitaetsmerkmale/
NHS England. Implementing the cancer taskforce recommendations: commissioning person centred care for people affected by cancer [Internet]. [cited 2022 Mar 7]. Available from: https://www.england.nhs.uk/wp-content/uploads/2016/04/cancer-guid-v1.pdf
Laviano A. The good physician and the great physician: why a physician should consider the ESMO guidelines on the management of cancer cachexia? ESMO Open. 2022;7(1):100383.
Keaver L, Connolly P, Richmond J. Providing nutrition advice in the oncology setting: a survey of current practice, awareness of guidelines and training needs of Irish healthcare professionals in three hospitals. Eur J Cancer Care (Engl). 2021;30(4):e13405.
Kiss N, Bauer J, Boltong A, Brown T, Isenring L, Loeliger J, et al. Awareness, perceptions and practices regarding cancer-related malnutrition and sarcopenia: a survey of cancer clinicians. Support Care Cancer. 2020;28(11):5263–70.
Deutz NEP, Ashurst I, Ballesteros MD, Bear DE, Cruz-Jentoft AJ, Genton L, et al. The underappreciated role of low muscle mass in the management of malnutrition. J Am Med Dir Assoc. 2019;20(1):22–7.
Osborn R, Moulds D, Squires D, Doty MM, Anderson C. International survey of older adults finds shortcomings in access, coordination, and patient-centered care. Health Aff (Millwood). 2014;33(12):2247–55.
Janssen G, Pourhassan M, Lenzen-Großimlinghaus R, Jäger M, Schäfer R, Spamer C, et al. The refeeding syndrome revisited: you can only diagnose what you know. Eur J Clin Nutr. 2019;73(11):1458–63.
Wirth R, Smoliner C, Spamer C, Marburger C, Schreiber FS, Willschrei HP, et al. Do doctors know how much nutrition patients need–a survey from Germany? Eur J Clin Nutr. 2014;68(7):840–3.
Prado CM, Purcell SA, Laviano A. Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle. 2020;11(2):366–80.
Communication from the Commission to the European Parliament and the Council Europe’s Beating Cancer Plan [Internet]. European Commission; [cited 2022 Mar 2]. Available from: https://ec.europa.eu/health/system/files/2022-02/eu_cancer-plan_en_0.pdf
Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(5):1187–96.
Muscaritoli M, Molfino A, Gioia G, Laviano A, Rossi FF. The ‘parallel pathway’: a novel nutritional and metabolic approach to cancer patients. Intern Emerg Med. 2011;6(2):105–12.
Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393(10188):2312–21.
Laviano A, Di Lazzaro L, Koverech A. Nutrition support and clinical outcome in advanced cancer patients. Proc Nutr Soc. 2018;77(4):388–93.
Britton B, Baker AL, Wolfenden L, Wratten C, Bauer J, Beck AK, et al. Eating as treatment (EAT): a stepped-wedge, randomized controlled trial of a health behavior change intervention provided by dietitians to improve nutrition in patients with head and neck cancer undergoing radiation therapy (TROG 12.03). Int J Radiat Oncol Biol Phys. 2019;103(2):353–62.
Warren S. The immediate causes of death in cancer. Am J Med Sci. 1932;184(5):610–5.
Kalantar-Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle. 2013;4(2):89–94.
Huillard O, Mir O, Peyromaure M, Tlemsani C, Giroux J, Boudou-Rouquette P, et al. Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br J Cancer. 2013;108:1034–41.
Ali R, Baracos VE, Sawyer MB, Bianchi L, Roberts S, Assenat E, et al. Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med. 2016;5:607–16.
Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol. 2010;21(8):1594–8.
Mir O, Coriat R, Blanchet B, Durand JP, Boudou-Rouquette P, Michels J, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS ONE. 2012;7:e37563.
Wendrich AW, Swartz JE, Bril SI, Wegner I, de Graeff A, Smid EJ, et al. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017;71:26–33.
Drami I, Pring ET, Gould L, Malietzis G, Naghibi M, Athanasiou T, et al. Body composition and dose-limiting toxicity in colorectal cancer chemotherapy treatment; a systematic review of the literature. Could muscle mass be the new body surface area in chemotherapy dosing? Clin Oncol. 2021;33(12):e540-52.
Mazzuca F, Onesti CE, Roberto M, Di Girolamo M, Botticelli A, Begini P, et al. Lean body mass wasting and toxicity in early breast cancer patients receiving anthracyclines. Oncotarget. 2018;9(39):25714–22.
Anandavadivelan P, Brismar TB, Nilsson M, Johar AM, Martin L. Sarcopenic obesity: a probable risk factor for dose limiting toxicity during neo-adjuvant chemotherapy in oesophageal cancer patients. Clin Nutr. 2016;35(3):724–30.
Arrieta O, De la Torre-Vallejo M, López-Macías D, Orta D, Turcott J, Macedo-Pérez EO, et al. Nutritional status, body surface, and low lean body mass/body mass index are related to dose reduction and severe gastrointestinal toxicity induced by Afatinib in patients with non-small cell lung cancer. Oncologist. 2015;20(8):967–74.
Auclin E, Bourillon C, De Maio E, By MA, Seddik S, Fournier L, et al. Prediction of everolimus toxicity and prognostic value of skeletal muscle index in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer. 2017;15:350–5.
Bruno KDA, Sobreira da Silva MJ, Chaves GV. Association of body composition with toxicity to first-line chemotherapy and three-year survival in women with ovarian adenocarcinoma. Acta Oncol. 2021;60(12):1611–20.
Bozzetti F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol. 2017;28(9):2107–18.
Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66:583–9.
Cousin S, Hollebecque A, Koscielny S, Mir O, Varga A, Baracos VE, et al. Low skeletal muscle is associated with toxicity in patients included in phase I trials. Invest New Drugs. 2014;32(2):382–7.
Chemama S, Bayar MA, Lanoy E, Ammari S, Stoclin A, Goéré D, et al. Sarcopenia is associated with chemotherapy toxicity in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer. Ann Surg Oncol. 2016;23:3891–8.
Cushen SJ, Power DG, Teo MY, MacEneaney P, Maher MM, McDermott R, et al. Body composition by computed tomography as a predictor of toxicity in patients with renal cell carcinoma treated with sunitinib. Am J Clin Oncol Cancer Clin Trials. 2017;40(1):47–52.
Catanese S, Aringhieri G, Vivaldi C, Salani F, Vitali S, Pecora I, et al. Role of baseline computed-tomography-evaluated body composition in predicting outcome and toxicity from first-line therapy in advanced gastric cancer patients. J Clin Med. 2021;10(5):1–13.
Daly LE, Power DG, O’Reilly Á, Donnellan P, Cushen SJ, O’Sullivan K, et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer. 2017;116(3):310–7.
Del Grande M, Rizzo S, Nicolino GM, Colombo I, Rossi L, Manganaro L, et al. Computed Tomography–Based Body Composition in Patients With Ovarian Cancer: Association With Chemotoxicity and Prognosis. Frontiers in Oncology [Internet]. 2021;11. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85120539830&doi=10.3389%2ffonc.2021.718815&partnerID=40&md5=3bb53f21bbc7a55186eb3ea006a90927
Dijksterhuis WPM, Pruijt MJ. Association between body composition, survival, and toxicity in advanced esophagogastric cancer patients receiving palliative chemotherapy. J Cachexia Sarcopenia Muscle. 2019;10:199–206.
Halvorsen TO, Valan CD, Slaaen M, Grønberg BH. Associations between muscle measures, survival, and toxicity in patients with limited stage small cell lung cancer. J Cachexia Sarcopenia Muscle. 2020;11(5):1283–90.
Heidelberger V, Goldwasser FNK. Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Invest New Drugs. 2017;35:436–41.
Jain R, Handorf E, Khare V, Blau M, Chertock Y, Hall MJ. Impact of baseline nutrition and exercise status on toxicity and outcomes in phase I and II oncology clinical trial participants. Oncologist. 2020;25(2):161–9.
Liu L, Erickson NT, Ricard I, von Weikersthal LF, Lerch MM, Decker T, et al. Early weight loss is an independent risk factor for shorter survival and increased side effects in patients with metastatic colorectal cancer undergoing first-line treatment within the randomized phase III trial FIRE-3 (AIO KRK-0306). Int J Cancer. 2022;150(1):112–23.
Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75(2):188–98.
Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007;13:3264–8.
Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35.
Butterworth CEJ. The skeleton in the hospital closet. Nutr Today. 1974;9(2):4–8.
Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla E, Prado CM. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016;75(2):199–211.
Health products in the pipeline from discovery to market launch for all diseases [Internet]. [cited 2022 Mar 7]. Available from: https://www.who.int/observatories/global-observatory-on-health-research-and-development/monitoring/health-products-in-the-pipeline-from-discovery-to-market-launch-for-all-diseases
Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of medical research council guidance. BMJ. 2021;30(374):n2061.
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;18(4):17105.
Molassiotis A, Brown T, Cheng HL, Byrnes A, Chan RJ, Wyld D, et al. The effects of a family-centered psychosocial-based nutrition intervention in patients with advanced cancer: the PiCNIC2 pilot randomised controlled trial. Nutr J. 2021;20(1):2.
de Schueren MAE, Laviano A, Blanchard H, Jourdan M, Arends J, Baracos VE. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann Oncol. 2018;29:1141–53.
Baldwin C, Spiro A, Ahern R, Emery PW. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:371–85.
Kleijnen S, Leonardo Alves T, Meijboom K, Lipska I, De Boer A, Leufkens HG, et al. The impact of quality-of-life data in relative effectiveness assessments of new anti-cancer drugs in European countries. Qual Life Res. 2017;26(9):2479–88.
Fearon K, Argiles JM, Baracos VE, Bernabei R, Coats A, Crawford J, et al. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle. 2015;6(4):272–4.
Garcia JM. What is next after anamorelin? Curr Opin Support Palliat Care. 2017;11:266–71.
Laird BJA, Balstad TR, Solheim TS. Endpoints in clinical trials in cancer cachexia: where to start? Curr Opin Support Palliat Care. 2018;12(4):445–52.
Ryan AM, Sullivan ES. Impact of musculoskeletal degradation on cancer outcomes and strategies for management in clinical practice. Proc Nutr Soc. 2021;80(1):73–91.
Richards J, Arensberg MB, Thomas S, Kerr KW, Hegazi R, Bastasch M. Impact of early incorporation of nutrition interventions as a component of cancer therapy in adults: a review. Nutrients. 2020;12(11):3403.
Del Fabbro E, Hui D, Dalal S, Dev R, Nooruddin ZI, Noorhuddin Z, et al. Clinical outcomes and contributors to weight loss in a cancer cachexia clinic. J Palliat Med. 2011;14:1004–8.
Bland K, Harrison M, Zopf E, Sousa M, Currow D, Ely M, et al. Quality of life and symptom burden improve in patients attending a multidisciplinary clinical service for cancer cachexia: a retrospective observational review. J Pain Symptom Manag. 2021;1:62.
Granda-Cameron C, DeMille D. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14:72–80.
Talwar B, Donnelly R, Skelly R, Donaldson M. Nutritional management in head and neck cancer: United Kingdom national multidisciplinary guidelines. J Laryngol Otol. 2016;130(S2):S32-40.
O’Neill LM, Guinan E, Doyle SL, Bennett AE, Murphy C, Elliott JA, et al. The RESTORE randomized controlled trial: impact of a multidisciplinary rehabilitative program on cardiorespiratory fitness in esophagogastric cancer survivorship. Ann Surg. 2018;268(5):747–55.
Vaughan VC, Harrison M, Dowd A, Eastman P, Martin P. Evaluation of a multidisciplinary cachexia and nutrition support service- the patient and carers perspective. J Patient Exp. 2021;8:2374373520981476.
Zhao Y, Pang D, Lu Y. The role of nurse in the multidisciplinary management of cancer cachexia. Asia Pac J Oncol Nurs. 2021;8(5):487–97.
Muscaritoli M, Molfino A, Scala F, Christoforidi K, Manneh-Vangramberen I, De Lorenzo F. Nutritional and metabolic derangements in Mediterranean cancer patients and survivors: the ECPC 2016 survey. J Cachexia Sarcopenia Muscle. 2019;10(3):517–25.
de Pinho NB, Martucci RB, Rodrigues VD, D’Almeida CA, Thuler LCS, Saunders C, et al. High prevalence of malnutrition and nutrition impact symptoms in older patients with cancer: results of a Brazilian multicenter study. Cancer. 2020;126(1):156–64.
Hall CC, Cook JMM. Combined exercise and nutritional rehabilitation in outpatients with incurable cancer: a systematic review. Support Care Cancer. 2019;27:2371–84.
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48.
Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, Ravasco P, et al. Cancer cachexia in adult patients: ESMO clinical practice guidelines☆. ESMO Open. 2021;6(3):100092.
Aapro M, Arends J, Bozzetti F, Fearon K, Grunberg SM, Herrstedt J, et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European school of oncology task force. Ann Oncol. 2014;25(8):1492–9.
Holistic Needs Assessment (HNA) | Healthcare professionals [Internet]. [cited 2022 Mar 7]. Available from: https://www.macmillan.org.uk/healthcare-professionals/innovation-in-cancer-care/holistic-needs-assessment
World Health Organization Regional Office for Europe. Vienna Declaration on Nutrition and Noncommunicable Diseases in the Context of Health 2020 [Internet]. Copenhagen; 2013 [cited 2022 Jul 13]. Available from: https://www.euro.who.int/__data/assets/pdf_file/0003/234381/Vienna-Declaration-on-Nutrition-and-Noncommunicable-Diseases-in-the-Context-of-Health-2020-Eng.pdf
Cardenas D. Ethical issues and dilemmas in artificial nutrition and hydration. Clin Nutr ESPEN. 2021;1(41):23–9.
Crowley J, Ball L, Hiddink GJ. Nutrition in medical education: a systematic review. Lancet Planetary Health. 2019;3(9):e379–89.
Cuerda C, Schneider SM, Van Gossum A. Clinical nutrition education in medical schools: results of an ESPEN survey. Clin Nutr. 2017;36(4):915–6.
Bozzetti F. ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clin Nutr. 2016;35(6):1577.
Schwartz DB, Posthauer ME, O’Sullivan Maillet J. Advancing Nutrition and Dietetics Practice: Dealing With Ethical Issues of Nutrition and Hydration. J Acad Nutr Diet [Internet]. 2020 Sep 25 [cited 2022 Mar 8]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7518202/
Tsai E. Withholding and withdrawing artificial nutrition and hydration. Paediatr Child Health. 2011;16(4):241–2.
European Charter of Patients’ Rights [Internet]. European Commission; 2002 [cited 2022 Mar 2]. Available from: https://ec.europa.eu/health/ph_overview/co_operation/mobility/docs/health_services_co108_en.pdf
Sullivan ES, Daly LE, Power DG, Ryan AM. Epidemiology of cancer-related weight loss and sarcopenia in the UK and Ireland: incidence, prevalence, and clinical impact. JCSM Rapid Commun. 2020;3(2):91–102.
von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle. 2016;7(5):507–9.
Hopkinson J. Psychosocial support in cancer cachexia syndrome: the evidence for supported self-management of eating problems during radiotherapy or chemotherapy treatment. Asia Pac J Oncol Nurs. 2018;5(4):358–68.
Oberholzer R, Hopkinson JB, Baumann K, Omlin A, Kaasa S, Fearon KC, et al. Psychosocial effects of cancer cachexia: a systematic literature search and qualitative analysis. J Pain Symptom Manag. 2013;46(1):77–95.
Haskins CP, Champ CE, Miller R, Vyfhuis MAL. Nutrition in cancer: evidence and equality. Adv Radiat Oncol. 2020;5(5):817–23.
Jagsi R, Ward KC. Unmet need for clinician engagement regarding financial toxicity after diagnosis of breast cancer. Cancer. 2018;124:3668–76.
Curtis LJ, Bernier P, Jeejeebhoy K, Allard J, Duerksen D, Gramlich L, et al. Costs of hospital malnutrition. Clin Nutr. 2017;36(5):1391–6.
Rabito EI, Marcadenti A, da Silva FJ, Figueira L, Silva FM. Nutritional risk screening 2002, short nutritional assessment questionnaire, malnutrition screening tool, and malnutrition universal screening tool are good predictors of nutrition risk in an emergency service. Nutr Clin Pract. 2017;32(4):526–32.
Kang MC, Kim JH, Ryu SW, Moon JY, Park JH, Park JK, et al. Prevalence of malnutrition in hospitalized patients: a multicenter cross-sectional study. J Korean Med Sci. 2018;33(2):e10.
Leiva Badosa E, Badia Tahull M, Virgili Casas N, Elguezabal Sangrador G, Faz Méndez C, Herrero Meseguer I, et al. Hospital malnutrition screening at admission: malnutrition increases mortality and length of stay. Nutr Hosp. 2017;34(4):907–13.
Fukuta A, Saito TSM. Impact of preoperative cachexia on postoperative length of stay in elderly patients with gastrointestinal cancer. Nutrition. 2019;58:65–8.
Tzeng YH, Wei J, Tsao TP, Lee YT, Lee KC, Liou HR, et al. Computed tomography-determined muscle quality rather than muscle quantity is a better determinant of prolonged hospital length of stay in patients undergoing transcatheter aortic valve implantation. Acad Radiol. 2020;27(3):381–8.
Elia M (on behalf of the Malnutrition Action Group of BAPEN and the National Institute for Health Research Southampton Biomedical Research Centre). The cost of malnutrition in England and potential cost savings from nutritional interventions: A report on the cost of disease-related malnutrition in England and a budget impact analysis of implementing the NICE clinical guidelines/quality standard on nutritional support in adults. BAPEN. London, United Kingdom. 2015. Available from: https://www.bapen.org.uk/resources-and-education/publications-and-reports/malnutrition/cost-of-malnutrition-in-england. Accessed 21 Feb 2023
Rice N, Normand C. The cost associated with disease-related malnutrition in Ireland. Public Health Nutr. 2012;15:1966–72.
Schuetz P, Sulo S, Walzer S, Vollmer L, Stanga Z, Gomes F, et al. Economic evaluation of individualized nutritional support in medical inpatients: secondary analysis of the EFFORT trial. Clin Nutr. 2020;39(11):3361–8.
Muscaritoli M, Krznarić Z, Singer P, Barazzoni R, Cederholm T, Golay A, et al. Effectiveness and efficacy of nutritional therapy: a systematic review following Cochrane methodology. Clin Nutr. 2017;36(4):939–57.
Toulson Davisson Correia MI, Castro M, de Oliveira TD, Farah D, Sansone D, de Morais Andrade TR, et al. Nutrition therapy cost-effectiveness model indicating how nutrition may contribute to the efficiency and financial sustainability of the health systems. J Parenter Enter Nutr. 2021;45(7):1542–50.
Hall BT, Englehart MS, Blaseg K, Wessel K, Stawicki SPA, Evans DC. Implementation of a dietitian-led enteral nutrition support clinic results in quality improvement, reduced readmissions, and cost savings. Nutr Clin Pract. 2014;29(5):649–55.
Freijer K, Tan SS, Koopmanschap MA, Meijers JMM, Halfens RJG, Nuijten MJC. The economic costs of disease related malnutrition. Clin Nutr. 2013;32(1):136–41.
Pimiento JM, Evans DC, Tyler R, Barrocas A, Hernandez B, Araujo-Torres K, et al. Value of nutrition support therapy in patients with gastrointestinal malignancies: a narrative review and health economic analysis of impact on clinical outcomes in the United States. J Gastrointest Oncol. 2021;12(2):864–73.
Bargetzi L, Brack C, Herrmann J, Bargetzi A, Hersberger L, Bargetzi M, et al. Nutritional support during the hospital stay reduces mortality in patients with different types of cancers: secondary analysis of a prospective randomized trial. Ann Oncol. 2021;32(8):1025–33.
Cipriano-Crespo C, Conde-Caballero D, Rivero Jiménez B, Mariano-Juárez L. Eating experiences and quality of life in patients with larynx cancer in Spain. A qualitative study. Int J Qual Stud Health Well-Being. 2021;16(1):1967262.
Gillis C, Hasil L, Kasvis P, Bibby N, Davies SJ, Prado CM, et al. Nutrition care process model approach to surgical prehabilitation in oncology. Front Nutr. 2021. https://doi.org/10.3389/fnut.2021.644706.
Laviano A. Current guidelines for nutrition therapy in cancer: the arrival of a long journey or the starting point? J Parenter Enter Nutr. 2021;45(S2):S12–5.
van den Berg MGA, Rasmussen-Conrad EL, Wei KH, Lintz-Luidens H, Kaanders JHAM, Merkx MAW. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104(6):872–7.
Martin L, de Schueren MAE, Blauwhoff-Buskermolen S, Baracos V, Gramlich L. Identifying the barriers and enablers to nutrition care in head and neck and esophageal cancers: an international qualitative study. JPEN J Parenter Enteral Nutr. 2016;40(3):355–66.
Nasrah R, Van Der Borch C, Kanbalian M, Jagoe RT. Defining barriers to implementation of nutritional advice in patients with cachexia. J Cachexia Sarcopenia Muscle. 2020;11(1):69–78.
Baier L, Hübner J, Kerschbaum E, Erickson N. Krebsdiäten: patientenzentrierte Kommunikationsstrategien. Onkologe. 2021;27(2):148–53.
Di Sebastiano KM, Murthy G, Campbell KL, Desroches S, Murphy RA. Nutrition and cancer prevention: why is the evidence lost in translation? Adv Nutr. 2019;10(3):410–8.
Mayne ST, Playdon MC, Rock CL. Diet, nutrition, and cancer: past, present and future. Nat Rev Clin Oncol. 2016;13(8):504–15.
Huebner J, Marienfeld S, Abbenhardt C, Ulrich C, Muenstedt K, Micke O, et al. Counseling patients on cancer diets: a review of the literature and recommendations for clinical practice. Anticancer Res. 2014;34(1):39–48.
Römer M, Dörfler J, Huebner J. The use of ketogenic diets in cancer patients: a systematic review. Clin Exp Med. 2021;21(4):501–36.
Erickson N, Boscheri A, Linke B, Huebner J. Systematic review: isocaloric ketogenic dietary regimes for cancer patients. Med Oncol. 2017;34(5):72.
Maschke J, Kruk U, Kastrati K, Kleeberg J, Buchholz D, Erickson N, et al. Nutritional care of cancer patients: a survey on patients’ needs and medical care in reality. Int J Clin Oncol. 2017;22(1):200–6.
Greenlee H, Santiago-Torres M, McMillen KK, Ueland K, Haase AM. Helping patients eat better during and beyond cancer treatment: continued nutrition management throughout care to address diet, malnutrition, and obesity in cancer. Cancer J. 2019;25(5):320–8.
Keaver L, Callaghan H, Walsh L, Houlihan C. Nutrition guidance for cancer patients and survivors—a review of the websites of Irish healthcare and charitable organisations and cancer centres. Eur J Cancer Care (Engl). 2020;29(2):e13216.
Barrett M, Uí Dhuibhir P, Njoroge C, Wickham S, Buchanan P, Aktas A, et al. Diet and nutrition information on nine national cancer organisation websites: a critical review. Eur J Cancer Care (Engl). 2020;29(5):e13280.
Balneaves LG, Wong ME, Porcino AJ, Truant TLO, Thorne SE, Wong ST. Complementary and alternative medicine (CAM) information and support needs of Chinese-speaking cancer patients. Support Care Cancer. 2018;26(12):4151–9.
Bauer F, Schmidt T, Eisfeld H, Dubois C, Kastrati K, Hochhaus A, et al. Information needs and usage of complementary and alternative medicine in members of a German self-help group for gastrointestinal stroma tumours, sarcoma, and renal cancer. Complement Ther Med. 2018;41:105–10.
Carey JM, Chi V, Flynn DJ, Nyhan B, Zeitzoff T. The effects of corrective information about disease epidemics and outbreaks: evidence from Zika and yellow fever in Brazil. Sci Adv. 2020. https://doi.org/10.1126/sciadv.aaw7449.
Bautista JR, Zhang Y, Gwizdka J. Healthcare professionals’ acts of correcting health misinformation on social media. Int J Med Inform. 2021;148:104375.
Chen L, Wang X, Peng TQ. Nature and diffusion of gynecologic cancer-related misinformation on social media: analysis of tweets. J Med Internet Res. 2018;20(10):e11515.
Grimes DR. The struggle against cancer misinformation. Cancer Discov. 2022;12(1):26–30.
Horneber M, van Ackeren G, Fischer F, Kappauf H, Birkmann J. Addressing unmet information needs: results of a clinician-led consultation service about complementary and alternative medicine for cancer patients and their relatives. Integr Cancer Ther. 2018;17(4):1172–82.
Murphy JL, Munir F, Davey F, Miller L, Cutress R, White R, et al. The provision of nutritional advice and care for cancer patients: a UK national survey of healthcare professionals. Support Care Cancer. 2021;29(5):2435–42.
Kruif J. Perceptions of Dutch health care professionals on weight gain during chemotherapy in women with breast cancer. Support Care Cancer. 2019;27:601–7.
Sandmæl JA, Bye A, Solheim TS, Balstad TR, Thorsen L, Skovlund E, et al. Physical rehabilitation in patients with head and neck cancer: impact on health-related quality of life and suitability of a post-treatment program. Laryngoscope Investig Otolaryngol. 2020;5(2):330–8.
Liposits G, Orrevall Y, Kaasa S, Österlund P, Cederholm T. Nutrition in cancer care: a brief, practical guide with a focus on clinical practice. JCO Oncol Pract. 2021. https://doi.org/10.1200/OP.20.00704.
Muscaritoli M, Arends J, Aapro M. From guidelines to clinical practice: a roadmap for oncologists for nutrition therapy for cancer patients. Ther Adv Med Oncol. 2019. https://doi.org/10.1177/1758835919880084.
Schneider N, Bäcker A, Brenk-Franz K, Keinki C, Hübner J, Brandt F, et al. Patient information, communication and competence empowerment in oncology (PIKKO)—evaluation of a supportive care intervention for overall oncological patients. Study protocol of a non-randomized controlled trial. BMC Med Res Methodol. 2020;20(1):120.
The Patients’ Charter on Patient Empowerment [Internet]. [cited 2022 Mar 2]. Available from: https://www.eu-patient.eu/globalassets/campaign-patient-empowerment/charter/epf_charter_pe_2016.pdf
Einarsson S, Laurell G, Tiblom EY. Experiences and coping strategies related to food and eating up to two years after the termination of treatment in patients with head and neck cancer. Eur J Cancer Care. 2019;28(2):e12964.
Larsson M, Hedelin B, Athlin E. Lived experiences of eating problems for patients with head and neck cancer during radiotherapy. J Clin Nurs. 2003;12(4):562–70.
Missel M, Hansen M, Jackson R, Siemsen M, Schønau MN. Re-embodying eating after surgery for oesophageal cancer: patients’ lived experiences of participating in an education and counselling nutritional intervention. J Clin Nurs. 2018;27(7–8):1420–30.
Laursen L, Schønau MN, Bergenholtz HM, Siemsen M, Christensen M, Missel M. Table in the corner: a qualitative study of life situation and perspectives of the everyday lives of oesophageal cancer patients in palliative care. BMC Palliat Care. 2019;18(1):60.
Sjeltoft JR, Donsel PO, Vad H, Larsen MK, Missel M. A radical change: a qualitative study of patients’ experiences of eating and daily living through the first year after oesophageal resection. Eur J Oncol Nurs. 2020;48:101800.
Arraras JI, Giesinger J, Shamieh O, Bahar I, Koller M, Bredart A, et al. Cancer patient satisfaction with health care professional communication: an international EORTC study. Psychooncology. 2021;31:541.
Bender JL, O’Grady L, Jadad AR. Supporting cancer patients through the continuum of care: a view from the age of social networks and computer-mediated communication. Curr Oncol. 2008;15(s2):107.
Keaver L. Irish cancer patients and survivors have a positive view of the role of nutritional care in cancer management from diagnosis through survivorship. Irish J Med Sci. 2021;190(4):1387–90.
Manneh-Vangramberen I, Molfino A, Scala F, De Lorenzo F, Geraghty H, Daly J, et al. (2018) Living well during cancer treatment: ECPC nutrition booklet—addressing cancer patients concerns [Internet]. Brussels: The European Cancer Patient Coalition (ECPC); 2018 [cited 2022 Jul 13]. Available from: https://ecpc.org/wp-content/uploads/2019/08/ecpc-nutrition-booklet-living-well-during-cancer-treatment-3.pdf
Cardone A, Gono P, Errando Calleja AL, Elkington C, Koukougianni M, Clark J, et al. Living well during cancer treatment: ECPC nutrition booklet—patient stories [Internet]. Vol. 2. Brussels: The European Cancer Patient Coalition (ECPC); 2021 [cited 2022 Jul 13]. Available from: https://ecpc.org/wp-content/uploads/2021/03/ECPC-Patients-stories-2021-final-3.pdf
Kiss N, Loeliger J, Findlay M, Isenring E, Baguley BJ, Boltong A, et al. Clinical oncology society of australia: position statement on cancer-related malnutrition and sarcopenia. Nutr Diet. 2020;77(4):416–25.
Cespedes Feliciano EM, Lee VS, Prado CM, Meyerhardt JA, Alexeeff S, Kroenke CH, et al. Muscle mass at the time of diagnosis of nonmetastatic colon cancer and early discontinuation of chemotherapy, delays, and dose reductions on adjuvant FOLFOX: The C-SCANS study. Cancer. 2017;123:4868–77.
O’Connor M, Drummond F, O’Donovan B, Donnelly C. The unmet needs of cancer survivors in ireland: a scoping review 2019. Cork: National Cancer Registry Ireland; 2019.
Sodergren SC, Wheelwright SJ, Permyakova NV, Patel M, Calman L, Smith PWF, et al. Supportive care needs of patients following treatment for colorectal cancer: risk factors for unmet needs and the association between unmet needs and health-related quality of life-results from the ColoREctal Wellbeing (CREW) study. J Cancer Surviv. 2019;13(6):899–909.
Mollica MA, Smith AW, Kent EE. Caregiving tasks and unmet supportive care needs of family caregivers: a U.S. population-based study. Patient Educ Couns. 2020;103(3):626–34.
Wang T, Molassiotis A, Chung BPM, Tan JY. Unmet care needs of advanced cancer patients and their informal caregivers: a systematic review. BMC Palliat Care. 2018;17(1):96.
Ashbury FD, Olver I. Cancer Symptoms, treatment side effects and disparities in supportive care. In: Olver I, editor. The MASCC textbook of cancer supportive care and survivorship. Cham: Springer International Publishing; 2018. p. 3–13.
Bennett S, Murphy C, Fanning M, Reynolds J, Doyle S, Donohoe C. The impact of nutrition and gastrointestinal symptoms on health-related quality of life in survivorship after oesophageal cancer surgery. Clin Nutr Open Sci. 2021;41:44.
Gegechkori N, Haines L, Lin JJ. Long-term and latent side effects of specific cancer types. Med Clin North Am. 2017;101:1053–73.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to preparation, data collection and analysis. The first draft of the manuscript was written by ESS and NE. All authors made equal contributions to content expansion, editing and revising. All authors and endorsing organizations read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
N. Erickson has received speaker honoraria from CSL Behring, Fresenius-Kabi GmbH, Baxter, Janssen-Cilag GmbH, Cognitando Gmbh, Nutricia and GHD. She served on the Expert advisory board at Baxter, received consulting fees from Fresenius-Kabi GmbH, and received compensation for writing articles for from Klarigo Verlag and Havas Lynxx Group. None of these activities were related to the content of this article. ES. Sullivan has received speakers’ honoraria from Fresenius Kabi and Nutricia, consulting fees from Abbott Laboratories and writing honoraria from Complete Nutrition and MedMedia. She is a current Irish Research Council Enterprise Partnership Scheme Postdoctoral Fellow, whose research is co-funded by Nualtra. A. Laviano has received speakers’ honoraria from Abbott, Baxter, Bbraun, Fresenius Kabi, Nestlé Health Science and Nutricia. Dr. Laviano received consulting fees from Abbott, Baxter, Bbraun, DSM, Nestlé Health Science and Nutricia. Dr. Laviano received a research grant from Fresenius Kabi. None of these activities were related to the content of this article. All remaining authors declared no conflict of interest.
Ethical approval
This consensus paper did not involve human or animal subjects.
Consent to publish
The European Cancer Patient Coalition (ECPC) provided written consent to adapt and publish patient quotes extracted from: Cardone A, Gono P, Errando Calleja AL, Elkington C, Koukougianni M, Clark J, et al. Living Well During Cancer Treatment: ECPC Nutrition Booklet—Patient Stories [Internet]. Vol. 2. Brussels: The European Cancer Patient Coalition (ECPC); 2021 [cited 2022 Jul 13]. Available from: https://ecpc.org/wp-content/uploads/2021/03/ECPC-Patients-stories-2021-final-3.pdf.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Endorsements from the following organisations The European Nutrition Health Alliance (ENHA), The European Federation of Associations of Dietitians (EFAD), The European Society for Clinical Nutrition and Metabolism (ESPEN), The European Cancer Patient Coalition (ECPC), The European Association for the Study of Obesity (EASO), The European Oncology Nursing Society (EONS), Lung Cancer Europe (LuCE), The German Clinical Nutrition Society (DGEM), The European Network of Dietetic Students (ENDietS).
Note regarding professional terminology Please note that due to variable legislative protections and differences in professional roles across the European Union, there exists significant variability in the titles and educational backgrounds of clinicians who are appropriately trained and licensed to provide specialist nutrition care in a clinical setting. Throughout this article, the term dietitian is used for consistency, but to avoid uncertainty, this should be interpreted as including the equivalent, accredited professional who is responsible for clinical nutrition care in the relevant jurisdiction. For example, this may include local translations of ‘Registered Dietitian’, or the protected title of a specialist physician responsible for clinical nutrition support.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erickson, N., Sullivan, E.S., Kalliostra, M. et al. Nutrition care is an integral part of patient-centred medical care: a European consensus. Med Oncol 40, 112 (2023). https://doi.org/10.1007/s12032-023-01955-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-01955-5