Abstract
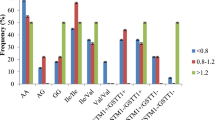
In the spite of the impressive results achieved with imatinib in chronic myeloid leukemia (CML) patients, differences in patient’s response are observed, which may be explained by interindividual genetic variability. It is known that cytochrome P450 enzymes play a major role in the metabolism of imatinib. The present study aimed to understand the functional impact of CYP2B6 15631G>T polymorphism on the response of imatinib in CML patients and its relation to CML susceptibility. We have genotyped CYP2B6 G15631T in 48 CML patients and 64 controls by PCR-RFLP. CYP2B6 15631G>T was not found to be a risk factor for CML (OR 95 % CI, 1.12, 0.6–2, p > 0.05). Hematologic response loss was higher in patients with 15631GG/TT genotype when compared with 15631GT (36.8 vs. 13.8 %; X 2 = 3.542, p = 0.063). Complete cytogenetic response was higher in 15631GG/GT genotype groups when compared with 15631TT (X 2 = 3.298, p = 0.024). Primary cytogenetic resistance was higher in patients carrying 15631GG/TT genotype when compared with 15631GT carriers (52.6 vs. 17.2 %; X 2 = 6.692, p = 0.010). Furthermore, side effects were more common for patients carrying 15631GG genotypes when compared with GT/TT carriers (36 vs. 13.8 %; X 2 = 8.3, p = 0.004). In light of our results, identification of 15631G>T polymorphism in CML patients might be helpful to predict therapeutic response to imatinib.




Similar content being viewed by others
References
Deininger MWN, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia Review article. Blood. 2000;96:3343–56.
Buchdunger E, Zimmermann J, Mett H, Lydon NB, Meyer T, Muller M, et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996;56:100–4.
Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15618470.
O’Brien SG, Guilhot F, Larson R a, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12637609.
Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11287972.
Branford S, Rudzki Z, Walsh S, Parkinson I, Grigg A, Szer J, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12623848.
Werner B, Lutz D, Brümmendorf TH, Traulsen A, Balabanov S. Dynamics of resistance development to imatinib under increasing selection pressure: a combination of mathematical models and in vitro data. PloS One. 2011;6:1–8. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3245228&tool=pmcentrez&rendertype=abstract.
Peng B, Hayes M, Resta D, Racine-Poon A, Druker BJ, Talpaz M, et al. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22:935–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14990650.
Lamba V, Lamba J, Yasuda K, Strom S, Davila J, Hancock ML, et al. Hepatic CYP2B6 expression : gender and ethnic differences and relationship to CYP2B6 genotype and CAR (constitutive androstane receptor) expression. J Pharmacol Exp Ther. 2003;307:906–22.
Saarikoski ST, Rivera SP, Hankinson O, Husgafvel-Pursiainen K. CYP2S1: a short review. Toxicol Appl pharmacol. 2005;207:62–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16054184.
Gonzalez F, Crespi C, Czerwinski M, Gelbon V. Tohoku J Exp Med. 1992;168:67–72.
Gervot L, Rochat B, Gautier JC, Bohnenstengel F, Kroemer H, de Berardinis V, et al. Human CYP2B6: expression, inducibility and catalytic activities. Pharmacogenetics. 1999;9:295–306. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10471061.
Ekins S, Wrighton S a. The role of CYP2B6 in human xenobiotic metabolism. Drug Metab Rev. 1999;31:719–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10461547.
Lang T, Klein K, Fischer J, Nüssler a K, Neuhaus P, Hofmann U, et al. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11:399–415. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11470993.
Lewis DF V. 57 varieties: the human cytochromes P450. Pharmacogenomics. 2004;5:305–18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15102545.
Villuendas R, Steegmann JL, Pollán M, Tracey L, Granda a, Fernández-Ruiz E, et al. Identification of genes involved in imatinib resistance in CML: a gene-expression profiling approach. Leukemia. 2006;20:1047–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16598311.
Deininger MW, O’Brien SG, Ford JM, Druker BJ. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21:1637–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12668652.
Kantarjian HM, O’Brien S, Cortes J, Giles FJ, Rios MB, Shan J, et al. Imatinib mesylate therapy improves survival in patients with newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukemia in the chronic phase: comparison with historic data. Cancer. 2003;98:2636–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14669283.
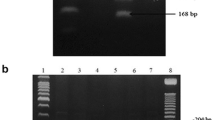
Miller S, Dykes D, Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
Berköz M, Yalin S. Association of CYP2B6 G15631T polymorphism with acute leukemia susceptibility. Leuk Res. 2009;33:919–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19144407.
Waxman DJ, Ko A, Walsh C. Regioselectivity and stereoselectivity of androgen hydroxylations catalyzed by cytochrome P-450 isozymes purified from phenobarbital-induced rat liver. J Biol Chem. 1983;258:11937–47.
Seree EJ, Pisano PJ, Placidi M, Rahmani R, Barra YA. Identification of the human and animal hepatic cytochromes P450 involved in clonazepam metabolism. Fundam Clin Pharmacol. 1993;7:69–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8486332.
Dehal SS, Kupfer D. Metabolism of the proestrogenic pesticide methoxychlor by hepatic P450 monooxygenases in rats and humans. Dual pathways involving novel ortho ring-hydroxylation by CYP2B. Drug Metab Dispos Biol Fate Chem. 1994;22:937–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7895613.
Evans WE, McLeod HL. Pharmacogenomics--drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538–49. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12571262.
Scripture CD, Sparreboom A, Figg WD. Modulation of cytochrome P450 activity: implications for cancer therapy. Lancet Oncol. 2005;6:780–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16198984.
Smires F, Moreau C, Habbal R, Siguret V, Fadili S, Golmard J, et al. Influence of genetics and non-genetic factors on acenocoumarol maintenance dose requirement in Moroccan patients. J Clin Pharm Ther. 2012;37:594–8.
Elmachad M, Elkabbaj D, Elkerch F, Laarabi F, Barkat A, Oualim Z, et al. Frequencies of CYP3A5 * 1/* 3 variants in a Moroccan population and effect on tacrolimus daily dose requirements in renal transplant. Genet Test Mol Biomarkers. 2012;16:644–7.
Belmouden A, Melki R, Hamdani M, Zaghloul K, Amraoui A, Nadifi S, et al. A novel frameshift founder mutation in the cytochrome P450 1B1 (CYP1B1) gene is associated with primary congenital glaucoma in Morocco. Clin Genet. 2002;62:334–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12372064.
Gan CQ, Wang XY, Cao YD, Ye WX, Liu H, Sun YY. Association of CYP2C19*3 gene polymorphism with breast cancer in Chinese women. Genet Mol Res GMR. 2011;10:3514–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22180071.
Levkovich NN, Gorovenko NG, Myasoedov D V. Association of polymorphic G1934A variant (allele *4) of CYP2D6 gene with increased risk of breast cancer development in Ukrainian women. Exp Oncol. 2011;33:136–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21956465.
Turpeinen M, Raunio H, Pelkonen O. The functional role of CYP2B6 in human drug metabolism: substrates and inhibitors in vitro, in vivo and in silico. Curr Drug Metab. 2006;7:705–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17073575.
Waris M, White LJ. Seasonality of respiratory syncytial virus infection. Clin Infect Dis Off Publ Infect Dis Soc Am. 2006;43:541–2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19713363.
Hofmann MH, Blievernicht JK, Klein K, Saussele T, Schaeffeler E, Schwab M, et al. Aberrant splicing caused by single nucleotide polymorphism c. 516G T [Q172H], a marker of CYP2B6 * 6, is responsible for decreased expression and activity of CYP2B6 in Liver. J Pharmacol Exp Ther. 2008;325:284–92.
Rakhmanina NY, van den Anker JN, Soldin SJ, van Schaik RH, Mordwinkin N, Neely MN. Can TDM improve pharmacotherapy of HIV infection in adolescents? Ther Drug Monit. 2010;32:273–81.
Xu C, Ogburn ET, Guo Y, Desta Z. Effects of the CYP2B6 * 6 allele on catalytic properties and inhibition of CYP2B6 in vitro: implication for the mechanism of reduced efavirenz metabolism and other CYP2B6 substrates in vivo (Abstract). 2012;40:717–25.
Yuan Z, Liu Q, Zhang Y, Liu H, Zhao J, Zhu P. CYP2B6 gene single nucleotide polymorphisms and leukemia susceptibility. Ann Hematol. 2011;90:293–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20878158.
Acknowledgments
We authors thank Hassan II academy of Science and Technology for providing us with financial support for this study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kassogue, Y., Quachouh, M., Dehbi, H. et al. Functional polymorphism of CYP2B6 G15631T is associated with hematologic and cytogenetic response in chronic myeloid leukemia patients treated with imatinib. Med Oncol 31, 782 (2014). https://doi.org/10.1007/s12032-013-0782-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0782-6