Abstract
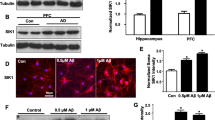
Inhibitory neurotransmission is important for brain function and requires specific transmitter receptors that are organized in synaptic domains. Gephyrin is a cytoskeletal organization protein that binds tubulin and plays an important role in clustering and organizing select inhibitory neurotransmitter receptors. Here, we tested if gephyrin is altered by protein accumulation stress that is common in age-related neurodegenerative disorders. For this, we used the hippocampal slice model that has been shown to exhibit chloroquine (CQN)-induced protein accumulation, microtubule destabilization, transport failure, and declines in excitatory neurotransmitter receptors and their responses. In addition to the decreases in excitatory receptor subunits and other glutamatergic markers, we found that gephyrin isoforms were reduced across the CQN treatment period. Associated with this decline in gephyrin levels was the production of three gephyrin breakdown products (GBDPs) of 30, 38, and 48 kDa. The induced effects on gephyrin were tested for evidence of recovery through enhancement of lysosomal function that is known to promote protein clearance and microtubule integrity. Using the lysosomal modulator Z-Phe-Ala-diazomethylketone (PADK), gephyrin levels were completely restored in correspondence with the recovery of excitatory glutamatergic components. In addition, GBDPs were significantly reduced after the 2-day PADK treatment, to levels that were at or below those measured in control cultures. These findings suggest that receptor-clustering mechanisms for inhibitory synapses are compromised during protein accumulation events. They also indicate that a lysosomal enhancement strategy can protect gephyrin integrity, which may be vital for the balance between inhibitory and excitatory signaling during age-related diseases.






Similar content being viewed by others
References
Bahr, B. A. (1995). Long-term hippocampal slices: A model system for investigating synaptic mechanisms and pathological processes. Journal of Neuroscience Research, 42, 294–305.
Bahr, B. A. (2003). Dysfunction and activation of the lysosomal system: Implications for and against Alzheimer’s disease. In E. M. Welsh (Ed.), Focus on Alzheimer's disease research (pp. 115–150). Hauppauge, NY: Nova Science.
Bahr, B. A., Abai, B., Gall, C. M., Vanderklish, P. W., Hoffman, K. B., Lynch, G. (1994). Induction of β-amyloid-containing polypeptides in hippocampus: Evidence for a concomitant loss of synaptic proteins and interactions with an excitotoxin. Experimental Neurology, 129, 81–94.
Bahr, B. A., & Bendiske, J. (2002). The neuropathogenic contributions of lysosomal dysfunction. Journal of Neurochemistry, 83, 481–489.
Bahr, B. A., Hoffman, K. B., Kessler, M., Hennegriff, M., Park, G. Y., Yamamoto, R. S. et al. (1996). Distinct distributions of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor subunits and a related 53,000 MR antigen (GR53) in brain tissue. Neuroscience, 74, 707–721.
Bahr, B. A., Kessler, M., Rivera, S., Vanderklish, P. W., Hall, R. A., Mutneja, M. S. et al. (1995). Stable maintenance of glutamate receptors and other synaptic components in long-term hippocampal slices. Hippocampus, 5, 425–439.
Bendiske, J., & Bahr, B. A. (2003). Lysosomal activation is a compensatory response against protein accumulation and associated synaptopathogenesis-an approach for slowing Alzheimer disease? Journal of Neuropathology and Experimental Neurology, 62, 481–489.
Bendiske, J., Caba, E., Brown, Q. B., & Bahr, B. A. (2002). Intracellular deposition, microtubule destabilization, and transport failure: an “early” pathogenic cascade leading to synaptic decline. Journal of Neuropathology and Experimental Neurology, 61, 640–650.
Bonde, C., Noraberg, J., Noer, H., & Zimmer, J. (2005). Ionotropic glutamate receptors and glutamate transporters are involved in necrotic neuronal cell death induced by oxygen-glucose deprivation of hippocampal slice cultures. Neuroscience, 136(3), 779–794.
Butler, D., Bendiske, J., Michaelis, M. L., Karanian, D. A., & Bahr, B. A. (2007). Microtubule-stabilizing agent prevents protein accumulation-induced loss of synaptic markers. European Journal of Pharmacology, 562, 20–27.
Butler, D., Brown, Q. B., Chin, D. J., Batey, L., Karim, S., Mutneja, M. S. et al. (2005). Cellular responses to protein accumulation involve autophagy and lysosomal enzyme activation. Rejuvenation Research, 8, 227–237.
Butler, D., Nixon, R. A., & Bahr, B. A. (2006). Potential compensatory responses through autophagic lysosomal pathways in neurodegenerative diseases. Autophagy, 2, 234–237.
Caba, E., & Bahr, B. A. (2004). Biphasic activation of NF-κB in the excitotoxic hippocampus. Acta Neuropathologica, 108, 173–182.
Caporaso, G. L., Gandy, S. E., Buxbaum, J. D., & Greengard, P. (1992). Chloroquine inhibits intracellular degradation but not secretion of Alzheimer/A4 amyloid precursor protein. Proceedings of the National Academy of Sciences of the United States of America, 89, 2252–2256.
Caspary, D. M., Schatleman, T. A., & Hughes, L. F. (2005). Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: Role of inhibitory inputs. Journal of Neuroscience, 25, 10952–10959.
Charrier, C., Ehrensperger, M. V., Dahan, M., Lèvi, S., & Triller, A. (2006). Cytoskeleton regulation of glycine receptor number at synapses and diffusion in the plasma membrane. Journal of Neuroscience, 26, 8502–8511.
Coleman, P., Federoff, H., & Kurlan, R. (2004). A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurology, 63, 1155–1162.
Collins, P. R., Stack, C. M., O’Neill, S. M., Doyle, S., Ryan, T., Brennan, G. P. et al. (2004). Cathepsin L1, the major protease involved in liver fluke (Fasciola hepatica) virulence: Propeptide cleavage sites and autoactivation of the zymogen secreted from gastrodermal cells. Journal of Biological Chemistry, 279, 17038–17046.
Craig, A. M., Banker, G., Chang, W., McGrath, M. E., & Serpinskaya, A. S. (1996). Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons. Journal of Neuroscience, 16, 3166–3177.
Craig, A. M., & Boudin, H. (2001). Molecular heterogeneity of central synapses: afferent and target regulation. Nature Neuroscience, 4, 569–578.
Danglot, L., Triller, A., & Bessis, A. (2003). Association of gephyrin with synaptic and extrasynaptic GABAA receptors varies during development in cultured hippocampal neurons. Molecular and Cellular Neurosciences, 23, 264–278.
Essrich, C., Lorez, M., Benson, J. A., Fritschy, J. M., & Luscher, B. (1998). Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nature Neuroscience, 1, 563–571.
Fremeau, R. T. Jr., Troyer, M. D., Pahner, I., Nygaard, G. O., Tran, C. H., Reimer, R. J. et al. (2001). The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron, 31, 247–260.
Fuhrmann, J. C., Kins, S., Rostaing, P., El Far, O., Kirsch, J., Sheng, M. et al. (2002). Gephyrin interacts with Dynein light chains 1 and 2, components of motor protein complexes. Journal of Neuroscience, 22(13), 5393–5402, Jul 1.
Graf, R. A., & Kater, S. B. (1998). Inhibitory neuronal activity can compensate for adverse effects of beta-amyloid in hippocampal neurons. Brain Research, 786, 1558–1569.
Hanus, C., Vannier, C., & Triller, A. (2004). Intracellular association of glycine receptor with gephyrin increases its plasma membrane accumulation rate. Journal of Neuroscience, 24(5), 1119–1128 (Feb 4).
Heinonen, O., Soininen, H., Sorvari, H., Kosunen, O., Paljarvi, L., Koivisto, E. et al. (1995). Loss of synaptophysin-like immunoreactivity in the hippocampal formation is an early phenomenon in Alzheimer’s disease. Neuroscience, 54, 375z-384.
Honer, W. G., Dickson, D. W., Gleeson, J., & Davies, P. (1992). Regional synaptic pathology in Alzheimer’s disease. Neurobiology of Aging, 13, 375–382.
Jacob, T. C., Bogdanov, Y. D., Magnus, C., Saliba, R. S., Kittler, J. T., Haydon, P. G. et al. (2005). Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. Journal of Neuroscience, 25, 10469–10478.
Karanian, D. A., Brown, Q. B., Makriyannis, A., & Bahr, B. A. (2005). Blocking cannabinoid activation of FAK and ERK1/2 compromises synaptic integrity in hippocampus. European Journal of Pharmacology, 508, 47–56.
Karanian, D. A., Brown, Q. B., Makriyannis, A., Kosten, T. A., & Bahr, B. A. (2005). Dual modulation of endocannabinoid transport and fatty acid amide hydrolase protects against excitotoxicity. Journal of Neuroscience, 25, 7813–7820.
Kawasaki, B. T., Hoffman, K. B., Yamamoto, R. S., & Bahr, B. A. (1997). Variants of the receptor/channel clustering molecule gephyrin in brain: Distinct distribution patterns, developmental profiles, and proteolytic cleavage by calpain. Journal of Neuroscience Research, 49, 381–388.
Kim, E. Y., Schrader, N., Smolinsky, B., Bedet, C., Vannier, C., Schwarz, G., et al. (2006). Deciphering the structural framework of glycine receptor anchoring by gephyrin. EMBO Journal, 25, 1385–1395.
Kirsch, J., & Betz, H. (1993). Widespread expression of gephyrin, a putative glycine receptor-tubulin linker protein, in rat brain. Brain Research, 621, 301–310.
Kirsch, J., Wolters, I., Triller, A., Betz, H. (1993). Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature, 366, 745–748.
Kneussel, M., & Betz, H. (2000). Clustering of inhibitory neurotransmitter receptors at developing postsynaptic sites: the membrane activation model. Trends in Neurosciences, 23, 429–435.
Kneussel, M., Brandstatter, J. H., Laube, B., Stahl, S., Muller, U., & Betz, H. (1999). Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. Journal of Neuroscience, 19, 9289–9297.
Legendre, P. (2001). The glycinergic inhibitory synapse. Cellular and Molecular Life Sciences, 58, 760–793.
Lévi, S., Logan, S. M., Tovar, K. R., & Craig, A. M. (2004). Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. Journal of Neuroscience, 24, 207–217.
Li, X., Serwanski, D. R., Miralles, C. P., Bahr, B. A., & De Blas, A. L. (2007). Two pools of Triton X-100-insoluble GABAA receptors are present in the brain, one associated to lipid rafts and another one to the postsynaptic GABAergic complex. Journal of Neurochemistry, 102, 1329–1345.
Masliah, E. (1995). Mechanisms of synaptic dysfunction in Alzheimer’s disease. Histology and Histopathology, 10, 509–519.
Meier, J., De Chaldee, M., Triller, A., Vannier, C. (2000). Functional heterogeneity of gephyrins. Molecular and Cellular Neurosciences, 16, 566–577.
Mielke, J. G., Murphy, M. P., Maritz, J., Bengualid, K. M., & Ivy, G. O. (1997). Chloroquine administration in mice increases β-amyloid immunoreactivity and attenuates kainate-induced blood-brain barrier dysfunction. Neuroscience Letters, 227, 169–172.
Mizukami, K., Ikonomovic, M. D., Grayson, D. R., Rubin, R. T., Warde, D., Sheffield, R. et al. (1997). Immunohistochemical study of GABAA receptor β2/3 subunits in the hippocampal formation of aged brains with Alzheimer-related neuropathologic changes. Experimental Neurology, 147, 333–345.
Niewiadomska, G., Baksalerska-Pazera, M., & Riedel, G. (2006). Cytoskeletal transport in the aging brain: focus on the cholinergic system. Reviews in the Neurosciences, 17(6), 581–618.
Nixon, R. A. (2000). A protease activation cascade" in the pathogenesis of Alzheimer’s disease. Annals of the New York Academy of Sciences, 924, 117–131.
Oyama, F., Murakami, N., & Ihara, Y. (1998). Chloroquine myopathy suggests that tau is degraded in lysosomes: Implication for the formation of paired helical filaments in Alzheimer’s disease. Neuroscience Research, 31, 1–8.
Poe, B. H., Linville, C., & Brunson-Bechtold, J. (2001). Age-related decline of presumptive inhibitory synapses in the sensimotor cortex as revealed by the physical dissector. Journal of Comparative Neurology, 439, 65–72.
Prior, P., Schmitt, B., Grenningloh, G., Pribilla, I., Multhaup, G., Beyreuther, K. et al. (1992). Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron, 8, 1161–1170.
Ramming, M., Kins, S., Werner, N., Hermann, A., Betz, H., & Kirsch, J. (2000). Diversity and phylogeny of gephyrin: tissue-specific splice variants, gene structure, and sequence similarities to molybdenum cofactor-synthesizing and cytoskeleton-associated proteins. Proceedings of the National Academy of Sciences of the United States of America, 97(18), 10266–10271.
Rissman, R. A., Mishizen-Eberz, A. J., Carter, T. L., Wolfe, B. B., De Blas, A. L., Miralles, C. P. et al. (2003). Biochemical analysis of GABAA receptor subunits α1, α5, β1, β2 in the hippocampus of patients with Alzheimer’s disease neuropathology. Neuroscience, 120, 695–704.
Rowland, A. M., Richmond, J. E., Olsen, J. G., Hall, D. H., Bamber, B. A. (2006). Presynaptic terminals independently regulate synaptic clustering and autophagy of GABAA receptors in Caenorhabditis elegans. Journal of Neuroscience, 26, 1711–1720.
Sabatini, D. M., Barrow, R. K., Blackshaw, S., Burnett, P. E., Lai, M. M., Field, M. E. et al. (1999). Interaction of RAFT1 with gephyrin required for rapamycin-sensitive signaling. Science, 284, 1161–1164.
Sassoé-Pognetto, M., Kirsch, J., Grunert, U., Greferath, U., Fritschy, J. M., Mohler, H. et al. (1995). Colocalization of gephyrin and GABAA-receptor subunits in the rat retina. Journal of Comparative Neurology, 357, 1–14.
Scornik, O. A. (1984). Effects of inhibitors of protein degradation on the rate of protein synthesis in Chinese hamster ovary cells. Journal of Cellular Physiology, 121, 257–262.
Scheff, S. W., Price, D. A., Schmitt, F. A., & Mufson, E. J. (2006). Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiology of Aging, 27, 1372–1384.
Stokin, G. B., Lillo, C., Falzone, T. L., Brusch, R. G., Rockenstein, E., Mount, S. L. et al. (2005). Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science, 307, 1282–1288.
Studler, B., Fritschy, J.-M., & Brunig, I. (2002). GABAergic and glutamatergic terminals differentially influence the organization of GABAergic synapses in rat cerebellar granule cells in vitro. Neuroscience, 114, 123–133.
Studler, B., Sidler, C., & Fritschy, J. M. (2005). Differential regulation of GABA(A) receptor and gephyrin postsynaptic clustering in immature hippocampal neuronal cultures. Journal of Comparative Neurology, 484, 344–355.
Takauchi, S., & Miyoshi, K. (1995). Cytoskeletal changes in rat cortical neurons induced by long-term intraventricular infusion of leupeptin. Acta Neuropathologica, 89, 8–16.
Terry, R. D., Masliah, E., Salmon, D. P., Butters, N., DeTeresa, R., Hill, R. et al. (1991). Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Annals of Neurology, 30, 572–580.
Tomita, S., Adesnik, H., Sekiguchi, M., Zhang, W., Wada, K., Howe, J. R. et al. (2005). Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature, 435, 1052–1058.
van Zundert, B., Albarran, F. A., & Aguayo, L. G. (2000). Effects of chronic ethanol treatment on gamma-aminobutyric acid(A) and glycine receptors in mouse glycinergic spinal neurons. Journal of Pharmacology and Experimental Therapeutics, 295, 423–429.
Vornov, J. J., Tasker, R. C., & Coyle, J. T. (1994). Delayed protection by MK-801 and tetrodotoxin in a rat organotypic hippocampal culture. Stroke, 25, 457–464.
Waldvogel, H. J., Baer, K., Snell, R. G., During, M. J., Faull, R. L. M., & Rees, M. I. (2003). Distribution of gephyrin in the human brain: an immunohistochemical analysis. Neuroscience, 116, 145–156.
Wheal, H. V., Chen, Y., Mitchell, S. M., Maerz, W., Wieland, H., van Rossum, D. et al. (1998). Molecular mechanisms that underlie structural and functional changes at the postsynaptic membrane during synaptic plasticity. Progress in Neurobiology, 55, 611–640.
Acknowledgments
The authors thank Professor Angel de Blas for generously providing GABA-A receptor antibodies, Malgorzata Michalowska and Kelly Zhang for excellent assistance, and Dr. David Karanian for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryzhikov, S., Bahr, B.A. Gephyrin Alterations Due to Protein Accumulation Stress are Reduced by the Lysosomal Modulator Z-Phe-Ala-Diazomethylketone. J Mol Neurosci 34, 131–139 (2008). https://doi.org/10.1007/s12031-007-9009-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-007-9009-7