Abstract
Background
Terson syndrome (TS), an intraocular hemorrhage associated with aneurysmal subarachnoid hemorrhage (aSAH), occurs in up to 46% of all patients with subarachnoid hemorrhage. Despite its high incidence, TS is underrepresented in the literature, and patients with aSAH are sometimes not systematically evaluated for the presence of TS in clinical practice. This work aims to raise awareness of TS, reevaluate previous scientific findings, describe risk factors associated with the occurrence of TS, and present our local diagnostic and treatment concept.
Methods
All patients with aSAH treated at our institution between October 2010 and May 2020 were included in this retrospective study. The frequency of ophthalmological screening by indirect funduscopy, as well as the results, was investigated. In addition, the collection and statistical analysis of epidemiological and clinical data was performed using χ2, Kruskal–Wallis, and analysis of variance testing; multivariate regression; and receiver operating characteristic analysis. The significance level was set at p < 0.05.
Results
A total of 617 patients were treated for aSAH in our institution. Of these, 367 patients (59.5%) were ophthalmologically examined for the presence of TS. The rate of TS in the examined patients was 21.3% (n = 78). Patients with TS had significantly higher Fisher and World Federation of Neurosurgical Societies (WFNS) scores (p < 0.0001). Regression analyses showed WFNS grade (p = 0.003) and the occurrence of seizures (p = 0.002) as independent predictors of TS, as did receiver operating characteristic analyses, which had a significant area under the curve of 0.66 for the combination of WFNS grade and seizures. For 12 (15.4%) patients, the TS had to be surgically treated by pars plana vitrectomy in a total of 14 eyes, which resulted in significant improvement of visual function in all patients: mean preoperative best-corrected visual acuity was 0.03 (± 0.08) versus 0.76 (± 0.21) postoperatively (p < 0.001).
Conclusions
TS is a common complication in patients with aSAH, affecting approximately one in five patients. A higher WFNS grade and the occurrence of seizures are associated with TS; therefore, screening for TS should be performed in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Terson syndrome (TS), first described by Moritz Litten in 1881 and named after the French ophthalmologist Albert Terson, is a relatively common complication after subarachnoid hemorrhage (SAH), with rates of 10.2–46% in the literature [1,2,3]. It refers to an intraocular hemorrhage, for example, into the subhyaloid or intraretinal layers of the retina, or a vitreous hemorrhage (VH) which represents 3–5% of cases in the literature [2, 4, 5].
Spontaneous clearance of TS occurs frequently. In cases of failing spontaneous clearance, ophthalmological surgery by pars plana vitrectomy (PPV) should be considered and has been described as a safe intervention with an immediate improvement of visual acuity, as a significant acceleration of the process can be achieved surgically [6,7,8].
In addition to the significance for the surviving patient in terms of visual function, the presence of TS also appears to play a prognostic role even in the initial stage of SAH. Sung et al. were able to show an association with increased mortality in patients with SAH with TS [9]. Risk factors for the occurrence of this complication have not been widely studied. Lee et al. were able to identify the initial Word Federation of Neurosurgical Societies (WFNS) grade, aneurysm size, and treatment modality of the ruptured aneurysm as risk factors [3]. The initial loss of consciousness has also been suggested as a predictor for the occurrence of TS [10].
The aim of this study was to raise awareness of TS, reevaluate previous scientific findings, describe risk factors associated with the occurrence of TS, and present our local diagnostic and treatment concept.
Methods
All 617 patients presenting to our intensive care unit with aneurysmal SAH (aSAH) between October 2010 and May 2020 were included in this study. SAH was confirmed by cranial computed tomography (cCT), magnetic resonance imaging, and/or lumbar puncture, as described before [11].
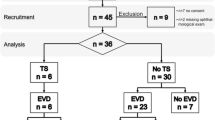
Patients were not screened for TS if they or their legal guardian did not consent to participate in our previous studies, if they died in-hospital, or were awake and fully oriented in the clinical investigations (and did not show any visual symptoms of TS, such as weakened or absent light reaction, subjective visual disturbances, or loss of red reflex) [11, 12]. In the end, 367 patients could be examined by an experienced neuro-ophthalmologist (VK and CS) by direct and indirect ophthalmoscopic examination after induced mydriasis (Tropicamid, Mydriaticum Stulln; Pharma Stulln GmbH, Stulln, Germany) prior to transfer to the rehabilitation unit or prior to discharge [11] (Fig. 1).
In the case of TS, necessary treatment was evaluated by the Department of Ophthalmology (CS) and determined according to the individual patient’s needs (watch and wait versus PPV), as described before [13]. In addition, best-corrected visual acuity (BCVA) was evaluated in awake patients scheduled for a PPV or watch and wait [14]. The perception of hand movement is stated with a BCVA of 0.005, whereas the ability to count fingers is stated with a BCVA of 0.014.
Patients demographics and aSAH-specific parameters were collected. The aneurysmal origin of SAH was proved by either digital subtraction angiography, cCT angiography, and/or magnetic resonance imaging angiography. These data included demographic information, aneurysm size and location, and distinct clinical evaluation scores (WFNS grading system, Fisher score). All events were assessed as seizures regardless of whether these occurred preclinically or during the inpatient course. If an epileptic seizure was observed outside the hospital, it was documented by a specialized emergency physician on-site and subsequently confirmed in the hospital by an Electroencephalography with epilepsy-typical potentials. Data were extracted from the intensive care unit’s electronic documentation system (Integrated Care Manager; Dräger Medical Deutschland GmbH, Lübeck, Germany).
Statistical analysis was performed using SPSS Statistics, Version 25 (IBM, Armonk, NY). Metric data are presented as the mean and standard deviation. Statistical analysis of the data was performed by a univariate analysis using χ2 tests, independent-samples Kruskal–Wallis tests, or analysis of variance tests, depending on the scale of the measurements and equality of variances. Logistic regression analyses were calculated to test the predictive power of clinical parameters previously described in the literature for the occurrence of TS. To evaluate the predictive performance of these predictors, receiver operating characteristic curves and corresponding areas under the curve (AUCs) were calculated. The statistical level of significance was set at p < 0.05.
This study was conducted according to the Declaration of Helsinki, as well as local and institutional laws, and was reported to the local ethical committee (Ethik-Kommission der Ärztekammer Hamburg, 2022-100874-WF). Parts of the data sets derive from the prospective studies with written consent of the patients and were anonymized after the studies were completed [11, 12]. Written informed consent was waived for this kind of study because of the anonymous recording and processing of data.
Results
Between October 2010 and May 2020, 617 patients with aSAH were treated in our clinic. Of these, 367 were examined ophthalmologically for the presence of TS. The rate of TS among the examined patients was 21.3% (78 of 367).
On univariate analysis, patients with TS and those without TS differed significantly in terms of the severity of the aSAH (Table 1). Patients with TS had significantly higher Fisher and WFNS grades.
In the next step, logistic regression analyses were performed to assess the independent predictive value of parameters that were significant in univariate analyses. Because of the correlation of Fisher grade and parenchymal hemorrhage (Pearson’s R = 0.35; p < 0.001) and WFNS grade and initial unconsciousness (Pearson’s R = 0.51; p < 0.001), of these, only Fisher grade and WFNS grade were considered for the regression analyses.
Regression analyses found WFNS grade and the occurrence of seizures to be independent predictors for the occurrence of TS (Table 2).
Receiver operating characteristic curves were calculated for the occurrence of TS using the significant parameters from regression analysis (Fig. 2). The AUC for WFNS grade was 0.66 (confidence interval [CI] 0.59–0.73; p < 0.001). The AUC for seizures was 0.61 (CI 0.54–0.69; p = 0.002). Combining both parameters resulted in an AUC of 0.70 (CI 0.63–0.76).
Furthermore, the patients with TS were examined for the necessity of surgical treatment and its outcome. There were 12 (15.4%) patients who had a total of 14 eyes treated surgically by PPV for TS. Postoperatively, visual function improved, as shown in Table 3. BCVA improved significantly in all patients at a postoperative mean of 0.76 (± 0.21), compared to 0.03 (± 0.08) preoperatively (p < 0.001). Surgeries were performed 6–8 weeks after the initial hemorrhage. No internal limiting membrane peeling was performed because no internal limiting membrane bleeds were present.
Discussion
TS is a long-known complication of aSAH and usually affects about every fifth patient, consistent with the 21.3% reported in this large cohort. It is even more astonishing that, on the one hand, patients in many hospitals are still not systematically examined for the presence of TS; on the other hand, there are still large gaps in the literature. The aim of this work is to bring TS back into the focus of neurosurgeons, neurologists, ophthalmologists, and intensive care physicians and to share our 10-year experiences.
The gold standard for the screening of TS is direct and indirect funduscopy by an ophthalmologist [11, 15]. However, various studies have shown that TS can also be diagnosed using ocular ultrasound (OU) and cCT [12, 16,17,18,19,20]. In one of our previous studies we found that sensitivity and specificity values for the detection of vitreous hemorrhage via OU increased with the investigator’s number of examinations up to 81.8% and 100%, respectively [12]. Galán et al. report the sensitivity and specificity of OU to be 87.5% and 98.5%, respectively, for the detection of vitreous hemorrhage [16]. Koskela et al. report a sensitivity of 42% for computed tomography findings regarding TS and a specificity of 97% [20].
Further diagnostic modalities were evaluated by Ramos-Estebanez et al. [15], showing the feasibility of bedside optic coherence tomography examination in patients with aSAH. The use of ultrasound and computed tomography provides increased the sensitivity and specificity, especially in the diagnosis of vitreous hemorrhage, making these methods particularly useful for identifying patients who should be treated surgically. Because ultrasound diagnosis in particular is ubiquitous in the intensive care unit, it is also suitable for hospitals in countries with limited resources or a shortage of ophthalmologists because it is easy to learn and can be performed quickly [12].
The question now arises as to which patients should be screened for TS, particularly in hospitals and health care systems with limited personnel and financial resources.
Several factors have been described in the literature to be associated with the occurrence of TS, such as high Hunt and Hess and WFNS grades, low Glasgow Coma Scale scores and high Fisher scale scores [3, 21,22,23,24,25]. An initial loss of consciousness and an initially raised intracranial pressure (ICP) have also been investigated as predictors for TS [10, 26]. In the work of Joswig et al. [26], all patients with TS presented with pathological ICP values of > 20 cm H2O.
These results partially align with this study’s results, as we were also able to show the association of a high WFNS grade with the occurrence of TS in our collective. We were also able to demonstrate an association between seizures and the occurrence of TS. In recent years, the literature has shown that seizures are associated with the severity of SAH and neuroinflammation and that these factors are also related to the possible pathomechanisms of TS [27, 28].
Because the pathophysiology of TS has not yet been conclusively clarified, a direct pathophysiological explanation for the occurrence of TS and an increased occurrence of seizures is missing [10, 29]. The favored pathomechanism in the literature is intraorbital venous congestion due to increased ICP and subsequent rupture of the small retinal vessels [29]. The increase in ICP certainly predisposes to the development of TS, yet not all patients with TS have been shown to have an increase, implying additional underlying pathomechanisms [10]. Other explanations are that intracranial blood penetrates directly into the vitreous cavity through the lamina cribrosa, the peripapillary and perivascular leak theory, and finally a primary vitreous origin [29,30,31,32,33].
In our opinion, different pathomechanisms for the development of TS should be suspected, as an increase in bleeding can be seen more than 14 days after SAH ictus, as shown previously by Vanderlinden et al. and by our group [5, 11].
For this reason, we screen for TS before patients are transferred to the rehabilitation units or before they are discharged. On the one hand, we are convinced of the reduced rate of overlooked TS with later development; on the other hand, however, there is the possibility of carrying out an ophthalmological treatment concept or PPV, if necessary.
The ideal point in time to perform a PPV is discussed controversially in the literature, as minor hemorrhages, as well as retinal hemorrhages, usually resolve spontaneously. Dense vitreous hemorrhages, on the other hand, lead to a loss of vision or relevant reduction of BCVA, which can also recover after a later PPV, but it seems obvious, even without evidence from studies, that an early improvement in BCVA should be aimed for because patients can then also take part in the rehabilitation programs in a more targeted and active manner [34]. Larger series have demonstrated that an early PPV can be performed safely and results in an immediate and significant improvement in vision [7, 13, 33, 35].
Limitations of the study are its partly retrospective character and the fact that not all patients with aSAH were screened for the presence of TS during the mentioned time period. After we conducted our prospective studies, patients who presented with a higher WFNS grade (III–V) and/or had neurological deficits before discharge or complained about subjective visual impairments were referred for an ophthalmological examination [11, 12]. Patients without any symptoms were not regularly examined by an ophthalmologist, as there was usually no need for treatment, even in the unlikely event that TS was detected, as described above, in which only minor hemorrhages were expected. Nonetheless, we were able to investigate a substantial number of TS cases, resulting in one of the largest TS cohorts published so far.
Summarizing the existing literature and the 10-year experience of our facility, we recommend the approach shown in Fig. 3.
Recommendation for the approach of patients with aneurysmal subarachnoid hemorrhage (aSAH) regarding potential Terson syndrome (TS). The figure shows the approach of screening and potential treatment of TS regarding optimal timing and methods. cCT cranial computed tomography, PPV pars plana vitrectomy, SAH subarachnoid hemorrhage
Conclusions
TS is a common complication of aSAH, affecting approximately one in five patients. Because of the associations shown between higher WFNS grades and the occurrence of seizures and TS we suggest consistent screening in, but not limited to, these particular patients.
References
Litten M. Ueber einige vom allgemein-klinischen Standpunkt aus interessante Augenveranderungen. Berl Klin Wochenschr. 1881;18:23–7.
Medele RJ, Stummer W, Mueller AJ, Steiger HJ, Reulen HJ. Terson’s syndrome in subarachnoid hemorrhage and severe brain injury accompanied by acutely raised intracranial pressure. J Neurosurg. 1998;88:851–4. https://doi.org/10.3171/jns.1998.88.5.0851.
Lee G-I, Choi K-S, Han M-H, Byoun H-S, Yi H-J, Lee B-R. Practical incidence and risk factors of Terson’s syndrome: a retrospective analysis in 322 consecutive patients with aneurysmal subarachnoid hemorrhage. J Cerebrovasc Endovasc Neurosurg. 2015;17:203. https://doi.org/10.7461/jcen.2015.17.3.203.
Castrén JA. Pathogeneses and treatment of terson-syndrome. Acta Ophthalmol. 1963;41:430–4. https://doi.org/10.1111/j.1755-3768.1963.tb03552.x.
Vanderlinden RG, Chisholm LD. Vitreous hemorrhages and sudden increased intracranial pressure. J Neurosurg. 1974;41:167–76. https://doi.org/10.3171/jns.1974.41.2.0167.
Clarkson JG, Flynn HW, Daily MJ. Vitrectomy in Terson’s syndrome. Am J Ophthalmol. 1980;90:549–52. https://doi.org/10.1016/S0002-9394(14)75027-5.
Garweg JG, Koerner F. Outcome indicators for vitrectomy in Terson syndrome. Acta Ophthalmol. 2009;87:222–6. https://doi.org/10.1111/j.1755-3768.2008.01200.x.
Schultz PN, Sobol WM, Weingeist TA. Long-term visual outcome in terson syndrome. Ophthalmology. 1991;98:1814–9. https://doi.org/10.1016/S0161-6420(91)32045-1.
Sung W, Arnaldo B, Sergio C, Juliana S, Michel F. Terson’s syndrome as a prognostic factor for mortality of spontaneous subarachnoid haemorrhage. Acta Ophthalmol. 2011;89:544–7. https://doi.org/10.1111/j.1755-3768.2009.01735.x.
Czorlich P, Skevas C, Knospe V, Vettorazzi E, Westphal M, Regelsberger J. Terson’s syndrome—pathophysiologic considerations of an underestimated concomitant disease in aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2016;33:182–6. https://doi.org/10.1016/j.jocn.2016.04.015.
Czorlich P, Skevas C, Knospe V, Vettorazzi E, Richard G, Wagenfeld L, Westphal M, Regelsberger J. Terson syndrome in subarachnoid hemorrhage, intracerebral hemorrhage, and traumatic brain injury. Neurosurg Rev. 2015;38:129–36. https://doi.org/10.1007/s10143-014-0564-4.
Czorlich P, Burkhardt T, Knospe V, Richard G, Vettorazzi E, Wagenfeld L, Westphal M, Regelsberger J, Skevas C. Ocular ultrasound as an easy applicable tool for detection of Terson’s syndrome after aneurysmal subarachnoid hemorrhage. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0114907.
Skevas C, Czorlich P, Knospe V, Stemplewitz B, Richard G, Westphal M, Regelsberger J, Wagenfeld L. Terson’s syndrome—rate and surgical approach in patients with subarachnoid hemorrhage: a prospective interdisciplinary study. Ophthalmology. 2014;121:1628–33. https://doi.org/10.1016/j.ophtha.2014.02.015.
Plainis S, Tzatzala P, Orphanos Y, Tsilimbaris MK. A modified ETDRS visual acuity chart for European-wide use. Optom Vis Sci. 2007. https://doi.org/10.1097/OPX.0b013e3180dc9a60.
Ramos-Estebanez C, Kohen M, Pace J, Bozorgi A, Manjila S, Alambyan V, Nwankwo I, DeGeorgia M, Bambakidis NC, Orge F. Bedside optical coherence tomography for Terson’s syndrome screening in acute subarachnoid hemorrhage: a pilot study. J Neurosurg. 2019;130:517–24. https://doi.org/10.3171/2017.7.JNS171302.
Mesa Galán LA, Henríquez Recine MA, Robles Caballero A, Yus Teruel S, García Martínez JR, Egea-Guerrero JJ, Quintana-Diaz M. Ultrasound diagnosis of Terson syndrome as an indicator of extreme severity in neurocritical care patients. Neurología (English Edition). 2022. https://doi.org/10.1016/j.nrleng.2020.04.026.
Bäuerle J, Gross NJ, Egger K, Neubauer J, Niesen WD, Buttler KJ, Lagrèze WA, Reinhard M. Terson’s syndrome: diagnostic comparison of ocular sonography and CT. J Neuroimaging. 2016;26:247–52. https://doi.org/10.1111/jon.12285.
Stewart MW, Hasan SA, Collins C, Stanko C, Summer J, Brazis PW, Tawk R, Freeman WD. Can baseline computed tomography scans be used to identify patients at high risk of vision loss due to Terson syndrome? Am J Ophthalmol. 2020;211:217–28. https://doi.org/10.1016/j.ajo.2019.09.016.
Swallow CE, Tsuruda JS, Digre KB, Glaser MJ, Davidson HC, Harnsberger HR. Terson syndrome: CT evaluation in 12 patients. Am J Neuroradiol. 1998;19:743–7.
Koskela E, Pekkola J, Kivisaari R, Kivelä T, Hernesniemi J, Setaälä K, Laakso A. Comparison of CT and clinical findings of Terson’s syndrome in 121 patients: a 1-year prospective study: clinical article. J Neurosurg. 2014;120:1172–8. https://doi.org/10.3171/2014.2.JNS131248.
Fountas KN, Kapsalaki EZ, Lee GP, Machinis TG, Grigorian AA, Robinson JS, Vergados I, Theodosiadis PG. Terson hemorrhage in patients suffering aneurysmal subarachnoid hemorrhage: predisposing factors and prognostic significance. J Neurosurg. 2008;109:439–44. https://doi.org/10.3171/JNS/2008/109/9/0439.
Frizzell RT, Kuhn F, Morris R, Quinn C, Fisher WS. Screening for ocular hemorrhages in patients with ruptured cerebral aneurysms: a prospective study of 99 patients. Neurosurgery. 1997;41:529–34. https://doi.org/10.1097/00006123-199709000-00004.
Garfinkle AM, Danys IR, Nicolle DA, Colohan ART, Brem S. Terson’s syndrome: a reversible cause of blindness following subarachnoid hemorrhage. J Neurosurg. 1992. https://doi.org/10.3171/jns.1992.76.5.0766.
Pfausler B, Belcl R, Metzler R, Mohsenipour I, Schmutzhard E. Terson’s syndrome in spontaneous subarachnoid hemorrhage: a prospective study in 60 consecutive patients. J Neurosurg. 1996;85:392–4. https://doi.org/10.3171/jns.1996.85.3.0392.
Stienen MN, Lücke S, Gautschi OP, Harders A. Terson haemorrhage in patients suffering aneurysmal subarachnoid haemorrhage: a prospective analysis of 60 consecutive patients. Clin Neurol Neurosurg. 2012;114:535–8. https://doi.org/10.1016/j.clineuro.2011.10.034.
Joswig H, Epprecht L, Valmaggia C, Leschka S, Hildebrandt G, Fournier JY, Stienen MN. Terson syndrome in aneurysmal subarachnoid hemorrhage—its relation to intracranial pressure, admission factors, and clinical outcome. Acta Neurochir (Wien). 2016;158:1027–36. https://doi.org/10.1007/s00701-016-2766-8.
Lanzino G, D’Urso PI, Suarez J. Seizures and anticonvulsants after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2011;15:247–56. https://doi.org/10.1007/s12028-011-9584-x.
Claassen J, Park S. Spontaneous subarachnoid haemorrhage. Lancet. 2022. https://doi.org/10.1016/S0140-6736(22)00938-2.
Aboulhosn R, Raju B, Jumah F, Majmundar N, Prenner J, Matin T, Roychowdhury S, Singla A, Khandelwal P, Nanda A, et al. Terson’s syndrome, the current concepts and management strategies: a review of literature. Clin Neurol Neurosurg. 2021. https://doi.org/10.1016/j.clineuro.2021.107008.
Hanai K, Hashimoto M, Sasaki M, Nakamura H. Microsurgical observation of the posterior vitreous in patients with vitreous hemorrhage caused by Terson syndrome. Am J Ophthalmol Case Rep. 2020. https://doi.org/10.1016/j.ajoc.2020.100613.
Ogawa T, Kitaoka T, Dake Y, Amemiya T. Terson syndrome: a case report suggesting the mechanism of vitreous hemorrhage. Ophthalmology. 2001;108:1654–6. https://doi.org/10.1016/S0161-6420(01)00673-X.
Sakamoto M, Nakamura K, Shibata M, Yokoyama K, Matsuki M, Ikeda T. Magnetic resonance imaging findings of Terson’s syndrome suggesting a possible vitreous hemorrhage mechanism. Jpn J Ophthalmol. 2010;54:135–9. https://doi.org/10.1007/s10384-009-0783-3.
Michalewska Z, Michalewski J, Nawrocki J. Possible methods of blood entrance in Terson syndrome. Ophthalmic Surg Lasers Imaging. 2010. https://doi.org/10.3928/15428877-20101031-15.
Wiethölter S, Steube D, Stotz HP. Terson syndrome: a frequently missed ophthalmologic complication in subarachnoid hemorrhage. Zentralblatt für Neurochir. 1998;59:166–70.
Nazarali S, Kherani I, Hurley B, Williams G, Fielden M, Adatia F, Kherani A. Outcomes of vitrectomy in Terson syndrome a multicenter Canadian perspective. Retina. 2020;40:1325–30. https://doi.org/10.1097/IAE.0000000000002570.
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding was received for this work.
Author information
Authors and Affiliations
Contributions
JG supervised the study, designed the study question, interpreted the data, and drafted the manuscript. VK collected the data. TS analyzed and interpreted the data. NS, JG, and MW helped to interpret the data and conceive the study questions. MSS contributed to the data interpretation. CS collected, analyzed, and interpreted the data. PC contributed to the overall design of the study, supervised the study, conceived the study question, designed the analysis plan, and analyzed and interpreted the data. All authors revised the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest related to the work.
Ethical Approval/Informed Consent
This study was conducted according to the Declaration of Helsinki and local and institutional laws and was reported to the local ethical committee (No. 2022-100874-WF). For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Göttsche, J., Knospe, V., Sauvigny, T. et al. Terson Syndrome in Patients with Aneurysmal Subarachnoid Hemorrhage: A 10-Year Single-Center Experience. Neurocrit Care 39, 155–161 (2023). https://doi.org/10.1007/s12028-023-01701-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01701-9