Abstract
Background
Endogenous apolipoprotein (apo) E mediates neuroinflammatory responses and recovery after brain injury. Exogenously administered apoE-mimetic peptides effectively penetrate the central nervous system compartment and downregulate acute inflammation. CN-105 is a novel apoE-mimetic pentapeptide with excellent evidence of functional and histological improvement in preclinical models of intracerebral hemorrhage (ICH). The CN-105 in participants with Acute supraTentorial intraCerebral Hemorrhage (CATCH) trial is a first-in-disease-state multicenter open-label trial evaluating safety and feasability of CN-105 administration in patients with acute primary supratentorial ICH.
Methods
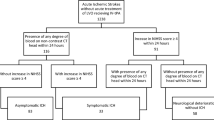
Eligible patients were aged 30–80 years, had confirmed primary supratentorial ICH, and were able to intiate CN-105 administration (1.0 mg/kg every 6 h for 72 h) within 12 h of symptom onset. A priori defined safety end points, including hematoma volume, pharmacokinetics, and 30-day neurological outcomes, were analyzed. For clinical outcomes, CATCH participants were compared 1:1 with a closely matched contemporary ICH cohort through random selection. Hematoma volumes determined from computed tomography images on days 0, 1, 2, and 5 and ordinal modified Rankin Scale score at 30 days after ICH were compared.
Results
In 38 participants enrolled across six study sites in the United States, adverse events occurred at an expected rate without increase in hematoma expansion or neurological deterioration. CN-105 treatment had an odds ratio (95% confidence interval) of 2.69 (1.31–5.51) for lower 30-day modified Rankin Scale score, after adjustment for ICH score, sex, and race/ethnicity, as compared with a matched contemporary cohort.
Conclusions
CN-105 administration represents an excellent translational candidate for treatment of acute ICH because of its safety, dosing feasibility, favorable pharmacokinetics, and possible improvement in neurological recovery.



Similar content being viewed by others
Change history
24 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12028-022-01553-9
References
Krishnamurthi RV, Moran AE, Forouzanfar MH, Bennett DA, Mensah GA, Lawes CM, et al. The global burden of hemorrhagic stroke: a summary of findings from the gbd 2010 study. Glob Heart. 2014;9:101–6.
Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 2009;40:394–9.
Roundtable Hemorrhagic Stroke Academia Industry, P. . Basic and translational research in intracerebral hemorrhage: limitations, priorities, and recommendations. Stroke. 2018;49:1308–14.
Hemorrhagic Stroke Academia Industry Roundtable P. Unmet needs and challenges in clinical research of intracerebral hemorrhage. Stroke. 2018;49:1299–1307.
Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, Martin RL, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375:1033–43.
Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–65.
Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor vii for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–37.
Baharoglu MI, Cordonnier C, Al-Shahi Salman R, de Gans K, Koopman MM, Brand A, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (patch): a randomised, open-label, phase 3 trial. Lancet. 2016;387:2605–13.
Sprigg N, Flaherty K, Appleton JP, Al-Shahi Salman R, Bereczki D, Beridze M, et al. Tranexamic acid for hyperacute primary intracerebral haemorrhage (tich-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391:2107–15.
Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (mistie iii): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393:1021–32.
Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908.
James ML, Blessing R, Bennett E, Laskowitz DT. Apolipoprotein e modifies neurological outcome by affecting cerebral oedema but not hematoma size after intracerebral hemorrhage in humans. J Stroke Cerebrovasc Dis. 2009;18:144–9.
Pang J, Peng J, Matei N, Yang P, Kuai L, Wu Y, et al. Apolipoprotein e exerts a whole-brain protective property by promoting m1? Microglia quiescence after experimental subarachnoid hemorrhage in mice. Transl Stroke Res. 2018;9:654–68.
Lei B, Mace B, Bellows ST, Sullivan PM, Vitek MP, Laskowitz DT, et al. Interaction between sex and apolipoprotein e genetic background in a murine model of intracerebral hemorrhage. Transl Stroke Res. 2012;3:94–101.
Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, et al. Apoe genotype and an apoe-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278:48529–33.
Qiu Z, Crutcher KA, Hyman BT, Rebeck GW. Apoe isoforms affect neuronal n-methyl-d-aspartate calcium responses and toxicity via receptor-mediated processes. Neuroscience. 2003;122:291–303.
Lawrence DW, Comper P, Hutchison MG, Sharma B. The role of apolipoprotein e episilon (epsilon)-4 allele on outcome following traumatic brain injury: a systematic review. Brain Inj. 2015;29:1018–31.
Li L, Bao Y, He S, Wang G, Guan Y, Ma D, et al. The association between apolipoprotein e and functional outcome after traumatic brain injury: a meta-analysis. Medicine (Baltimore). 2015;94:e2028.
Cao F, Jiang Y, Wu Y, Zhong J, Liu J, Qin X, et al. Apolipoprotein e-mimetic cog1410 reduces acute vasogenic oedema following traumatic brain injury. J Neurotrauma. 2016;33:175–82.
Hoane MR, Kaufman N, Vitek MP, McKenna SE. Cog1410 improves cognitive performance and reduces cortical neuronal loss in the traumatically injured brain. J Neurotrauma. 2009;26:121–9.
Hoane MR, Pierce JL, Holland MA, Birky ND, Dang T, Vitek MP, et al. The novel apolipoprotein e-based peptide cog1410 improves sensorimotor performance and reduces injury magnitude following cortical contusion injury. J Neurotrauma. 2007;24:1108–18.
James ML, Sullivan PM, Lascola CD, Vitek MP, Laskowitz DT. Pharmacogenomic effects of apolipoprotein e on intracerebral hemorrhage. Stroke. 2009;40:632–9.
Kaufman NA, Beare JE, Tan AA, Vitek MP, McKenna SE, Hoane MR. Cog1410, an apolipoprotein e-based peptide, improves cognitive performance and reduces cortical loss following moderate fluid percussion injury in the rat. Behav Brain Res. 2010;214:395–401.
Laskowitz DT, Lei B, Dawson HN, Wang H, Bellows ST, Christensen DJ, et al. The apoe-mimetic peptide, cog1410, improves functional recovery in a murine model of intracerebral hemorrhage. Neurocrit Care. 2012;16:316–26.
Tukhovskaya EA, Yukin AY, Khokhlova ON, Murashev AN, Vitek MP. Cog1410, a novel apolipoprotein-e mimetic, improves functional and morphological recovery in a rat model of focal brain ischemia. J Neurosci Res. 2009;87:677–82.
Wang H, Anderson LG, Lascola CD, James ML, Venkatraman TN, Bennett ER, et al. Apolipoproteine mimetic peptides improve outcome after focal ischemia. Exp Neurol. 2013;241:67–74.
Wu Y, Pang J, Peng J, Cao F, Vitek MP, Li F, et al. An apoe-derived mimic peptide, cog1410, alleviates early brain injury via reducing apoptosis and neuroinflammation in a mouse model of subarachnoid hemorrhage. Neurosci Lett. 2016;627:92–9.
Laskowitz DT, Song P, Wang H, Mace B, Sullivan PM, Vitek MP, et al. Traumatic brain injury exacerbates neurodegenerative pathology: Improvement with an apolipoprotein e-based therapeutic. J Neurotrauma. 2010;27:1983–95.
Krishnamurthy K, Cantillana V, Wang H, Sullivan PM, Kolls BJ, Ge X, et al. Apoe mimetic improves pathology and memory in a model of alzheimer’s disease. Brain Res. 2020;1733:146685.
Guptill JT, Raja SM, Boakye-Agyeman F, Noveck R, Ramey S, Tu TM, et al. Phase 1 randomized, double-blind, placebo-controlled study to determine the safety, tolerability, and pharmacokinetics of a single escalating dose and repeated doses of cn-105 in healthy adult subjects. J Clin Pharmacol. 2017;57:770–6.
Woo D, Rosand J, Kidwell C, McCauley JL, Osborne J, Brown MW, et al. The ethnic/racial variations of intracerebral hemorrhage (erich) study protocol. Stroke. 2013;44:e120-125.
Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ich score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–7.
Urday S, Beslow LA, Goldstein DW, Vashkevich A, Ayres AM, Battey TW, et al. Measurement of perihematomal oedema in intracerebral hemorrhage. Stroke. 2015;46:1116–9.
Aono M, Lee Y, Grant ER, Zivin RA, Pearlstein RD, Warner DS, et al. Apolipoprotein e protects against nmda excitotoxicity. Neurobiol Dis. 2002;11:214–20.
Laskowitz DT, Goel S, Bennett ER, Matthew WD. Apolipoprotein e suppresses glial cell secretion of tnf alpha. J Neuroimmunol. 1997;76:70–4.
Guttman M, Prieto JH, Handel TM, Domaille PJ, Komives EA. Structure of the minimal interface between apoe and lrp. J Mol Biol. 2010;398:306–19.
Misra UK, Adlakha CL, Gawdi G, McMillian MK, Pizzo SV, Laskowitz DT. Apolipoprotein e and mimetic peptide initiate a calcium-dependent signaling response in macrophages. J Leukoc Biol. 2001;70:677–83.
Sheng Z, Prorok M, Brown BE, Castellino FJ. N-methyl-d-aspartate receptor inhibition by an apolipoprotein e-derived peptide relies on low-density lipoprotein receptor-associated protein. Neuropharmacology. 2008;55:204–14.
Aono M, Bennett ER, Kim KS, Lynch JR, Myers J, Pearlstein RD, et al. Protective effect of apolipoprotein e-mimetic peptides on n-methyl-d-aspartate excitotoxicity in primary rat neuronal-glial cell cultures. Neuroscience. 2003;116:437–45.
Laskowitz DT, Fillit H, Yeung N, Toku K, Vitek MP. Apolipoprotein e-derived peptides reduce cns inflammation: implications for therapy of neurological disease. Acta Neurol Scand Suppl. 2006;185:15–20.
Laskowitz DT, Thekdi AD, Thekdi SD, Han SK, Myers JK, Pizzo SV, et al. Downregulation of microglial activation by apolipoprotein e and apoe-mimetic peptides. Exp Neurol. 2001;167:74–85.
Laskowitz DT, Vitek MP. Apolipoprotein e and neurological disease: therapeutic potential and pharmacogenomic interactions. Pharmacogenomics. 2007;8:959–69.
James ML, Komisarow JM, Wang H, Laskowitz DT. Therapeutic development of apolipoprotein e mimetics for acute brain injury: augmenting endogenous responses to reduce secondary injury. Neurotherapeutics. 2020;17:475–83.
Laskowitz DT, Wang H, Chen T, Lubkin DT, Cantillana V, Tu TM, et al. Neuroprotective pentapeptide cn-105 is associated with reduced sterile inflammation and improved functional outcomes in a traumatic brain injury murine model. Sci Rep. 2017;7:46461.
Lei B, James ML, Liu J, Zhou G, Venkatraman TN, Lascola CD, et al. Neuroprotective pentapeptide cn-105 improves functional and histological outcomes in a murine model of intracerebral hemorrhage. Sci Rep. 2016;6:34834.
Liu J, Zhou G, Kolls BJ, Tan Y, Fang C, Wang H, et al. Apolipoprotein e mimetic peptide cn-105 improves outcome in a murine model of sah. Stroke Vasc Neurol. 2018;3:222–30.
Tu TM, Kolls BJ, Soderblom EJ, Cantillana V, Ferrell PD, Moseley MA, et al. Apolipoprotein e mimetic peptide, cn-105, improves outcomes in ischaemic stroke. Ann Clin Transl Neurol. 2017;4:246–65.
Biffi A, Anderson CD, Jagiella JM, Schmidt H, Kissela B, Hansen BM, et al. Apoe genotype and extent of bleeding and outcome in lobar intracerebral haemorrhage: a genetic association study. Lancet Neurol. 2011;10:702–9.
Brouwers HB, Biffi A, Ayres AM, Schwab K, Cortellini L, Romero JM, et al. Apolipoprotein e genotype predicts hematoma expansion in lobar intracerebral hemorrhage. Stroke. 2012;43:1490–5.
Warner DS, James ML, Laskowitz DT, Wijdicks EF. Translational research in acute central nervous system injury: lessons learned and the future. JAMA Neurol. 2014;71:1311–8.
Wang H, Faw TD, Lin Y, Huang S, Venkatraman TN, Cantillana V, et al. Neuroprotective pentapeptide, cn-105, improves outcomes in translational models of intracerebral hemorrhage. Neurocrit Care. 2021. https://doi.org/10.1007/s12028-020-01184-y. Epub ahead of print.
de Gans K, de Haan RJ, Majoie CB, Koopman MM, Brand A, Dijkgraaf MG, et al. Patch: platelet transfusion in cerebral haemorrhage: Study protocol for a multicentre, randomised, controlled trial. BMC Neurol. 2010;10:19.
Poon MT, Fonville AF, Al-Shahi SR. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:660–7.
Selim M, Foster LD, Moy CS, Xi G, Hill MD, Morgenstern LB, et al. Deferoxamine mesylate in patients with intracerebral haemorrhage (i-def): a multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2019;18:428–38.
Acknowledgements
The authors would like to express their sincere gratitude to the full list of CATCH Investigators (Supplemental Table 1) for their tireless efforts in executing this trial.
Author information
Authors and Affiliations
Consortia
Contributions
MLJ designed the research, analyzed and interpreted the data, wrote the manuscript, and prepared the figures. JT designed portions of the research, analyzed and interpreted the data, wrote portions of the manuscript, and prepared the figures. NN analyzed and interpreted data, wrote portions of the manuscript, and offered critical edits to the manuscript. JK, CBS, KT, KH, MAB, BBW, and CA performed the research and collected data, and offered critical edits to the manuscript. DW designed part of the research, performed the research and collected data, and offered critical edits to the manuscript. PGK and Lascola analyzed and interpreted data, wrote portions of the manuscript, and offered critical edits to the manuscript. MM wrote portions of the manuscript and offered critical edits to the manuscript. DTL designed portions of the research, analyzed and interpreted the data, wrote portions of the manuscript, and prepared the figures. Authorship requirements have been met, and the final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Source of Support
The United States Food and Drug Administration provided grant funding for this trial (FDA FD-R-5387; DTL, MM). Aegis-CN provided the study drug, CN-105, and funding for this trial.
Conflict of interest
DTL is an officer and has equity in Aegis-CN. Duke University has equity and an intellectual property stake in CN-105 and might benefit if proven effective and successful commercially. MLJ serves as Principal Investigator for the CATCH trial, receiving grant funding for the trial from Aegis-CN. JT received consulting fees from Aegis-CN during the conduct of this trial. MM is an officer in Aegis-CN. All other authors have no conflicts to report.
Ethical Approval/Informed Consent
CATCH was approved by central institutional review board, Copernicus Group Independent Review Board, and by each participating site’s institutional review board. The study was performed in accordance with ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all individual participants or their legal authorized representatives prior to engagement in study procedures. Because of potential conflicts of interest with Duke University, which holds a portion of intellectual property, CATCH was required to have all primary data provenance and statistical analyses performed by a third party not financially related to Duke University (Pharpoint). Further, oversight of the study was performed using Data Safety and Monitoring Board Plus format, for which all participants were external to Duke and whose express duties included examining any potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
the CATCH Investigators: Full list in Supplemental Table 1.
This article was updated to replace an incorrect Supplemental Table 1.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
James, M.L., Troy, J., Nowacki, N. et al. CN-105 in Participants with Acute Supratentorial Intracerebral Hemorrhage (CATCH) Trial. Neurocrit Care 36, 216–225 (2022). https://doi.org/10.1007/s12028-021-01287-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01287-0