Abstract
Background
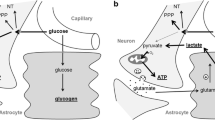
There has been growing interest in the use of hypertonic sodium lactate (HSL) solution following traumatic brain injury (TBI) in humans. However, little is known about the effects of HSL on functional deficits with respect to the hyperosmotic nature of HSL.
Methods
We have compared the effects of HSL solution and isotonic saline solution using sensorimotor and cognitive tests for 14 days post-trauma in animals. Thirty minutes after trauma (impact-acceleration model), anesthetized rats were randomly allocated to receive a 2-h infusion of isotonic saline solution (TBI-saline group) or HSL (TBI-HSL group) (n = 10 rats per group). In another series of experiments using a similar protocol, the effects of equiosmolar doses of HSL and hypertonic saline solution (HSS) were compared in TBI rats (n = 10 rats per group). Blood lactate and ion concentrations were measured during the 2-h infusions.
Results
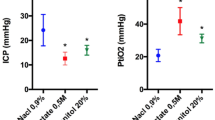
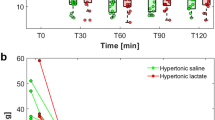
Compared to the TBI-saline group, the TBI-HSL group had a reduced latency to complete the adhesive removal test: 6 s (5–9) (median [25–75th centiles]) versus 13 s (8–17) on day 7, and 5 s (5–9) versus 11 s (8–26) on day 14 (P < 0.05), respectively, and a shorter delay to complete the radial arm maze test on day 7: 99 s (73–134) versus 176 s (127–300), respectively (P < 0.05). However, no differences were found between the TBI-HSL and TBI-HSS groups in neurocognitive tests performance. Compared to the TBI-saline group, the HSL and HSS groups had higher serum osmolality: 318 mOsm/Kg (315–321) and 315 mOsm/Kg (313–316) versus 307 mOsm/Kg (305–309), respectively (P < 0.05), and the HSL group had a higher serum lactate concentration: 6.4 mmol/L (5.3–7.2) versus 1.5 mmol/L (1.1–1.9) and 1.6 mmol/L (1.5–1.7), respectively (P < 0.05).
Conclusions
These results indicate that improvements in cognitive and sensorimotor tests with HSL infusion post-TBI could be related to elevation of serum osmolality, not to exogenous administration of lactate.




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- BVf:
-
Blood volume fraction
- HSL:
-
Hypertonic sodium lactate
- HSS:
-
Hypertonic saline solution
- StO2 :
-
Brain tissue oxygen saturation
- TBI:
-
Traumatic brain injury
- VSI:
-
Vessel size index
References
White H, Venkatesh B. Clinical review: ketones and brain injury. Crit Care. 2011;15:219.
Bouzat P, Oddo M. Lactate and the injured brain: friend or foe? Curr Opin Crit Care. 2014;20:133–40.
Greco T, Vespa PM, Prins ML. Alternative substrate metabolism depends on cerebral metabolic state following traumatic brain injury. Exp Neurol. 2020;329:113289.
Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science. 1988;240:1326–8.
Gallagher CN, Carpenter KL, Grice P, et al. The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain. 2009;132:2839–49.
Berthet C, Lei H, Thevenet J, Gruetter R, Magistretti PJ, Hirt L. Neuroprotective role of lactate after cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:1780–9.
Meierhans R, Brandi G, Fasshauer M, et al. Arterial lactate above 2 mM is associated with increased brain lactate and decreased brain glucose in patients with severe traumatic brain injury. Minerva Anestesiol. 2012;78:185–93.
Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–306.
Millet A, Cuisinier A, Bouzat P, et al. Hypertonic sodium lactate reverses brain oxygenation and metabolism dysfunction after traumatic brain injury. Br J Anaesth. 2018;120:1295–303.
Ichai C, Armando G, Orban JC, et al. Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intensive Care Med. 2009;35:471–9.
Ichai C, Payen JF, Orban JC, et al. Half-molar sodium lactate infusion to prevent intracranial hypertensive episodes in severe traumatic brain injured patients: a randomized controlled trial. Intensive Care Med. 2013;39:1413–22.
Bouzat P, Sala N, Suys T, et al. Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive Care Med. 2014;40:412–21.
Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–9.
Alessandri B, Schwandt E, Kamada Y, Nagata M, Heimann A, Kempski O. The neuroprotective effect of lactate is not due to improved glutamate uptake after controlled cortical impact in rats. J Neurotrauma. 2012;29:2181–91.
Mannino C, Glenn TC, Hovda DA, et al. Acute glucose and lactate metabolism are associated with cognitive recovery following traumatic brain injury. J Neurosci Res. 2018;96:696–701.
Rice AC, Zsoldos R, Chen T, et al. Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Res. 2002;928:156–9.
Holloway R, Zhou Z, Harvey HB, et al. Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat. Acta Neurochir (Wien). 2007;149:919–27.
Bisri T, Utomo BA, Fuadi I. Exogenous lactate infusion improved neurocognitive function of patients with mild traumatic brain injury. Asian J Neurosurg. 2016;11:151–9.
Sell SL, Avila MA, Yu G, et al. Hypertonic resuscitation improves neuronal and behavioral outcomes after traumatic brain injury plus hemorrhage. Anesthesiology. 2008;108:873–81.
Schreibman DL, Hong CM, Keledjian K, et al. Mannitol and hypertonic saline reduce swelling and modulate inflammatory markers in a rat model of intracerebral hemorrhage. Neurocrit Care. 2018;29:253–63.
Bouzat P, Millet A, Boue Y, et al. Changes in brain tissue oxygenation after treatment of diffuse traumatic brain injury by erythropoietin. Crit Care Med. 2013;41:1316–24.
Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg. 1994;80:291–300.
Christen T, Bouzat P, Pannetier N, et al. Tissue oxygen saturation mapping with magnetic resonance imaging. J Cereb Blood Flow Metab. 2014;34:1550–7.
Tropres I, Pannetier N, Grand S, et al. Imaging the microvessel caliber and density: Principles and applications of microvascular MRI. Magn Reson Med. 2015;73:325–41.
Beaumont A, Marmarou A, Czigner A, et al. The impact-acceleration model of head injury: injury severity predicts motor and cognitive performance after trauma. Neurol Res. 1999;21:742–54.
Schallert T, Upchurch M, Lobaugh N, et al. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacol Biochem Behav. 1982;16:455–62.
Thompson HJ, Marklund N, LeBold DG, et al. Tissue sparing and functional recovery following experimental traumatic brain injury is provided by treatment with an anti-myelin-associated glycoprotein antibody. Eur J Neurosci. 2006;24:3063–72.
Olton DS, Samuelson RJ. Remembrance of places passed: spatial memory in rats. J Exp Psychol Anim Behav Process. 1976;2:97–116.
Soblosky JS, Tabor SL, Matthews MA, Davidson JF, Chorney DA, Carey ME. Reference memory and allocentric spatial localization deficits after unilateral cortical brain injury in the rat. Behav Brain Res. 1996;80:185–94.
Foda MA, Marmarou A. A new model of diffuse brain injury in rats Part II: Morphological characterization. J Neurosurg. 1994;80:301–13.
O'Connor WT, Smyth A, Gilchrist MD. Animal models of traumatic brain injury: a critical evaluation. Pharmacol Ther. 2011;130:106–13.
Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14:128–42.
Sebastian V, Diallo A, Ling DS, Serrano PA. Robust training attenuates TBI-induced deficits in reference and working memory on the radial 8-arm maze. Front Behav Neurosci. 2013;7:38.
Wenk GL (2004) Assessment of spatial memory using the radial arm maze and Morris water maze. Curr Protoc Neurosci; Chapter 8:Unit 8 5A
van Hall G, Stromstad M, Rasmussen P, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–9.
Schilte C, Bouzat P, Millet A, et al. Mannitol improves brain tissue oxygenation in a model of diffuse traumatic brain injury. Crit Care Med. 2015;43:2212–8.
Millet A, Bouzat P, Trouve-Buisson T, et al. Erythropoietin and its derivates modulate mitochondrial dysfunction after diffuse traumatic brain injury. J Neurotrauma. 2016;33:1625–33.
Barzo P, Marmarou A, Fatouros P, Hayasaki K, Corwin F. Biphasic pathophysiological response of vasogenic and cellular edema in traumatic brain swelling. Acta Neurochir Suppl. 1997;70:119–22.
Nimmo AJ, Cernak I, Heath DL, Hu X, Bennett CJ, Vink R. Neurogenic inflammation is associated with development of edema and functional deficits following traumatic brain injury in rats. Neuropeptides. 2004;38:40–7.
Tanino M, Kobayashi M, Sasaki T, et al. Isoflurane Induces Transient Impairment of Retention of Spatial Working Memory in Rats. Acta Med Okayama. 2016;70:455–60.
Acknowledgements
The authors wish to thank Vasile Stupar and Nora Collomb (IRMaGe) for their technical help with the experiments.
Funding
This study was conducted at the INSERM U1216/Grenoble Institut des Neurosciences and was partly funded by the “Fondation des Gueules Cassées” (Paris, France). Brain MRI was performed at the Grenoble MRI Facility (IRMaGe) that is partly funded by the French program “Investissement d'Avenir” (Agence Nationale de la Recherche, Grant Infrastructure d'Avenir en Biologie Santé ANR-11-INBS-0006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
The study design was approved by the Internal Evaluation Committee for Animal Welfare and Rights (Ref#2017030614362942). Experiments were performed in accordance with the guidelines of the French Government (licenses 380819 and B3851610008). All applicable institutional and national guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Crespy, T., Durost, M., Fricault, P. et al. Hypertonic Sodium Lactate to Alleviate Functional Deficits Following Diffuse Traumatic Brain Injury: An Osmotic or a Lactate-Related Effect?. Neurocrit Care 34, 795–803 (2021). https://doi.org/10.1007/s12028-020-01090-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-01090-3